Abstract
Background
The pathology of delusions in patients with Alzheimer’s disease (AD) associated with white matter (WM) abnormalities is poorly understood. In addition, whether the abnormalities in WM integrity that underlie the delusions develop before the onset of the delusions remains unclear. In this study, we used a diffusion tensor imaging approach to examine the existence of baseline abnormalities in WM integrity in AD patients who developed delusions and AD patients who did not develop delusions.
Methods
Using the Neuropsychiatric Inventory, we identified patients with AD who exhibit delusions during a 1-year period. All the patients underwent a magnetic resonance imaging (MRI) examination at baseline. We conducted fractional anisotropy using tract-based spatial statistics software and compared the results of AD patients who developed delusions with those who did not develop delusions.
Results
Compared with the AD patients who did not develop delusions (n = 15), the AD patients who developed delusions (n = 10) exhibited two relatively large clusters and one minimal cluster of significantly lower fractional anisotropy results. The first cluster was located in the left parieto-occipital region and included several fibers: the left inferior longitudinal fasciculus, the inferior fronto-occipital fasciculus, the posterior corona radiate, and the forceps major of the corpus callosum. The second cluster was located on the body of the corpus callosum. A third minimal cluster was located on the superior temporal gyrus white matter.
Conclusion
Abnormalities in WM integrity involving several fibers may be crucial to the development of delusions in AD patients.
Introduction
Recent magnetic resonance imaging (MRI) studies have revealed that many patients with Alzheimer’s disease (AD) have coexisting white matter hyperintensities. Although the significance of white matter hyperintensities in AD patients is poorly understood, several studies have suggested that the existence of white matter hyperintensities is associated with specific neuropsychiatric symptoms, such as delusions and nighttime disturbances.Citation1–Citation3 Furthermore, delusions, unlike other neuropsychiatric symptoms, are known to be associated with a poor prognosis.Citation4
Diffusion tensor imaging (DTI), a relatively new MRI imaging procedure, has been developed to examine the integrity of white matter (WM) fiber bundles. The DTI technique has clarified that WM abnormalities are widespread throughout WM fiber bundles in AD patients.Citation5 Fractional anisotropy (FA) is a widely used metric in studies of AD patients. Two reports have indicated that a reduction in FA in specific fiber tracts occurs during the early stage of AD.Citation6,Citation7 Together, these findings suggest that some neuropsychiatric symptoms, such as delusions, in patients with AD may be associated with a reduction in FA in several fiber bundles.
A 2-year prospective longitudinal study in AD patients has reported that the cumulative incidence of psychotic symptoms (delusions, hallucinations) was 36.9%.Citation8 This study suggested that not all AD patients develop psychotic symptoms during the course of AD. Whether the baseline FA value in AD patients without delusions can predict future progression to delusions is a clinically important issue. Thus, we hypothesized that FA abnormalities may exist prior to the onset of delusions in AD patients who subsequently develop delusions. We performed DTI using baseline data for AD patients without delusions who were enrolled in a 1-year prospective longitudinal study. Then, after the 1-year follow-up period, we compared the FA values between AD patients who developed delusions and the AD patients who did not develop delusions, using tract-based spatial statistics.
Methods
Participants
The baseline sample consisted of Japanese AD patients with mild functional severity who attended the outpatient clinic of Nagoya City University Hospital between June 2009 and December 2010. The study inclusion criteria were (1) a diagnosis of probable AD according to the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer Disease and Related Disorders Association criteria,Citation9 (2) no history of medication with antipsychotic medications, and (3) no psychosis symptoms, as assessed using the Japanese version of the Neuropsychiatric Inventory (NPI).Citation10,Citation11 The NPI is a semiquantitative assessment based on information provided by the caregiver. The questionnaire consists of ten behavior-related questions concerning delusions, hallucinations, depression, anxiety, agitation, disinhibition, euphoria, irritability, apathy, and aberrant motor activity. The score for each item is obtained by multiplying the severity with the frequency. The total score is 120. In accordance with our previous study,Citation12 the psychosis symptoms were defined as delusions, hallucinations, agitation, disinhibition, irritability, and aberrant motor activity.
In 2011, the National Institute on Aging and the Alzheimer’s Association proposed a revision of the criteria for AD.Citation13 However, we started this study in June 2009. The revised criteria require the examination of multiple biomarkers, including the need for a positron emission tomography (PET) examination, and the widespread clinical adoption of the revised criteria remains controversial.Citation14 Thus, we did not adopt the revised criteria that were updated in 2011.
