Abstract
Background
Acetylcholinesterase inhibitors are considered standard of care for Alzheimer’s disease in many countries. Galantamine is an acetylcholinesterase inhibitor that may also act via allosteric modulation of nicotinic acetylcholine receptors. Therefore, it may provide benefits compared with other acetylcholinesterase inhibitors. The present study compared galantamine (n = 116) with donepezil (n = 117) in a double-blind trial at nine hospitals in China.
Methods
After washout of any previous acetylcholinesterase inhibitors, subjects with mild to moderate Alzheimer’s disease received galantamine or donepezil for 16 weeks.
Results
Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-cog/11) scores improved significantly from baseline in both treatment arms, with a significant difference in favor of galantamine on the “language” functional area (P = 0.035). Significantly more galantamine-treated patients responded to treatment (defined as a reduction in ADAS-cog/11 score of >4, >7, or >10 points; all P < 0.05), and had an ADAS-cog/11 score < 20 at end point (P = 0.015). Both treatments were well tolerated, although fewer galantamine-treated patients experienced gastrointestinal adverse events compared with donepezil (30% versus 48%).
Conclusion
Cognitive function improved significantly in subjects with mild to moderate Alzheimer’s disease treated with galantamine or donepezil, and both treatments were generally well tolerated. Significant benefits for galantamine over donepezil were observed for language and response to treatment.
Introduction
Alzheimer’s disease is a progressive neurodegenerative disorder with a mean duration of around 8.5 years between onset of clinical symptoms and death. Brain regions that are associated with higher mental functions, particularly the neocortex and hippocampus, are those most affected by the characteristic pathology of Alzheimer’s disease. Subsequent discoveries of reduced choline uptake, acetylcholine release, and loss of cholinergic perikarya from the nucleus basalis of Meynert confirmed a substantial presynaptic cholinergic deficit. Thus, it was proposed that degeneration of cholinergic neurons in the basal forebrain, and the associated loss of cholinergic neurotransmission in the cerebral cortex and other areas, contribute significantly to the deterioration in cognitive function seen in patients with Alzheimer’s disease. As a result, acetylcholinesterase inhibitors were developed for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease, and are considered standard of care in many countries worldwide.Citation1–Citation4
Galantamine is an acetylcholinesterase inhibitor that, in addition to acetylcholinesterase inhibition, may also act through allosteric modulation of nicotinic acetylcholine receptors.Citation5 Therefore, it may provide additional benefits compared with donepezil or rivastigmine.
Several attempts have been made to differentiate between acetylcholinesterase inhibitors on the basis of safety and efficacy. A meta-analysis by the Cochrane Collaboration found that all acetylcholinesterase inhibitors produced improvements in cognitive function when compared with placebo, with an average change (improvement) in the 11-point Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-cog/11) of −2.7 points (95% confidence interval −3.0 to −2.3).Citation6 In one study of galantamine versus placebo, the improvement over placebo was 3.09 points.Citation7 The safety profile of donepezil and galantamine did not differ significantly in the Cochrane analysis.Citation6
Two rater-blinded, randomized comparative trials of donepezil and galantamine have been conducted. In a 52-week study in the UK comparing galantamine and donepezil, 182 subjects receiving galantamine maintained Mini-Mental State Examination scores at baseline levels throughout the study, compared with a significant deterioration in those receiving donepezil (P < 0.0005 versus baseline).Citation8 Activities of daily living and behavioral outcomes were similar between the two treatment arms, although more caregivers of subjects receiving galantamine reported reductions in burden compared with donepezil. In a 12-week multinational study in which 120 subjects were treated with donepezil or galantamine, ADAS-cog/11 and Disability Assessment in Dementia scores were significantly improved in the donepezil arm compared with galantamine at week 12.Citation9 Furthermore, fewer donepezil-treated subjects reported gastrointestinal adverse events.
The objective of the present double-blind study was to compare cognitive outcomes in patients with mild to moderate Alzheimer’s disease receiving either galantamine or donepezil, based on the hypothesis that galantamine would be noninferior to donepezil.
Materials and methods
Study design
GAL-CH-100 was a randomized, double-blind, parallel-group, 16-week study conducted at nine hospitals in China. Subjects first completed a screening period of up to 14 days. Those previously receiving acetylcholinesterase inhibitors then entered a 4-week, single-blind placebo washout period, while those not previously receiving acetylcholinesterase inhibitors proceeded directly to the double-blind phase.
