Abstract
Background
Up to 30% of depressed patients are partially or totally resistant to antidepressant therapy. The administration of triiodothyronine (T3) to antidepressant nonresponders can be an effective augmentation strategy, although the mechanism is not fully understood.
Methods
In vivo microdialysis was used to examine the effect of T3 augmentation of the antidepressant, milnacipran. Basal extracellular serotonin, norepinephrine, and dopamine levels were measured before and after acute milnacipran administration in the medial prefrontal cortex and amygdala of rats which had received subchronic (7 days) T3 treatment or control saline.
Results
Subchronic administration of T3 at 0.1 mg/kg significantly increased basal extracellular levels of serotonin in the medial prefrontal cortex, but not in the amygdala. In contrast, subchronic administration of T3 at 0.2 mg/kg did not alter basal extracellular serotonin levels in the medial prefrontal cortex. Basal extracellular levels of norepinephrine and dopamine were not modified by either dose of T3 in either region. Acute administration of milnacipran, a serotonin-norepinephrine reuptake inhibitor, to control animals resulted in a significant increase of extracellular levels of serotonin, norepinephrine, and dopamine. When administered to animals treated subchronically with T3 at 0.1 mg/kg, milnacipran produced an additional increase in extracellular serotonin levels but not in levels of norepinephrine or dopamine in the medial prefrontal cortex of rats.
Conclusion
These results suggest that the mechanism of the augmentation effect of milnacipran by T3 administration occurs via enhancement of serotonergic neurotransmission, but not through noradrenergic or dopaminergic neurotransmission.
Introduction
Major depression is considered by the World Health Organization to be the fourth most disabling disorder worldwide.Citation1 Despite successive generations of antidepressant drugs, on average, 30%–45% of depressed patients fail to respond adequately to their initial antidepressant treatment and only 25%–35% achieve full symptom remission.Citation2 It has been estimated that 10%–30% of depressed patients are partially or totally resistant to antidepressant therapy.Citation3
Among the treatment strategies proposed for nonresponders or partial responders to antidepressants, adjunctive administration of triiodothyronine (T3) has been shown to be an effective augmentation therapy.Citation4–Citation7 STAR*D research has shown that the augmentation effects of T3 in patients with refractory depression taking selective serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors, or bupropion gave similar remission rates in all cases.Citation8,Citation9 However, a recent meta-analysis of four trials where T3 was initiated simultaneously with an selective serotonin reuptake inhibitor failed to show any superiority for the augmentation therapy.Citation10
The mechanism of T3 augmentation is still unclear. One week of T3 administration has been shown to result in desensitization of 5-HT1A receptors and an increase in extracellular serotonin levels in the frontal cortex of rats.Citation11,Citation12 Coadministration of imipramine and T3 results in desensitization of 5-HT2A receptors.Citation13 Thus, the effect of T3 augmentation of antidepressant effects has been assumed to involve serotonin neurotransmission.Citation14 However, to our knowledge, there has been no study investigating extracellular levels of serotonin, norepinephrine, and dopamine simultaneously after T3 treatment. A role of the norepinephrine or dopamine neurotransmitter cannot thus be ruled out.
Previous mechanistic studies have investigated the augmentation effects of T3 with tricyclic antidepressants. However, tricyclic antidepressants are not ideal for clarification of the augmentative effects of T3 because these agents block several receptors in addition to their effects on monoamine transporters.Citation15 Therefore, we chose to study the effect of the augmentation effects of T3 on the antidepressant, milnacipran, which inhibits reuptake of serotonin and norepinephrine with similar efficacy but without any receptor interactions.Citation16–Citation18 A recent reviewCitation19 has suggested hyperactivity of the amygdala and hypoactivity of the prefrontal cortex in depressed patients.
In this study we used in vivo microdialysis in the medial prefrontal cortex and amygdala to measure the basal extracellular levels of serotonin, norepinephrine, and dopamine and levels after acute administration of milnacipran in rats which had received subchronic treatment (for one week) with T3 or with control saline.