Patients were excluded if (1) other neurological diseases were present, (2) the patient had a previous history of mental illness or substance abuse before the onset of dementia, (3) either an MRI or a CT scan had revealed focal brain lesions, (4) the patient’s Mini-Mental State Examination (MMSE)Citation15 score was less than 11, or (5) reliable informed consent could not be obtained from the patient and/or his/her relatives. The study protocol was approved by the Ethics Committee of Nagoya City University Medical School. Both the subjects and the caregivers were informed of the purpose and procedures of this study and were asked to sign a consent form.
Follow-up assessment
The follow-up assessment was conducted within one year after the baseline assessment. The presence of delusions was diagnosed based on the delusion score of the NPI. In agreement with previous studies,Citation8 a score of greater than 3 was regarded as indicating the presence of delusions. An independent psychiatrist who was unaware of the MRI results conducted the NPI evaluation. Both the MMSE and the NPI were assessed at 3-month intervals from the baseline examination.
MRI image acquisition
DTI data were acquired using single-shot, spin-echo echo-planar sequences on a 1.5-T MRI system (Gyoroscan Intera; Royal Philips Electronics, Amsterdam, The Netherlands) with a 33-m Tm−1 gradient and a receiver-only, six-channel phased-array head coil. The scanning parameters were as follows: echo time = 80 ms; repetition time = 6000 ms; 128 × 128 matrices, field of view = 224 × 224 mm2, 45 continuous axial slices of 3.0 mm thickness, 15 noncollinear axis motion probing gradient, b = 800 s · mm−2. Scanning was repeated two times to enhance the signal-to-noise ratio. The MRI studies were performed in the Department of Radiology of Nagoya City University Hospital. The MRI examination was conducted at baseline.
Data processing and analysis
DTI data processing was performed using FSL Ver 4.1 (Neuroimaging Informatics Tools and Research Clearinghouse [NITRC], Washington, DC, USA). All the data sources were corrected for eddy currents and head motion by registering all the data with the first b = 0 image of the first repetition, using affine transformation. A voxel-wise statistical analysis of the FA data was performed using tract-based spatial statistics (FMRIB, Oxford, UK).Citation16 FA maps were calculated using the FMRIB’s Diffusion Tool program. Both the AD patients who developed delusions and the AD patients who did not develop delusions were tested with 10,000 permutations. The statistical threshold was set at P < 0.05, and the results were corrected for multiple comparisons using threshold-free cluster enhancement.Citation16 Threshold-free cluster enhancement produces an output image in which the voxel value represents the weighed sum of the local clustered signal. We performed group comparisons using an analysis of covariates with age and gender, and MMSE score as nuisance covariates.
Results
Twenty-eight AD patients were enrolled in this study. Three AD patients had been lost to follow up at the end of the 1-year period; at the 1-year follow-up examinations, the remaining 25 AD patients had completed the study. Among these patients, ten AD patients had developed delusions, and the remaining 15 AD patients had not developed delusions during the follow-up period. The types of delusions in the AD patients, as identified by the NPI, were persecutory delusions (n = 9) and misidentification delusions (n = 1). The mean time until the development of delusions from the baseline assessment was 7.2 months (minimum of 3 months, maximum of 12 months).
The demographic data at baseline for the AD patients are summarized in . No significant differences in the demographic variables were found between the AD patients who developed delusions (n = 10) and those who did not develop delusions (n = 15).
Table 1 Demographic data of AD patients who did or did not develop delusions
Regarding the NPI subscale scores, with the exception of depression/dysphoria and apathy, the two groups of AD patients both scored 0 at baseline. Regarding the depression/dysphoria and apathy subscales, no significant differences were found between the two groups of AD patients (). During the 1-year study period, no significant differences in the depression/dysphoria and apathy subscales were found between the two groups of AD patients at the time of the appearance of delusions (depression/dysphoria: AD patients with delusions, 0.4 ± 0.5; AD patients without delusions, 0.3 ± 0.4; apathy: AD patients with delusions, 3.5 ± 1.6; AD patients without delusions, 3.9 ± 1.9). However, during the 1-year study period, the AD patients with delusions had significantly higher scores (P < 0.01) for both agitation and irritability on the NPI than did the AD patients without delusions, at the time of the appearance of delusions (agitation: AD patients with delusions, 2.4 ± 01.1; AD patients without delusions, 0.1 ± 0.3; irritability: AD patients with delusions, 2.5 ± 0.9; AD patients without delusions, 0.1 ± 0.3).