At double-blind baseline, subjects were randomly allocated (1:1) to galantamine or donepezil. Galantamine was dosed at 8 mg/day for 4 weeks, followed by 16 mg/day for 4 weeks. In weeks 9–12, subjects received flexible-dose galantamine (6–24 mg/day) at the investigator’s discretion, followed by a fixed galantamine dose of 16 or 24 mg/day in weeks 13–16, depending on tolerability of the dose they received in weeks 9–12. Donepezil was dosed at 5 mg/day for 8 weeks, followed by flexible dosing (5–10 mg/day) in weeks 9–12. In weeks 13–16, subjects received a fixed donepezil dose of 5 or 10 mg/day depending on dose in weeks 9–12 and tolerability.
Patients
Eligible subjects were men or women aged 40–90 years with a diagnosis of probable Alzheimer’s disease, according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association, and a Mini-Mental State Examination score of 10–24. Subjects were also required to have a permanent caregiver during the study. Those with neurodegenerative diseases, encephalosis, or vascular dementia were excluded. Additional exclusion criteria included epilepsy, depression, schizophrenia, active peptic ulcers, significant liver, kidney, lung, metabolic, or endocrine anomalies, significant urinary obstructions, and cardiovascular disease. Written informed consent was obtained from subjects, or their legal representatives, and their caregivers.
End points
The primary efficacy outcome measure was the ADAS-cog/11. Prespecified secondary analyses included six functional areas of the ADAS-cog/11 (operation, memory, orientation, visual space, language, attention), and the response to treatment, defined as a reduction in ADAS-cog/11 score of >0, >4, >7, or >10 points. In addition, efficacy was assessed using the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory and the Neuropsychiatric Inventory. Safety was assessed in terms of adverse events, laboratory tests, physical examination, and vital signs.
Statistical analysis
The sample size for the study was based on an expected between-group difference of 2.47, with a standard deviation of 7.1. With α = 0.05 and 80% power of a test, 103 subjects are required for each treatment arm. With an estimated dropout rate of 20%, a sample size of 258 was required.
Efficacy was assessed in the full analysis set, which was defined as all subjects who were randomized and received at least one dose of double-blind study drug, and had at least one post-baseline primary end point assessment. Safety was assessed in the safety population, defined as all subjects who were randomized and received at least one dose of double-blind study drug.
The primary end point was the change on ADAS-cog/11 at end point versus baseline at week 16, and was analyzed by analysis of covariance with treatment as a factor and site as a covariate. Secondary end points were analyzed using analysis of covariance, adjusted for study center, to evaluate the differences of efficacy between galantamine and donepezil. The analysis of response to treatment was analyzed using the Cochran-Mantel-Haenszel test, adjusted for study center. No missing data were imputed.
Results
Subjects
In total, 233 subjects were randomly allocated to treatment (galantamine, n = 116; donepezil, n = 117), which was lower than the predetermined sample size for the study. The 16-week study was completed by 198 subjects (85%, ). The full analysis set included 218 subjects (galantamine, n = 110; donepezil, n = 108), of whom 198 (91%) completed the study. Baseline characteristics were similar between the two study arms (). The majority of patients in the study were aged 65–85 years, with no significant difference in mean age, duration of cognitive impairment, or duration of Alzheimer’s disease between the treatment arms (). At the end of treatment, 80 galantamine-treated (69%) and 71 donepezil-treated (61%) subjects were receiving the highest maintenance dose (galantamine 24 mg/day or donepezil 10 mg/day).
Table 1 Baseline characteristics (FAS)
Efficacy
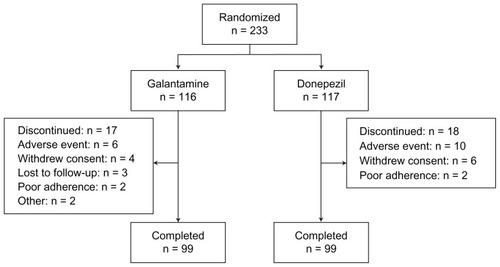
At week 16, mean ADAS-cog/11 scores improved (decreased) significantly from baseline in both treatment arms (). While there was no significant difference between the galantamine and donepezil arms overall, improvement was numerically greater with galantamine than with donepezil. Furthermore, galantamine was superior to donepezil for improving the “language” functional area of the ADAS-cog/11 (P = 0.035, ). No significant differences between galantamine and donepezil were observed for the other ADAS-cog/11 functional areas.
Figure 2 Mean change from baseline in Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-cog/11) score.
Note: *P < 0.05 vs baseline.