Materials and methods
Animals
Male Sprague-Dawley rats, obtained from the Shizuoka Laboratory Animal Centre (Shizuoka, Japan), were housed in groups of four and maintained on a 12-hour light-dark cycle (light phase 6.30 am to 6.30 pm) in a temperature-controlled environment (22°C ± 1°C) with free access to food and water. Experiments began after a 10-day period of acclimatization. The Hokkaido University School of Medicine Animal Care and Use Committee approved all procedures, which complied with the Guide for the Care and Use of Laboratory Animals, Hokkaido University School of Medicine.
Drugs
3,3′,5-Triiodo-L-thyronine sodium salt (T3; O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-L-tyrosine sodium salt; Sigma, St Louis, MO) was dissolved in 1 M NaOH with saline, which was used to dilute the solution to the required concentrations (0.1 and 0.2 mg/mL). The final concentration of NaOH was 5 mM for each dose of T3; 5 mM NaOH was used as the vehicle for T3. Milnacipran (Asahi Kasei Corporation, Shizuoka, Japan) was dissolved in distilled water, which was used as the vehicle for milnacipran, to achieve a final concentration of 10 mg/mL.
Drug administration
The animals were divided into two groups. Control group rats were given the vehicle subcutaneously once daily in the morning for 7 days and the T3 group rats were given T3 0.1 mg/kg or 0.2 mg/kg subcutaneously once daily in the morning for 7 days. On day 7, 2 hours after the last subcutaneous injection, the animals were implanted stereotactically in the medial prefrontal cortex or the amygdala with guide cannulae and microdialysis probes (see below). The rats were administered milnacipran 10 mg/kg intraperitoneally or saline 180 minutes after the first dialysate samples were collected. The 0.1 mg/kg and 0.2 mg/kg doses of T3 were chosen because administration of these doses for one week has been shown to increase extracellular serotonin levels in the frontal cortex of rats.Citation11
Microdialysis procedures
The experiments were performed according to a procedure described previously.Citation20 Under anesthesia with pentobarbital (30 mg/kg intraperitoneally), AG-4 and AG-8 guide cannulae (Eicom Corporation, Kyoto, Japan) were stereotactically implanted into rats at the surface of the medial prefrontal cortex or amygdala at the following coordinates relative to bregma from the stereotaxic atlas of Paxinos and Watson;Citation21 A + 3.2, ML + 0.8, DV +1.0 mm; A − 2.8, ML + 5.0, + DV 7.4 mm, respectively. Dialysis probes with a 0.22 mm outer diameter (A-I-4-03, A-I-8-02; Eicom Corporation) were then inserted into the guide cannulae so that 3.0 mm and 2.0 mm of the probes were exposed, respectively, in the medial prefrontal cortex and amygdala tissues. Rats were housed individually after the operation. Microdialysis was performed the next day in the freely moving rats. Perfusion was started 20 hours after surgery using artificial cerebrospinal fluid (145 mM NaCl, 3.0 mM KCl, 1.3 mM CaCl2, 1.0 mM MgCl2) at a flow rate of 2 μL per minute. Following an initial perfusion for 2 hours, dialysate samples were collected in vials containing 50 μL of 50 mM acetic acid every 20 minutes for 480 minutes. The recovery rates of serotonin, norepinephrine, and dopamine in vitro at 30°C were as follows: medial prefrontal cortex, 14.5%, 12.9%, and 14.1%, respectively; amygdala, 9.4%, 10.4%, and 11.2%, respectively (from Eicom Corporation).