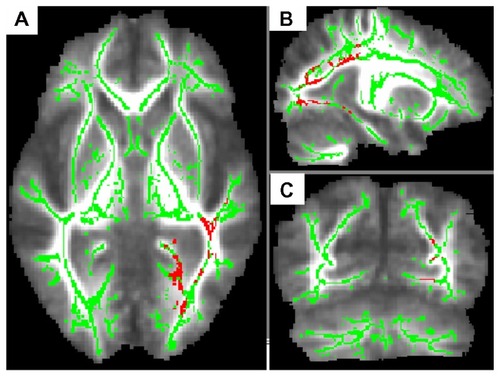
In the group comparison, the AD patients who developed delusions showed three clusters of significantly lower FA values compared with the AD patients who did not develop delusions. The first cluster (maximum cluster) was located in the left parieto-occipital region (peak MNI coordinates, x = −56, y = −37, z = 25; cluster size = 3741) (). These areas include several fibers, the left inferior longitudinal fasciculus (ILF), the left inferior fronto-occipital fasciculus (IFOF), the left posterior corona radiate, and the left forceps major of the corpus callosum. The second cluster was located on the body of the corpus callosum (CC) (peak MNI coordinates, x = 5, y = 8, z = 24; cluster size = 935). The third cluster (minimum cluster) was located on the right superior temporal gyrus white matter (peak MNI coordinates, x = 43, y = −30, z = 4; cluster size = 58).
Figure 1 AD patients who developed delusions (n = 10) exhibited lower FA values than those who did not develop delusions (n = 15). To aid visualization, the regions with significant FA reductions (P < 0.05, corrected by TFCE) have been thickened (pink-red). The results (left inferior longitudinal fasciculus, left inferior fronto-occipital fasciculus, left posterior corona radiate, left forceps major of the corpus callosum) are shown overlaid on the mean FA map and the FA skeleton (green); (A) axial slice; (B) sagittal slice; (C) coronal slice.
Discussion
To our knowledge, this is the first DTI study to use tract-based spatial statistics to examine white matter abnormalities at baseline, between AD patients who developed delusions and those who did not develop delusions. We identified two relatively large clusters of reduced FA in AD patients who developed delusions: one in the left parieto-occipital region, and the other in the body of the corpus callosum. The former cluster contains multiple fiber tracts, such as the left ILF and the IFOF. Several recent studies have suggested that abnormally low FA exists in late-myelinating fibers, such as the ILF, IFOF, and CC, in AD patients.Citation17,Citation18 Thus, it is clinically important to note that these significant FA reductions were observed in the AD patients who developed delusions relative to what was observed in AD patients who did not develop delusions, in this study.
A few, but significant, neuroimaging studies have demonstrated that both attentional, visuoperceptual, and memory deficits contribute to delusions in AD patients.Citation19,Citation20 The widespread attentional network may play an important role in both anterior and posterior associations in the development of delusions. Both ILF and IFOF are large fibers connected to corticortical pathways. The former association fiber connects the occipital and temporal lobes, while the latter connects the occipital and frontal lobes. These fibers are thought to play a role in the emotional valence of visual processing.Citation21,Citation22 Recent studiesCitation23,Citation24 have reported an association between delusion in AD and cognitive impairments such as visuospatial and executive functions. Unfortunately, we did not perform detailed neuropsychological tests other than the MMSE in the AD patients. No significant differences in the MMSE scores were found between the AD patients who developed delusions (n = 10) and those who did not develop delusions (n = 15). Thus, the association between detailed cognitive impairments and delusion in AD was unclear in this study. Further studies are needed to clarify the influence of neuropsychological function on AD patients who develop delusions.
The CC is the largest interhemispheric white matter commissure connecting the cerebral hemispheres.Citation25 Thus, the breakdown of connectivity between widespread areas may contribute to the development of delusions. Our study indicated that AD patients who develop delusions may have vulnerable WM structures in several fiber tracts. Several previous functional neuroimaging studies (either single photon emission computed tomography [SPECT] or PET) have suggested that a right hemispheric pathology is associated with delusions in AD patients.Citation26 The difference in laterality in this study (left dominance) may be attributed to methodological differences (ie, DTI analysis in this study vs functional neuroimaging in previous studies). In addition, recent studies using SPECT have demonstrated that the neural basis underlying persecutory delusions and misidentification delusions may differ in AD patients with delusions. Fukuhara et alCitation20 reported that the regional cerebral blood flow in the right posterior parietal region was lower in AD patients with persecutory delusions than in AD patients without delusions. However, Nakano et alCitation27 reported a lower regional cerebral blood flow in bilateral anterior cingulate gyri in AD patients with persecutory delusions compared with AD patients with misidentification delusions. In our study, the limited number of AD patients with misidentification delusions (n = 1) did not allow such an analysis. Thus, we should be cautious when interpreting the findings of this study, since only a limited number of AD patients were included in this pilot study. DTI analyses in further longitudinal, large-sample studies of AD patients who develop delusions are needed to support the findings of the present study.
Acknowledgments
The authors gratefully acknowledge the grant from a Grant-in-Aid for Scientific Research (c) (22530750, 22591293) from the Ministry of Education, Culture, Sports, Sciences and Technology in Japan.
