Abstract
Background
This post hoc subgroup analysis of a randomized, double-blind trial evaluated the response to treatment with two long-acting injectable atypical antipsychotics, ie, paliperidone palmitate and risperidone long-acting injectable (RLAI), in subjects with schizophrenia experiencing clinically significant symptoms despite recent treatment with oral risperidone only or other oral antipsychotics.
Methods
Adult subjects were eligible for the 13-week, double-blind, double-dummy trial (NCT00589914) if they had an established diagnosis of schizophrenia for at least one year and a Positive and Negative Syndrome Scale (PANSS) total score of 60–120 inclusive at screening. Subjects received either paliperidone palmitate (234 mg, day 1; 156 mg, day 8; then once-monthly flexible dosing) or RLAI (25–50 mg biweekly, with oral risperidone supplementation on days 1–28), plus matched placebo injections/tablets.
Results
This post hoc analysis reports data on 747 subjects who, within 2 weeks of starting double-blind study medication, had reportedly received oral risperidone only (paliperidone palmitate group, n = 126; RLAI group, n = 107), other oral antipsychotics (paliperidone palmitate group, n = 199; RLAI group, n = 203), or no antipsychotic (paliperidone palmitate group, n = 56; RLAI group, n = 56). Mean PANSS total scores improved significantly at end point across all subgroups (mean change from baseline ranged from −17.5 to −19.5, all P < 0.0001). Clinical Global Impression-Severity and Personal and Social Performance scale measures also significantly improved from baseline (all P < 0.0001).
Conclusion
Treatment with paliperidone palmitate or RLAI resulted in a significant reduction in the symptoms of schizophrenia irrespective of previous recent treatment with oral risperidone only or other oral antipsychotics. For subjects who had previously received oral risperidone only, the difference in formulation was the main change in the intervention because the molecule delivered remained the same or similar. These data support the contribution of a long-acting formulation to improving the treatment response and suggest that nonadherence may be a significant contributor to inadequate efficacy of oral formulations in subjects with schizophrenia.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Treatment options tailored to patient and clinician choice are an important aspect of therapy for schizophrenia. Unfortunately, adherence to treatment with oral antipsychotics is poor,Citation1 and this is associated with clinical and functional deterioration, increased risk of relapse, rehospitalization, and increased risk for suicidal behavior.Citation2–Citation5 Simplified antipsychotic regimens that can provide continuous long-term symptom relief may be helpful in improving these outcomes. In this regard, long-acting injectable antipsychotics may improve adherence over oral antipsychotics by reducing the requirement from daily dosing to biweekly or monthly dosing.Citation3,Citation6–Citation9 This reduces the requirement for patients to remember to take their medication from 365 times annually for once-daily oral dosing, to 26 times annually for biweekly dosing or 12 times annually for monthly dosing. Further, health care providers can be certain of their patients’ level of adherence to their medications, and resources are not wasted on medication that is discarded or forgotten.
Paliperidone palmitate and risperidone long-acting injection (RLAI) are two long-acting, injectable, atypical antipsychotics that are effective in treating schizophrenia.Citation10–Citation15 They deliver related molecules (paliperidone [9-hydroxy risperidone] and risperidone, respectively) using formulations with different pharmacologic and release profiles and different initiation and maintenance regimens. Paliperidone palmitate is the palmitate ester of paliperidone.Citation16–Citation18 Treatment with paliperidone palmitate is initiated with deltoid injections (234 mg on day 1 and 156 mg on day 8), followed by once-monthly injections (deltoid or gluteal, 39–234 mg), without oral supplementation.Citation19 RLAI is a microsphere formulation of risperidone and is administered intramuscularly biweekly (25–50 mg).Citation20 Because less than 1% of risperidone is released during the first 3 weeks of treatment with RLAI, oral supplementation with risperidone (or another antipsychotic) should accompany the first RLAI dose and continue for the initial 3 weeks of RLAI treatment.Citation20
Until novel therapies are developed that offer new mechanisms of action for treating schizophrenia, improving delivery of effective agents and addressing the problem of daily adherence remain important strategies to improve outcomes for these individuals. However, because of the pharmacologic relationships between risperidone and paliperidone palmitate and among the active entities of their oral and injectable formulations, questions may be raised about the efficacy of RLAI and paliperidone palmitate in subjects who have recently been treated with oral risperidone but continue to experience symptoms of schizophrenia. This post hoc analysis was undertaken to compare treatment responses to RLAI and paliperidone palmitate in subjects who had recently been treated with oral risperidone only, who had been treated with other antipsychotics, or who were not receiving any antipsychotic treatment at the time they entered the study. These exploratory findings are informative about whether the long-acting formulations of these agents offer benefit to subjects with persistent symptoms despite recent antipsychotic therapy with an oral version of the same or a similar product.