Extracellular serotonin, norepinephrine, and dopamine levels were determined using high-performance liquid chromatography (HPLC) with electrochemical detection. The HPLC system comprised a liquid chromatography pump (EP-300; Eicom Corporation), a degasser (DG-300; Eicom Corporation), a reverse phase ODS column (Eicompak PPODS 30 4.6 mm; Eicom Corporation), an electrochemical detector (ECD-300; Eicom Corporation), and a data acquisition system (PowerChrom; AD Instruments Pty Ltd, Sydney, Australia). For serotonin and dopamine analysis, 20 μL of dialysate was injected into the HPLC system, that used a 0.1 M phosphate buffer (pH 6.0) mobile phase containing 1% (v/v) methanol, 50 mg/L Na2 ethylenediamine tetra-acetic acid and 500 mg/L sodium L-decanesulfonate. Separations were conducted at 25°C with a flow rate of 0.5 mL per minute. In the electrochemical detector, the oxidation potential was set at of 400 mV. Standard solutions for serotonin and dopamine were injected every working day, and the peak heights for the standards were used to determine the amounts of serotonin and dopamine in the samples.
Norepinephrine concentrations were measured using the same equipment with the exception of a different reverse-phase ODS column (Eicompak CA-5ODS 150 2.1 mm; Eicom Corporation). For norepinephrine analysis, 30 μL of dialysate was injected into the HPLC system that used a 0.1 M phosphate buffer (pH 6.0) mobile phase containing 5% (v/v) methanol, 50 mg/L Na2 ethylenediamine tetra-acetic acid and 500 mg/L L-octanesulfonic acid. Separation was conducted at 25°C with a flow rate of 0.23 mL per minute. The electrochemical detector was set at an oxidation potential of 550 mV. Standard norepinephrine solutions were injected every working day. The peak heights for the standard were used to determine the amount of norepinephrine in the samples.
Statistical analysis
All data are given as the mean values ± standard error of the mean of individual rats from each group. The serotonin, norepinephrine, and dopamine contents of dialysate samples are expressed as absolute values (pg/fraction). The mean absolute values in nine consecutive samples (−160 to 0 minutes) before milnacipran administration were used to determine basal levels. Differences between the two groups for extracellular serotonin, norepinephrine, and dopamine concentrations after milnacipran administration were calculated using a repeated-measures analysis of variance (ANOVA) for absolute values to examine the interaction between pretreatment (T3) and time factors (0–300 minutes). When the interaction effects were found to be significant, post hoc comparisons (differences in absolute values at each time point between the two groups, ie, the control and T3 groups) were analyzed using an unpaired Student’s t-test (two-tailed). The area under the curve for the 0–180-minute periods was compared between the T3 0.1 mg/kg group and the control group using an unpaired Student’s t-test (two-tailed). Differences were considered to be statistically significant at P < 0.05.
Results
Effect of subchronic T3 treatment on basal extracellular concentrations of serotonin, norepinephrine, and dopamine in the medial prefrontal cortex and amygdala
In the medial prefrontal cortex, basal extracellular serotonin concentrations in the T3 0.1 mg/kg group were significantly higher than those of the control group (P < 0.05, ). No significant differences in basal extracellular concentrations of norepinephrine or dopamine were found between the control and T3 0.1 mg/kg groups. No significant differences were found between the control and T3 0.2 mg/kg groups in basal extracellular levels of any of the monoamines. In the amygdala, no significant difference was found in basal extracellular serotonin, norepinephrine or dopamine concentrations between the control and the T3 0.1 mg/kg groups.
Table 1 Effect of subchronic treatment with T3 (for 7 days) on baseline concentrations of serotonin, norepinephrine, and dopamine in the medial prefrontal cortex and the amygdale
Effect of acute milnacipran administration on extracellular concentrations of serotonin, norepinephrine, and dopamine in the medial prefrontal cortex and amygdala after control and subchronic T3 treatments
Acute intraperitoneal administration of milnacipran 10 mg/kg to saline-treated (control) animals resulted in an increase in extracellular concentrations of serotonin, norepinephrine, and dopamine in the medial prefrontal cortex ( and ) and in the amygdala ().