Table 2 Mean scores on the six ADAS-cog/11 functional areas at baseline and week 16
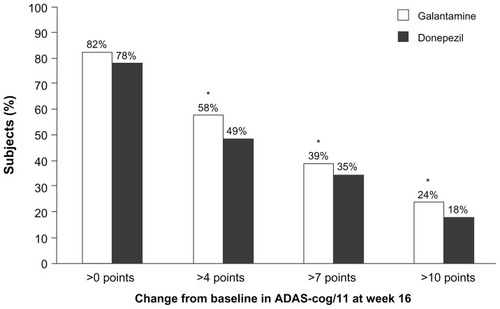
The proportion of subjects who maintained ADAS-cog/11 at or above baseline levels was 82% with galantamine (n = 90) and 78% with donepezil (n = 84). Response to treatment, as determined by reductions in ADAS-cog/11 score of >4, >7, and >10 points, was achieved by significantly more subjects receiving galantamine (). Significantly more subjects receiving galantamine had an ADAS-cog/11 score < 20 at end point compared with donepezil (76% [n = 78] versus 58% [n = 60]; P = 0.015).
Figure 3 Percentage of patients with an improvement from baseline of >0, >4, >7, and >10 points on the Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-cog/11).
Mean (± standard deviation) improvements from baseline in Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory score at week 16 were similar with galantamine and donepezil (2.0 ± 12.1 versus 3.1 ± 10.1), as were mean improvements in Neuropsychiatric Inventory score (0.4 ± 8.3 versus 0.3 ± 8.0).
Safety
Of the 233 subjects, 51 (44%) in the galantamine arm and 54 (47%) in the donepezil arm reported at least one adverse event, of which most were transient and mild to moderate in severity. The most common adverse events in both treatment arms were nausea, vomiting, and dizziness (), although fewer galantamine-treated patients experienced adverse gastrointestinal events compared with donepezil (30% versus 48%). Adverse events considered by the investigator to be possibly, probably, or almost certainly related to treatment were reported by 28% and 40% of patients receiving galantamine or donepezil, respectively.
Table 3 Adverse events occurring in ≥3% of subjects in either treatment arm
Discontinuations because of adverse events occurred in six patients (5%) in the galantamine arm and 10 patients (8.4%) in the donepezil arm. Serious adverse events considered to be possibly related to treatment occurred in one patient in each treatment arm (purpura and thrombocytopenia with galantamine; abnormal liver function tests with donepezil). No deaths were reported during the study. No clinically significant differences between treatment groups were reported for laboratory tests, vital signs, or physical examination.
Discussion
Few comparative studies of galantamine and donepezil have been conducted previously, with only two rater-blinded trials comparing the two drugs over 16 and 52 weeks.Citation8,Citation9 While the shorter of these (a 16-week trial) yielded more positive results for donepezil, the longer trial (12 months) showed superiority of galantamine over donepezil with regard to cognition. The present 16-week multicenter, double-blind trial demonstrated that improvements in cognitive function (ADAS-cog/11), daily living capabilities (Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory), and neuropsychiatric symptoms (Neuropsychiatric Inventory) in Chinese patients with Alzheimer’s disease are broadly similar with galantamine (16–24 mg/day) and donepezil (5–10 mg/day). However, the overall level of response in terms of ADAS-cog/11 score (ie, improvement of >4, >7, or >10 points) was significantly better with galantamine than with donepezil. Furthermore, improvement in the ADAS-cog/11 functional area of language ability, a composite of the ADAS-cog/11 items “spoken language ability,” “word-finding difficulty”, and “comprehension in spontaneous speech”, was significantly greater with galantamine than with donepezil. Previous data on language performance in patients with Alzheimer’s disease receiving galantamine or donepezil are limited. A 24-week study in 1467 patients with moderate to severe Alzheimer’s disease showed no benefit of donepezil 10 mg/day on language assessed using the Severe Impairment Battery – Language scale.Citation10 However, significant improvements in language were seen when the donepezil dose was increased to 23 mg/day. In a secondary analysis of a 4-month, placebo-controlled trial of galantamine in 130 community-dwelling patients with mild to moderate Alzheimer’s disease, significantly more patients receiving galantamine experienced a reduction in verbal repetition compared with those receiving placebo (58% versus 24%, P < 0.01).Citation11
In the present study, as in placebo-controlled studies,Citation7,Citation12–Citation15 galantamine was well tolerated. Furthermore, unlike the previous 16-week comparative study of galantamine and donepezil,Citation9 the present study showed a lower overall adverse event rate for galantamine and a lower frequency of gastrointestinal adverse events. The slight differences in outcomes of the different comparative studies may be attributable to treatment duration, study design, and patient variability.