Disclosure
The authors report no conflicts of interest in this work.
References
- HironoNKitagakiHKazuiHHashimotoMMoriEImpact of white matter changes on clinical manifestation of Alzheimer’s disease: A quantitative studyStroke20003192182218810978049
- LeeDYChooIHKimKWWhite matter changes associated with psychotic symptoms in Alzheimer’s disease patientsJ Neuropsychiatry Clin Neurosci200618219119816720796
- OgawaYHashimotoMYatabeYAssociation of cerebral small vessel disease with delusions in patients with Alzheimer’s diseaseInt J Geriatr Psychiatry Epub March 7, 2012
- ScarmeasNBrandtJAlbertMDelusions and hallucinations are associated with worse outcome in Alzheimer diseaseArch Neurol200562101601160816216946
- OishiKMielkeMMAlbertMLyketsosCGMoriSDTI analyses and clinical applications in Alzheimer’s diseaseJ Alzheimers Dis201126Suppl 328729621971468
- ZhangYSchuffNJahngGHDiffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer diseaseNeurology2007681131917200485
- MielkeMMKozauerNAChanKCRegionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer’s diseaseNeuroimage2009461475519457371
- AaltenPde VugtMELousbergRBehavioral problems in dementia: a factor analysis of the neuropsychiatric inventoryDement Geriatr Cogn Disord20031529910512566599
- McKhannGDrachmanDFolsteinMKatzmanRPriceDStadlanEMClinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services. Task Force on Alzheimer’s DiseaseNeurology19843479399446610841
- CummingsJLMegaMGrayKRosenberg-ThompsonSCarusiDAGornbeinJThe Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementiaNeurology19944412230823147991117
- HironoNMoriEIkejiriYJapanese version of the Neuropsychiatric Inventory – a scoring system for neuropsychiatric disturbance in dementia patientsNo To Shinkei1997493266271 Japanese9125732
- MatsuiTNakaakiSMurataYDeterminants of the quality of life in Alzheimer’s disease patients as assessed by the Japanese version of the Quality of Life-Alzheimer’s disease scaleDement Geriatr Cogn Disord200621318219116401890
- McKhannGMKnopmanDSChertkowHThe diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s diseaseAlzheimers Dement20117326326921514250
- FrisoniGBWinbladBO’BrienJTRevised NIA-AA criteria for the diagnosis of Alzheimer’s disease: a step forward but not yet ready for widespread clinical useInt Psychogeriatrics201123811911196
- FolsteinMFFolsteinSEMcHughPR“Mini-mental state”. A practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res19751231891981202204
- SmithSMNicholsTEThreshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inferenceNeuroimage2009441839818501637
- TeipelSJBornCEwersMMultivariate deformation-based analysis of brain atrophy to predict Alzheimer’s disease in mild cognitive impairmentNeuroimage2007381132417827035
- StrickerNHSchweinsburgBCDelano-WoodLDecreased white matter integrity in late-myelinating fiber pathways in Alzheimer’s disease supports retrogenesisNeuroimage2009451101619100839
- StaffRTShanksMFMacintoshLPestellSJGemmellHGVenneriADelusions in Alzheimer’s disease: spet evidence of right hemispheric dysfunctionCortex199935454956010574080
- FukuharaRIkedaMNebuAAlteration of rCBF in Alzheimer’s disease patients with delusions of theftNeuroreport200112112473247611496132
- CataniMJonesDKDonatoRFfytcheDHOccipito-temporal connections in the human brainBrain2003126Pt 92093210712821517
- MiyataJYamadaMNamikiCReduced white matter integrity as a neural correlate of social cognition deficits in schizophreniaSchizophr Res20101191–323223920097045
- Perez-MadrinanGCookSESaxtonJAAlzheimer disease with psychosis: excess cognitive impairment is restricted to the misidentification subtypeAm J Geriatr Psychiatry200412544945615353383
- NagataTIshiiKItoTCorrelation between a reduction in Frontal Assessment Battery scores and delusional thoughts in patients with Alzheimer’s diseasePsychiatry Clin Neurosci200963444945419460120
- NakamaeTNarumotoJSakaiYDiffusion tensor imaging and tract-based spatial statistics in obsessive-compulsive disorderJ Psychiatr Res201145568769020965515
- IsmailZNguyenMQFischerCESchweizerTAMulsantBHNeuroimaging of delusions in Alzheimer’s diseasePsychiatry Res20122022899522703622
- NakanoSYamashitaFMatsudaHKodamaCYamadaTRelationship between delusions and regional cerebral blood flow in Alzheimer’s diseaseDement Geriatr Cogn Disord2006211162116254426