Figure 1 Effect of acute intraperitoneal administration of milnacipran 10 mg/kg on extracellular concentrations of serotonin (A), norepinephrine (B), and dopamine (C) in the medial prefrontal cortex after subchronic treatment with T3 0.1 mg/kg.
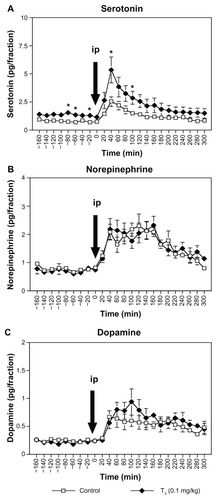
Figure 2 Effect of acute intraperitoneal administration of milnacipran 10 mg/kg on extracellular concentrations of serotonin (A), norepinephrine (B), and dopamine (C) in the medial prefrontal cortex after subchronic treatment with T3 0.2 mg/kg.
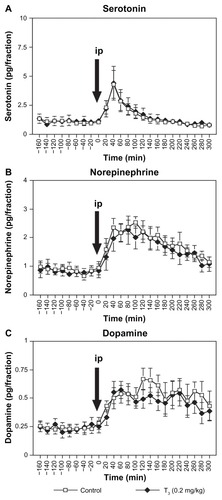
Figure 3 Effect of acute intraperitoneal administration of milnacipran 10 mg/kg on extracellular concentrations of serotonin (A), norepinephrine (B), and dopamine (C) in the amygdala after subchronic treatment with T3 0.1 mg/kg.
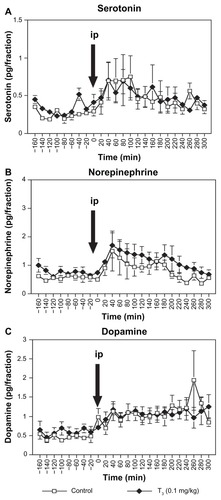
Following intraperitoneal administration of milnacipran 10 mg/kg to rats treated with subchronic T3, two-way ANOVA with repeated measures (0–300 minutes) of extracellular serotonin concentrations in the medial prefrontal cortex () showed significant interaction between T3 treatment and time after milnacipran administration [F(15,165) = 1.992, P = 0.0185] and a significant main effect of time [F(15,165) = 13.111, P < 0.0001]. However, the main effect of T3 treatment after milnacipran administration was not significant [F(1,11) = 4.758, P = 0.0518]. The T3 0.1 mg/kg group showed significantly higher concentrations of extracellular serotonin than those of the control group at two time points, ie, at 40 and 100 minutes (P < 0.05, ).
The area under the curve (0–180 minutes) for extracellular serotonin in the subchronic T3 0.1 mg/kg group was significantly higher than that of the control group (527.4 ± 101.1 and 282.9 ± 46.5 pg * minutes, respectively, P < 0.05). The area under the curve (0–180 minutes) for extracellular norepinephrine and dopamine were not significantly different between the T3 and control groups.
For the extracellular norepinephrine concentrations in the medial prefrontal cortex (), two-way ANOVA with repeated measures (0–300 minutes) showed a significant main effect of time [F(15,165) = 15.234, P < 0.0001]. There was neither a significant main effect of T3 0.1 mg/kg treatment after milnacipran administration [F(1,11) = 0.25, P = 0.8769] nor an interaction between subchronic T3 0.1 mg/kg treatment and time after milnacipran administration [F(15,165) = 1.107, P = 0.3537].
For the extracellular dopamine concentrations in the medial prefrontal cortex (), two-way ANOVA with repeated measures (0–300 minutes) showed a significant main effect of time [F(15,165) = 8.449, P < 0.0001]. There was neither a significant main effect of subchronic T3 0.1 mg/kg treatment after milnacipran administration [F(1,11) = 0.504, P = 0.4926] nor an interaction between subchronic T3 0.1 mg/kg treatment and time after milnacipran administration [F(15,165) = 1.536, P = 0.0979].