Materials and methods
Study design
This was a post hoc analysis of a 13-week, double-blind, double-dummy, multicenter study (NCT00589914). The original study was designed to evaluate the efficacy and safety of paliperidone palmitate treatment as compared with RLAI in adult subjects with schizophrenia and demonstrated the noninferiority of paliperidone palmitate versus RLAI in the primary efficacy variable in subjects with schizophrenia; details of the original study population and results of the noninferiority analysis are published elsewhere.Citation21 This post hoc analysis was performed to assess the efficacy of a long-acting injectable antipsychotic (either paliperidone palmitate or RLAI) in those subjects from the original trial who had been treated within 2 weeks before starting double-blind study medication with oral risperidone only or with other antipsychotics. Subjects who were not taking oral antipsychotics immediately prior to the trial were also included in the analysis. Previous long-acting injectable antipsychotic treatment was not part of this subgroup analysis because the original study excluded subjects who had received an injectable antipsychotic within one injection interval before screening. Subjects who had received oral paliperidone previously were excluded because the sample size was too small (n = 18).
Subjects
Adult men and women aged ≥18 years were eligible for the original study if they had met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision,Citation22 criteria for schizophrenia for at least one year; had a screening Positive and Negative Syndrome Scale (PANSS)Citation23 total score between 60 and 120, inclusive; and had a body mass index of 17–40 kg/m2, inclusive. All subjects provided written informed consent before study entry, and the original study protocol was reviewed by an independent ethics committee or an institutional review board at each study site. The trial was conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with Good Clinical Practices and applicable regulatory requirements.
Study medication
Subjects were randomly assigned in a 1:1 ratio to receive paliperidone palmitate or RLAI. Paliperidone palmitate was administered via deltoid injection on day 1 (234 mg) and again on day 8 (156 mg), followed by once-monthly deltoid or gluteal injections according to subject choice on days 36 (78 or 156 mg) and 64 (78, 156, or 234 mg), with RLAI-matched placebo gluteal injections. RLAI was administered as biweekly gluteal injections at day 8 (25 mg), then day 22 (25 mg), days 36 and 50 (25 or 37.5 mg), and days 64 and 78 (25, 37.5, or 50 mg), with paliperidone palmitate–matched placebo deltoid or gluteal injections. RLAI subjects received oral risperidone supplementation (1–6 mg/day, days 1–28; optional thereafter with dose increases); paliperidone palmitate subjects received oral placebo. Where flexible doses of medication were permitted, the choice was determined by the treating physician based on perceived risk versus benefit.
Paliperidone palmitate doses may also be expressed as milligram equivalents (mg eq) of paliperidone, with 39, 78, 117, 156, and 234 mg of paliperidone palmitate being equivalent to 25, 50, 75, 100, and 150 mg eq of paliperidone, respectively.Citation16,Citation19 This report expresses paliperidone palmitate as milligrams.