Overall, the results of this study in Chinese patients are consistent with those from randomized, double-blind, placebo-controlled trials of galantamine, which were performed mostly in Caucasian patients.Citation7,Citation12–Citation15 These studies show that treatment significantly improves cognitive function, daily functioning, and behavior. Open-label data from Chinese individuals are available from a small (n = 32) 2-year study in which galantamine was effective in slowing cognitive decline compared with historical controls.Citation16
The main strengths of the present study are its double-blind, active-comparator design, and its reporting of key outcomes, subgroup analyses, and adverse event profiles. The main weakness of the study is its sample size, which was four patients per treatment arm smaller than determined in the sample size calculation. However, the dropout rate in the study (15%) was lower than that used in the sample size calculation (20%). Additional limitations of the study are the lack of data imputation (although the rate of discontinuation was similar in the two treatment arms), the inclusion (albeit with a washout period) of patients who had previously received cholinesterase inhibitors, the relatively short follow-up, and the lack of data on APP, PSEN1, PSEN2, and ApoE genotypes among the participants.
In conclusion, while improvements in cognition were generally similar in the galantamine and donepezil arms, response to treatment (reductions in ADAS-cog/11 score >4, >7, and >10 points), language ability (in terms of the “language” functional area of the ADAS-cog/11), and the percentage of patients with low cognitive impairment (ADAS-cog/11 score < 20) were significantly greater with galantamine.
Disclosure
The GAL-CH-100 study was sponsored by Xian-Janssen Pharmaceutical Ltd, Beijing, China. Editorial support with drafting and completion of the manuscript was provided by Daniel Booth (Bioscript Medical Ltd, London, UK) and funded by Janssen-Cilag EMEA. Some data from this study have previously been published in Chinese (Hong X, et al. Zhonghua Shen Jing Ge Za Zhi. 2006;39:379–382). LY is an employee of Xi’an Janssen Pharmaceutical Ltd, Beijing, China. MG is an employee of Janssen-Cilag EMEA, Neuss, Germany. BS is a former employee of Janssen-Cilag EMEA, Neuss, Germany. UR is an employee of Janssen-Cilag AG, Baar, Switzerland.
References
- National Institute for Health and Clinical ExcellenceDonepezil, Galantamine, Rivastigmine (Review) and Memantine for the Treatment of Alzheimer’s Disease (Amended)London, UKNational Institute for Health and Clinical Excellence2007
- Deutsche Gesellschaft für Psychiatrie Psychotherapie und Nervenheilkunde, Deutsche Gesellschaft für Neurologie. S3-Leitlinie “Demenzen”German Association for Psychiatry and Psychotherapy; German Society of Neurology. S3 guideline “Dementia”2009 Available from: http://www.dgn.org/images/stories/dgn/leitlinien/ll_demenz/ll-demenz-kurz-170210.pdfAccessed January 15, 2011
- SocialstyrelsenNationella riktlinjer för vård och omsorg vid demenssjukdom 2010 – stöd för styrning och ledningThe National Board of Health and Welfare (Sweden). National guidelines for care in dementia 2010 – support for governance and managementVästeråsEdita Västra Aros2010
- American Psychiatric AssociationPractice Guideline for the Treatment of Patients with Alzheimer’s Disease and Other Dementias2nd edArlington, VAAmerican Psychiatric Publishing Inc2007
- SamochockiMHoffleAFehrenbacherAGalantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptorsJ Pharmacol Exp Ther20033051024103612649296
- BirksJCholinesterase inhibitors for Alzheimer’s diseaseCochrane Database Syst Rev20061CD00559316437532
- RaskindMAPeskindERWesselTGalantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study GroupNeurology2000542261226810881250
- WilcockGHoweIColesHA long-term comparison of galantamine and donepezil in the treatment of Alzheimer’s diseaseDrugs Aging20032077778912875613
- JonesRWSoininenHHagerKA multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer’s diseaseInt J Geriatr Psychiatry200419586714716700
- FerrisSHSchmittFASaxtonJAnalyzing the impact of 23 mg/day donepezil on language dysfunction in moderate to severe Alzheimer’s diseaseAlzheimers Res Ther201132221689411
- RockwoodKFaySJarrettPEffect of galantamine on verbal repetition in AD: a secondary analysis of the VISTA trialNeurology2007681116112117404193
- RockwoodKMintzerJTruyenLEffects of a flexible galantamine dose in Alzheimer’s disease: a randomised, controlled trialJ Neurol Neurosurg Psychiatry20017158959511606667
- TariotPNSolomonPRMorrisJCA 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study GroupNeurology2000542269227610881251
- WilcockGKLilienfeldSGaensEEfficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. Galantamine International-1 Study GroupBMJ20003211445144911110737
- BrodatyHCorey-BloomJPotocnikFCGalantamine prolonged-release formulation in the treatment of mild to moderate Alzheimer’s diseaseDement Geriatr Cogn Disord20052012013215990426
- ChuLWYikPYMokWA 2-year open-label study of galantamine therapy in Chinese Alzheimer’s disease patients in Hong KongInt J Clin Pract20076140341017313606