In rats treated with subchronic T3 0.2 mg/kg (), two-way ANOVA with repeated measures (0–300 minutes) showed a significant main effect of time for the extracellular serotonin, norepinephrine, and dopamine concentrations [F(15,150) = 15.068, P < 0.0001; F(15,150) = 12.058, P < 0.0001; F(15,150) = 4.241, P < 0.0001, respectively]. However, there was neither a main effect of subchronic T3 0.2 mg/kg treatment after milnacipran administration [F(1,10) = 0.036, P = 0.853; F(1,10) = 0.206, P = 0.660; F(1,10) = 0.325, P = 0.581, respectively] nor an interaction between subchronic T3 0.2 mg/kg treatment and time after milnacipran administration [F(15,150) = 0.046, P > 0.999; F(15,150) = 0.463, P = 0.955; F(15,150) = 0.664, P = 0.816, respectively].
In the amygdala (), for extracellular serotonin and norepinephrine concentrations, two-way ANOVA with repeated measures (0–300 minutes) showed a significant main effect of time [F(15,75) = 1.843, P = 0.044; F(15,75) = 5.197, P < 0.0001, respectively]. There was neither a main effect of subchronic T3 0.1 mg/kg treatment after milnacipran administration [F(1,5) = 0.0008, P = 0.993; F(1,5) = 0.848, P = 0.399, respectively] nor an interaction between subchronic T3 0.1 mg/kg treatment and time after milnacipran administration [F(15,75) = 0.547, P = 0.905; F(15,75) = 0.342, P = 0.988, respectively].
For the extracellular dopamine concentrations in the amygdala (), there was neither a main effect of subchronic T3 0.1 mg/kg treatment after milnacipran administration [F(1,5) = 0.165, P = 0.701] nor an interaction between subchronic T3 0.1 mg/kg treatment and time after milnacipran administration [F(15,75) = 1.325, P = 0.209]. The main effect of time was not significant [F(15,75) = 1.217, P = 0.279].
Discussion
Subchronic (7 days) administration of T3 0.1 mg/kg significantly increased basal extracellular levels of serotonin in the medial prefrontal cortex (). This finding is consistent with previous studiesCitation11,Citation12 which have shown that T3 administered daily for 7 days over the range 0.02–0.2 mg/kg increases basal extracellular levels of serotonin in the rat cortex, in a dose-dependent manner. Subchronic administration of T3 at 0.1 mg/kg had no effect on basal extracellular levels of norepinephrine or dopamine (). Although several studies have investigated the role of serotonin in the mechanism of T3 augmentation,Citation11–Citation13 to our knowledge, the present study is the first to examine extracellular serotonin, norepinephrine, and dopamine levels simultaneously after T3 treatment. It is currently believed that the effect of T3 on serotonin neurotransmission is integral to the mechanism of T3 augmentation of antidepressant effects.14 The results of the present study strongly support this notion, and the absence of effect on the levels of norepinephrine or dopamine makes it unlikely that these neurotransmitters are involved.
The present study found no effects on basal extracellular serotonin levels in the amygdala () after subchronic administration of T3 0.1 mg/kg, in contrast with the increase found in the medial prefrontal cortex (). A previous studyCitation11 has reported that subchronic administration of T3 at 0.1 mg/kg increased basal extracellular serotonin levels in the frontal cortex of rats, but not in the hippocampus. The effects of T3 on increases in extracellular serotonin thus appear to be sensitive, not only to dose but to the brain region studied. A more detailed study of brain regions sensitive to the effects of T3 administration might be helpful in clarifying the mechanism of action of T3 augmentation.
Results of the present study showed that, in contrast with the effect of subchronic administration of T3 0.1 mg/kg, similar administration of T3 0.2 mg/kg did not alter basal extracellular serotonin levels (). This differs from previous findings.Citation12 However, higher doses of T3 0.5 mg/kg have been shown to have no effect on extracellular serotonin levels in the frontal cortex of rats,Citation12 suggesting a bell-shaped relationship between the dose of T3 and the increase in serotonin levels. The nature of the bell-shaped curve has not been studied in detail, but it is possible that experimental differences between studies (strain of rats, for example) could result in a leftward shift of the bell-shaped curve, offering a possible explanation of the different results obtained.