Concomitant medication
Subjects were allowed to continue receiving antidepressants (except for nonselective and irreversible monoamine oxidase inhibitors) if they had been on a stable dose for at least 30 days before screening. Antiparkinsonian medication was washed out before study entry but could be reintroduced by the investigator if extrapyramidal symptoms emerged or worsened during the study; allowed antiparkinsonian medications were trihexyphenidyl, benztropine, biperiden, and antihistamines with anticholinergic properties. Oral benzodiazepines (at permitted maximum daily doses) were also allowed, preferably lorazepam. Mood stabilizers (including lithium and all anticonvulsants) and any prescription, herbal, or over-the-counter agents with psychotropic actions were not allowed during the double-blind treatment period.
Study assessments
Assessments included change from baseline to end point in PANSS total, PANSS factors,Citation24 Clinical Global Impression–Severity (CGI-S),Citation25 and Personal and Social Performance (PSP)Citation26 scale scores. Responder rate (defined as those subjects with a 30% or greater improvement in PANSS total score from baseline) was also determined. Adverse event reports were collected at each visit from the time an informed consent form was obtained until completion of the last study-related procedure.
Statistical analysis
For this post hoc subgroup analysis, data were analyzed separately for the paliperidone palmitate and RLAI subpopulations. There was no statistical comparison between the two treatment arms of the study because subjects had not been randomly assigned within the subgroups, and this was not the objective of the analysis. For the initial analysis of the overall study, four analysis sets were defined, ie, the safety analysis set, the intent-to-treat analysis set, the per-protocol analysis set, and the pharmacokinetic analysis set.Citation21 For this post hoc subgroup comparison, the per-protocol analysis set, originally used only for the primary efficacy analysis,Citation21 was considered appropriate for analysis of all efficacy and safety assessments as the comparison was exploratory and did not look at early time points. The per-protocol set was defined as all subjects with a baseline and at least one post randomization PANSS measure, minimum exposure of 36 days to the double-blind treatment regimen, and no major protocol violations.Citation21 Within each treatment group, subjects were analyzed based on whether they had received oral risperidone treatment only, treatment with another antipsychotic, or no previous antipsychotic treatment in the 2 weeks before starting double-blind study medication. Mean (±standard deviation), median, minimum, and maximum were used for summary of continuous variables; percentage and frequency were used for categorical variables. Within-group differences were evaluated using a paired t-test. All statistical tests were two-sided, and no adjustments were made for multiplicity. The analysis used last-observation-carried-forward methodology.
Results
Subject disposition, baseline demographics, and clinical characteristics
A total of 747 subjects (61% of those randomized) from the original study were included for this subgroup analysis (). Two hundred thirty-three subjects received oral risperidone only within 2 weeks before starting double-blind study medication (paliperidone palmitate treatment arm, n = 126; RLAI treatment arm, n = 107). A further 402 subjects had received some other oral antipsychotic within 2 weeks before starting double-blind study medication (paliperidone palmitate, n = 199; RLAI, n = 203), and 112 subjects were not receiving any antipsychotics during the 2 weeks before starting double-blind study medication (n = 56 for both study arms).
Table 1 Baseline demographics and clinical characteristics
Thirteen-week completion rates ranged from 79% to 88% across the subgroups analyzed (). The most common reason for discontinuation in subjects who had received oral risperidone only during the 2 weeks before starting double-blind study medication was withdrawal of consent (6.3% in the paliperidone palmitate group; 5.6% in the RLAI group), whereas in subjects who had received other antipsychotics, the reason was lack of efficacy (7.5% in the paliperidone palmitate group; 6.4% in the RLAI group). Discontinuation rates due to adverse events were low across all subgroups analyzed (range 0%–3.6%, ). One subject from the original study who died met the inclusion criteria for this analysis. Details have been reported elsewhere.Citation21 Exposure to the study medication (paliperidone palmitate or RLAI) is outlined in . For subjects in the prior risperidone only population, the mean modal dose of risperidone within 2 weeks before the start of double-blind study medication was 5.2 ± 3.2 mg for the paliperidone palmitate group and 5.0 ± 3.1 mg for the RLAI group.