As we have reported previously,20,22 acute administration of the antidepressant, milnacipran, to rats receiving saline resulted in an increase in basal extracellular concentrations of serotonin, norepinephrine, and dopamine in the medial prefrontal cortex ( and ) and amygdala (). In rats treated subchronically with T3 0.1 mg/kg, acute administration of milnacipran resulted in an additional increase in extracellular serotonin levels, but not in norepinephrine or dopamine levels. This suggests that the augmentation effect of T3 administration on a selective serotonin and norepinephrine reuptake inhibitor, such as milnacipran, occurs through enhancement of serotonergic neurotransmission, but not through noradrenergic and dopaminergic neurotransmission.
The present study suffers from several weaknesses. A one-week administration of T3 was used since this is the duration typically used in similar animal studies.Citation11,Citation13,Citation23 However, a recent study reported that coadministration of T3 and the selective serotonin reuptake inhibitor, fluoxetine, for 3 weeks caused greater increases in neurogenesis in the hippocampus than fluoxetine administration alone.Citation24 The effects of one week of administration of T3 may thus not be optimal for extrapolating to the effects occurring with long-term treatment as used clinically.
In addition, in the present study, the effect of milnacipran was studied acutely. Subchronic administration of milnacipran to rats has been shown,25 however, to enhance noradrenergic transmission beyond that achieved with acute administration while no such effect is seen on serotonergic or dopaminergic transmission. Thus, it may be interesting to study longer durations of administration of both T3 and milnacipran in future studies.
Another weakness of the present study is that it is exclusively neurochemical. Simultaneous behavioral studies could possibly give a “functional window” for studying the phenomenon. Unfortunately, the constraints of in vivo microdialysis are not compatible with behavioral paradigms.
In conclusion, subchronic administration of T3 0.1 mg/kg significantly increased basal extracellular levels of serotonin in the medial prefrontal cortex, but not in the amygdala. Subchronic administration of T3 at 0.2 mg/kg showed no effect on extracellular levels of serotonin in the medial prefrontal cortex, suggesting a bell-shaped dose-effect relationship. Acute administration of milnacipran, which in control animals significantly increased levels of serotonin, norepinephrine, and dopamine, caused an additional increase in extracellular serotonin levels, but not in norepinephrine or dopamine levels, in the medial prefrontal cortex in rats treated subchronically with T3 0.1 mg/kg. This suggests that the augmentation effect of T3 administration on selective serotonin and norepinephrine reuptake inhibitors, such as milnacipran, occurs via enhancement of serotonergic neurotransmission, but not through noradrenergic and dopaminergic neurotransmission.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (21591478 to TI and 17790800 to YK) from the Japanese Ministry of Education, Culture, Sports, Science and Technology. TK has received honoraria from Pfizer, Astellas, and Eli Lilly, and has received a research grant from Dainippon Sumitomo Pharma, Pfizer, Astellas, and GSK. He is an advisory board member for GSK.
Disclosure
The authors report no conflicts of interest in this work.