Table 2 Patient disposition and study medication exposure
Efficacy
Baseline scores for each of the efficacy measures for both the RLAI and paliperidone palmitate treatment groups are outlined in . Improvement in PANSS total, PANSS factors, and CGI-S scale scores from baseline to end point was significant for both paliperidone palmitate and RLAI treatment groups (all P ≤ 0.0002), regardless of whether subjects had received recent prior treatment with oral risperidone only, other antipsychotics, or no antipsychotic in the 2 weeks before starting double-blind study medication (). Responder rates at end point ranged from 48.2% to 66.1% across groups (). The distribution of categorical CGI-S scores () showed improvements that were similar by inspection from baseline to end point for both the paliperidone palmitate and RLAI subpopulations, regardless of whether subjects had previously received risperidone only, other antipsychotics, or no antipsychotics.
Figure 1 Categorical CGI-S scores from baseline to end point.
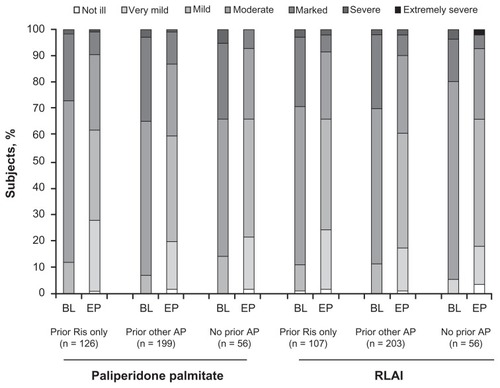
Table 3 Efficacy outcomes
Functioning
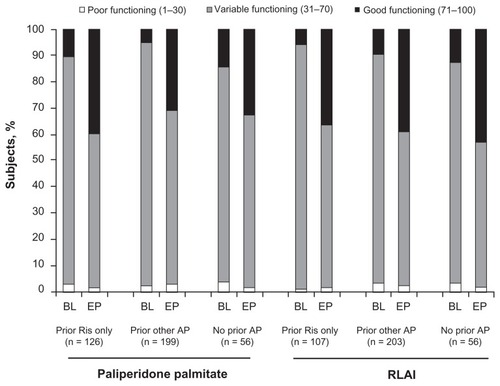
PSP scale scores significantly improved from baseline to end point among all previous treatment subgroups for both paliperidone palmitate and RLAI treatment groups (all P < 0.0001, ). The distribution of categorical PSP scale scores () showed improvement across all groups.
Safety
From 52% to 61% of subjects across the six subgroups analyzed experienced at least one treatment-emergent adverse event. The most common adverse events, and those of particular interest (extrapyramidal symptoms, prolactin-related and glucose-related adverse events), are outlined in . Common adverse events for paliperidone palmitate subjects were insomnia, headache, and injection site pain, irrespective of prior oral risperidone status. Common adverse events for all RLAI subjects were insomnia and headache.
Table 4 Treatment-emergent adverse events
Discussion
This post hoc subgroup analysis of a 13-week trial demonstrates that treatment with monthly paliperidone palmitate or biweekly RLAI effectively reduced symptoms of schizophrenia independently of whether previous treatment consisted of oral risperidone only or oral antipsychotics, or whether no immediate previous treatment had been received. The robustness of this result is supported by findings of consistent symptom improvement across all efficacy measures used (PANSS and CGI-S) and by the functioning outcome measured by the PSP scale. Of particular interest is the finding that symptom reduction was similar for the groups receiving an oral form of an identical or chemically related molecule both before and after randomization to long-acting injection (risperidone followed by RLAI, and risperidone followed by paliperidone palmitate). This supports the clinical value of initiating long-acting injectable treatment in subjects who have not achieved adequate symptom control with an identical or chemically related oral antipsychotic, despite the similarities in mechanisms of action. Of note, our analysis is consistent with other studies of injectable formulations of paliperidone palmitate and risperidone that report successful symptom reduction in subjects who had remained symptomatic after therapy with a related oral molecule.Citation27–Citation31 However, it is relevant to note here that published findings from direct comparisons of long-acting injectable and oral antipsychotics have yielded conflicting results.Citation9,Citation32,Citation33
This analysis included data on patients who were not taking oral antipsychotics immediately before entering the trial. This clinically relevant patient population might be expected to respond well after starting paliperidone palmitate or RLAI, or even better than those patients who were symptomatic despite treatment with oral antipsychotics. The similarity in response to paliperidone palmitate and RLAI seen in these patients compared with those who had been receiving risperidone only or other antipsychotics suggests that the lack of efficacy that the majority of patients experienced using oral antipsychotics before study entry, particularly risperidone, was due to some reason other than lack of pharmacologic action.
Poor adherence to prescribed treatment is a well-recognized problem for subjects with schizophrenia. At least one-third of subjects struggle to adhere adequately to treatment after only a few weeks of therapy, and only one-quarter remain fully adherent 2 years after initiating therapy.Citation3,Citation7 Nonadherence to medication can result in increased hospitalizations (both psychiatric and nonpsychiatric),Citation5 exacerbation of schizophrenia symptoms, and poor functional outcomes.Citation7 Subjects who remained symptomatic after oral risperidone may have benefited from treatment with extended-release oral formulations of paliperidone palmitate because of improved pharmacokinetics and tolerability.Citation34,Citation35 However, this formulation still requires daily dosing. Long-acting injectable medications can further help overcome problems with nonadherence by removing the need for daily dosing and by simplifying treatment.Citation3,Citation7 Because the health care provider can know with certainty whether a patient has received an injection, use of long-acting injectable antipsychotics provides clinicians with definitive information on patient adherence to medication. This removes the need for ongoing discussion of the need for medication and careful adherence to its use,Citation3,Citation36 and patient contact with treatment teams can be more focused on providing psychoeducation and social skills training.Citation3 Further, there is a potential health economic advantage of knowing that a prescribed medication has been taken rather than discarded or left unused in a medicine cabinet.
Another possible reason for improved treatment response to paliperidone palmitate or RLAI in subjects with a recent history of suboptimal response to treatment with oral risperidone is that long-acting injectable formulations provide more continuous delivery of medication without daily peaks and troughs.Citation37,Citation38 Depot injections improve the bioavailability of antipsychotics, which typically have variable bioavailability when taken orally because of nonspecific metabolism in the gut wall and first-pass hepatic metabolism.Citation37,Citation39 Increased bioavailability means that lower total drug doses may be required to achieve similar clinical outcomes because a greater portion of the dose is available to the central nervous system.Citation39 The reduction in daily peak and trough blood levels compared with oral compounds may contribute to fewer adverse events and again to better long-term compliance.Citation37 Interestingly, discontinuations due to lack of efficacy were no more frequent in the prior oral risperidone only subgroups than in those receiving alternative prior treatments. Lack of efficacy might have been expected to be greater in these subjects because only the method of delivery of similar molecules was changed. This finding highlights the importance of formulation considerations when treating subjects with schizophrenia.
Tolerability and safety in this subgroup analysis were consistent with findings from the overall study population.Citation21 Injection site pain was reported more frequently by subjects in the paliperidone palmitate subgroups than by those receiving RLAI (range 3.0–10.7% vs 0–1.0%, respectively). This may have been due to the initial injection site for the active treatment (deltoid for paliperidone palmitate vs gluteal for RLAI), because gluteal injections have been reported to be somewhat better tolerated than deltoid injections.Citation40
Limitations
The original study was not designed to examine subjects by prior oral antipsychotic treatment and, therefore, complete information on prior treatment (including dose, duration, and adherence) was not systematically collected. Available information was dependent on retrospective subject or clinician reports. For this reason, it was not possible to determine whether the duration and dose of prior treatment with risperidone only or other antipsychotics had been optimized before switching to the long-acting study medication. In addition, it is possible that the observed improvements with paliperidone palmitate and RLAI were due in part to study participation and regression to the mean. However, given that improvements were seen across all treatment groups and were consistent across multiple measures (psychotic symptoms, global status, and functioning), this seems unlikely. A comparator group with oral antipsychotic treatment could have helped clarify these limitations to interpretation. Finally, although numerical differences were noted in the distribution of gender and race between the subgroups, significant improvements in schizophrenia symptoms were seen in every subgroup analyzed, making further exploration of these baseline characteristics unnecessary.