References
- MurrayCJLLopezADThe Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Disease, Injuries, and Risk Factors in 1990 and Projected to 2020Cambridge, MAHarvard University Press19961
- FavaMDiagnosis and definition of treatment-resistant depressionBiol Psychiatry20035364965912706951
- JoffeRTLevittAJSokolovSTAugmentation strategiesJ Clin Psychiatry19965725318690693
- ConnollyKRThaseMEIf at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategiesDrugs201171436421175239
- Cooper-KazazRLererBEfficacy and safety of triiodothyronine supplement in patients with major depressive disorder treated with specific serotonin reuptake inhibitorsInt J Neuropsychopharmacol20081168569918047754
- AbrahamGMilevRStuartJLT3 augmentation of SSRI resistant depressionJ Affect Disord20069121121516483669
- AltshulerLLBauerMFryeMADoes thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literatureAm J Psychiatry20011581617162211578993
- NierenbergAAFavaMTrivediMHA comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D reportAm J Psychiatry20061631519153016946176
- RushAJTrivediMHWisniewskiSRAcute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D reportAm J Psychiatry20061631905191717074942
- PapakostasGICooper-KazazRAppelhofBCSimultaneous initiation (coinitiation) of pharmacotherapy with triiodothyronine and a selective serotonin reuptake inhibitor for major depressive disorder: a quantitative synthesis of double-blind studiesInt Clin Psychopharmacol200924192519092448
- GurELererBNewmanMEChronic clomipramine and triiodothyronine increases serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activityJ Pharmacol Exp Ther199928881879862756
- GurELifschytzTLererBNewmanMEEffects of triiodothyronine and imipramine on basal 5-HT levels and 5-HT(1) autoreceptor activity in rat cortexEur J Pharmacol2002457374312460641
- MoreauXJeanningrosRMazzola-PomiettoPChronic effects of triiodothyronine in combination with imipramine on 5-HT transporter, 5-HT(1A) and 5-HT(2A) receptors in adult rat brainNeuropsychopharmacology20012465266211331145
- BauerMHeinzAWhybrowPCThyroid hormones, serotonin and mood: of synergy and significance in the adult brainMol Psychiatry2002714015611840307
- RichelsonEInteractions of antidepressants with neurotransmitter transporters and receptors and their clinical relevanceJ Clin Psychiatry200364Suppl 1351214552650
- MoretCCharveronMFinbergJPCouzinierJPBrileyMBiochemical profile of midalcipran (F 2207), 1-phenyl-1-diethyl-aminocarbonyl-2-aminomethyl-cyclopropane (Z) hydrochloride, a potential fourth generation antidepressant drugNeuropharmacology198524121121193005901
- MochizukiDTsujitaRYamadaSNeurochemical and behavioural characterization of milnacipran, a serotonin and noradrenaline reuptake inhibitor in ratsPsychopharmacology (Berl)200216232333212122491
- VaishnaviSNNemeroffCBPlottSJRaoSGKranzlerJOwensMJMilnacipran: a comparative analysis of human monoamine uptake and transporter binding affinityBiol Psychiatry20045532032214744476
- QuidéYWitterveenABEl-HageWVeltmanDJOlffMDifferences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: a systematic reviewNeurosci Biobehav Rev20123662664421963442
- KitaichiYInoueTNakagawaSIzumiTKoyamaTEffect of milnacipran on extracellular monoamine concentrations in the medial prefrontal cortex of rats pre-treated with lithiumEur J Pharmacol200551621922615963494
- PaxinosGWatsonCThe Rat Brain in Stereotaxic Coordinates3rd edSan Diego, CAAcademic Press1997
- KitaichiYInoueTIzumiTEffect of co-administration of a serotonin-noradrenaline reuptake inhibitor and a dopamine agonist on extracellular monoamine concentrations in ratsEur J Pharmacol200858428529018336812
- LifschytzTGoltser-DubnerTLandshutGLererBEffect of triiodothyronine on 5-HT1A and 5-HT1B receptor expression in rat forebrain and on latency to feed in the novelty suppressed feeding testProg Neuropsychopharmacol Biol Psychiatry20103463263820206658
- EitanRLandshutGLifschytzTEinsteinOBen-HurTLererBThe thyroid hormone, triiodothyronine, enhances fluoxetine-induced neurogenesis in rats: possible role in antidepressant-augmenting propertiesInt J Neuropsychopharmacol20101355356119835665
- KitaichiYInoueTIzumiTNakagawaSKatoAKoyamaTSubchronic milnacipran treatment increases basal extracellular noradrenaline concentrations in the medial prefrontal cortex of ratsEur J Pharmacol2005520374216153636