In conclusion, this post hoc analysis of a 13-week trial suggests that treatment with paliperidone palmitate or RLAI can be effective in subjects regardless of which oral antipsychotic treatment they have received. For patients who had previously received oral risperidone only, the difference in formulation was the main change in the intervention, because the molecule delivered remained the same or similar. These data support the contribution of a long-acting formulation to improved treatment response, and suggest that nonadherence may be a significant contributor to inadequate efficacy of oral formulations in subjects with schizophrenia.
Acknowledgment
The authors wish to acknowledge Matthew Grzywacz and Alison Comer of ApotheCom (funded by Janssen Scientific Affairs LLC) for providing writing and editorial assistance.
Disclosure
This study was supported by Janssen Scientific Affairs LLC. LA, D-JF, CAB, JKS, and JH are employees of Janssen Scientific Affairs LLC, and are Johnson & Johnson stockholders. Y-WM is an employee of Janssen Research & Development LLC, and is a Johnson & Johnson stockholder.
References
- LiebermanJAStroupTSMcEvoyJPEffectiveness of antipsychotic drugs in patients with chronic schizophreniaN Engl J Med20053531209122316172203
- LindenmayerJPLiu-SeifertHKulkarniPMMedication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior responseJ Clin Psychiatry20097099099619497244
- NasrallahHAThe case for long-acting antipsychotic agents in the post-CATIE eraActa Psychiatr Scand200711526026717355516
- NovickDHaroJMSuarezDPerezVDittmannRWHaddadPMPredictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophreniaPsychiatry Res201017610911320185182
- WeidenPJKozmaCGroggALocklearJPartial compliance and risk of rehospitalization among California Medicaid patients with schizophreniaPsychiatr Serv20045588689115292538
- BuchananRWKreyenbuhlJKellyDLThe 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statementsSchizophr Bull201036719319955390
- VelliganDIWeidenPJSajatovicMThe expert consensus guideline series: adherence problems in patients with serious and persistent mental illnessJ Clin Psychiatry20097014619686636
- LeuchtCHeresSKaneJMKisslingWDavisJMLeuchtSOral versus depot antipsychotic drugs for schizophrenia – a critical systematic review and meta-analysis of randomised long-term trialsSchizophr Res2011127839221257294
- TiihonenJHaukkaJTaylorMHaddadPMPatelMXKorhonenPA nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophreniaAm J Psychiatry201116860360921362741
- KaneJMEerdekensMLindenmayerJPKeithSJLesemMKarcherKLong-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychoticAm J Psychiatry20031601125113212777271
- SimpsonGMMahmoudRALasserRAA 1-year double-blind study of 2 doses of long-acting risperidone in stable patients with schizophrenia or schizoaffective disorderJ Clin Psychiatry2006671194120316965196
- FleischhackerWWEerdekensMKarcherKTreatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychoticJ Clin Psychiatry2003641250125714658976
- KramerMLitmanRHoughDPaliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety studyInt J Neuropsychopharmacol20101363564719941696
- GopalSHoughDWXuHEfficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response studyInt Clin Psychopharmacol20102524725620389255
- PandinaGJLindenmayerJPLullJA randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophreniaJ Clin Psychopharmacol20103023524420473057
- CitromeLPaliperidone palmitate – review of the efficacy, safety and cost of a new second-generation depot antipsychotic medicationInt J Clin Pract20106421623919886879
- GopalSGassmann-MayerCPalumboJSamtaniMNShiwachRAlphsLPractical guidance for dosing and switching paliperidone palmitate treatment in patients with schizophreniaCurr Med Res Opin20102637738720001492
- HoughDGopalSVijapurkarULimPMorozovaMEerdekensMPaliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled studySchizophr Res201011610711719959339
- Janssen Pharmaceuticals IncInvega® Sustenna® (paliperidone palmitate) extended-release injectable suspensionTitusville, NJJanssen Pharmaceuticals Inc82012
- Janssen Pharmaceuticals IncRisperdal® Consta® (risperidone) long-acting injectionTitusville, NJJanssen Pharmaceuticals Inc62012
- PandinaGLaneRGopalSA double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophreniaProg Neuropsychopharmacol Biol Psychiatry20113521822621092748
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text RevisionWashington, DCAmerican Psychiatric Association2000
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull1987132612763616518
- MarderSRDavisJMChouinardGThe effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trialsJ Clin Psychiatry1997585385469448657
- GuyWECDEU Assessment Manual for Psychopharmacology (028 Clinical Global Impressions [CGI])1976218222
- MorosiniPLMaglianoLBrambillaLUgoliniSPioliRDevelopment, reliability and acceptability of a new version of the DSM-IV social and occupational functioning assessment scale (SOFAS) to assess routine social functioningActa Psychiatr Scand200010132332910782554
- SliwaJKBossieCAMaYWAlphsLEffects of acute paliperidone palmitate treatment in subjects with schizophrenia recently treated with oral risperidoneSchizophr Res2011132283421775106
- MollerHJLlorcaPMSacchettiEMartinSDMedoriRParelladaEEfficacy and safety of direct transition to risperidone long-acting injectable in patients treated with various antipsychotic therapiesInt Clin Psychopharmacol20052012113015812261
- van OsJBossieCALasserRAImprovements in stable patients with psychotic disorders switched from oral conventional antipsychotics therapy to long-acting risperidoneInt Clin Psychopharmacol20041922923215201570
- De MarinisTSaleemPTGluePSwitching to long-acting injectable risperidone is beneficial with regard to clinical outcomes, regardless of previous conventional medication in patients with schizophreniaPharmacopsychiatry20074025726318030649
- SchmaussMSacchettiEKahnJPMedoriREfficacy and safety of risperidone long-acting injectable in stable psychotic patients previously treated with oral risperidoneInt Clin Psychopharmacol200722859217293708
- Grimaldi-BensoudaLRouillonFAstrucBDoes long-acting injectable risperidone make a difference to the real-life treatment of schizophrenia? Results of the Cohort for the General study of Schizophrenia (CGS)Schizophr Res201213418719422130111
- RosenheckRAKrystalJHLewRLong-acting risperidone and oral antipsychotics in unstable schizophreniaN Engl J Med201136484285121366475
- CanusoCMGrinspanAKalaliAMedication satisfaction in schizophrenia: a blinded-initiation study of paliperidone extended release in patients suboptimally responsive to risperidoneInt Clin Psychopharmacol20102515516420216424
- CanusoCMYoussefEABossieCATurkozISchreinerASimpsonGMPaliperidone extended-release tablets in schizophrenia patients previously treated with risperidoneInt Clin Psychopharmacol20082320921518545059
- KaneJMGarcia-RiberaCClinical guideline recommendations for antipsychotic long-acting injectionsBr J Psychiatry Suppl200952S63S6719880920
- EreshefskyLMascarenasCAComparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamicsJ Clin Psychiatry200364182314680415
- SamtaniMNVermeulenAStuyckensKPopulation pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychoticClin Pharmacokinet20094858560019725593
- McEvoyJPRisks versus benefits of different types of long-acting injectable antipsychoticsJ Clin Psychiatry200667 Suppl 5151816822092
- HoughDLindenmayerJPGopalSSafety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophreniaProg Neuropsychopharmacol Biol Psychiatry2009331022103119481579