Abstract
Background
We aimed to investigate the association of post-thrombolytic D-dimer elevation with symptomatic intracranial hemorrhage (sICH) and functional outcome in AIS patients receiving intravenous thrombolysis.
Methods
We retrospectively reviewed our database for patients with AIS who received intravenous thrombolysis between August 2018 and December 2021. ΔD-dimer was calculated as follow-up D-dimer minus baseline D-dimer. Poor functional outcome was defined as 3 months modified Rankin score (mRS) 3–6. sICH was defined as cerebral hemorrhagic transformation in combination with clinical deterioration of National Institutes of Health Stroke Scale (NIHSS) score ≥4 points at 24 hours. Binary logistic regression analysis was used to investigate the association of post-thrombolytic D-dimer parameters with sICH and poor functional outcome. The receiver operating characteristic (ROC) curve derived optimal cut-off of different D-dimer parameters was determined at the maximal Youden’s Index.
Results
A total of 325 patients were finally included. After controlling for clinical variables, follow-up D-dimer level (OR 1.230; 95% CI 1.119 to 1.351; P < 0.001) and ΔD-dimer (OR 1.347; 95% CI 1.165 to 1.559; P < 0.001) were independently associated with poor functional outcome. Additionally, follow-up D-dimer level (OR 1.095; 95% CI 1.009 to 1.188; P = 0.030) was independently related to sICH. The optimal cut-off value of follow-up D-dimer level for predicting sICH was 4185 μg/L (area under the curve 0.760; sensitivity 76.0%; specificity 81.3%); and the optimal cut-off value of follow-up D-dimer level and ΔD-dimer as a predictor for poor functional outcome was projected to be 3838 μg/L and 2190 μg/L, which yielded a sensitivity and a specificity of 62.3%, 84.5% and 73.8%, 85.2%, respectively.
Conclusion
Elevated follow-up D-dimer levels are associated with sICH and poor functional outcome in AIS patients following intravenous rt-PA. Moreover, post-thrombolytic D-dimer elevation, measured by ΔD-dimer, was a better predictive biomarker for long-term outcome at 3 months.
Introduction
Intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) has been the established standard treatment in acute ischemic stroke (AIS) patients within 4.5 hours of stroke onset.Citation1 However, intravenous thrombolysis also increases the risk of post-thrombolytic hemorrhagic transformation (HT), particularly symptomatic intracranial hemorrhage (sICH), which is closely related to unfavorable outcome.Citation2–5 Therefore, it is crucial that the physician be able to assess and predict outcomes. Previous studies demonstrated that various risk factors may be attributed to poor functional outcome or the occurrence of HT after intravenous thrombolysis, including old age, severe neurological symptoms on admission, high serum glucose or diabetes mellitus history.Citation6 There are, however, few studies that have focused on the effect of coagulation factors on outcomes of AIS patients with intravenous thrombolysis.
Since rt-PA works by activating the clot-bound plasminogen, converting plasminogen to plasmin, which in turn breaks the cross-linking strands of fibrin, and eventually dissolves the blood clot.Citation7 It suggested that fibrinolysis parameters might have a non-negligible impact on the outcomes of AIS patients receiving intravenous thrombolysis. D-dimer is formed during activation of the fibrinolytic system and derived from the degradation of cross-linked fibrinogen.Citation8,Citation9 As a result, the levels of D-dimer are increased after the application of rt-PA. A previous study involving 159 patients found that increased D-dimer levels within 24 hours of stroke onset were independently associated with the occurrence of sICH and poor functional outcome at 3 months.Citation10 However, D-dimer data before intravenous thrombolysis were not available in the study. And how intravenous rt-PA affects D-dimer levels remains unclear. Considering that post-thrombolytic D-dimer levels are a dynamically changing process, it may seem more reasonable to use ΔD-dimer (follow-up D-dimer minus baseline D-dimer) to ascertain the causality. Meanwhile, few studies have investigated the relationship between post-thrombolytic D-dimer changes and outcomes in AIS patients with intravenous thrombolysis.
In view of these considerations, we aimed to investigate the association between post-thrombolytic ΔD-dimer changes and outcomes in AIS patients with intravenous rt-PA and evaluate its predictive value for sICH and poor functional outcome.
Materials and Methods
Ethics Statement
The human ethics committee of Zhejiang Provincial People’s Hospital approved the protocol of this study. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. All subjects had given written informed consent prior to the study.
Study Subjects
We retrospectively reviewed our database for consecutive acute ischemic stroke patients who received intravenous thrombolysis with rt-PA between August 2018 and December 2021. The inclusion criteria were (1) age ≥18 years; (2) a diagnosis of AIS and treatment with intravenous rt-PA within 4.5 hours of symptom onset; (3) D-dimer level on admission and within 24 hours after thrombolysis were collected; (4) Follow-up computed tomography (CT) or magnetic resonance imaging (MRI) was performed within 24–36 hours after thrombolysis; and (5) had modified Rankin Scale (mRS) score at 3 months. Given that endovascular thrombectomy (EVT) may have a significant effect on functional outcome and postoperative hemorrhagic transformation. For example, EVT may cause damage vascular endothelial injury, the glycoprotein-IIb/IIIa inhibitor tirofiban might be used to prevent early reocclusion due to endothelial injury. We thus excluded patients with EVT in order to reduce study heterogeneity. Recombinant tissue-type plasminogen activator (alteplase, 0.9 mg/kg up to a maximum of 90 mg/kg) was used with 10% of the total dosage as a bolus and the rest >1 hour.Citation4,Citation11
We retrieved demographic and clinical data, including age, sex; and body mass index (BMI), comorbid conditions such as history of smoking, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, prior antiplatelet usage; onset-to-needle time (ONT); baseline National Institutes of Health Stroke Scale (NIHSS) score, baseline systolic blood pressure (SBP) and diastolic blood pressure (DBP); time interval between rt-PA infusion and follow-up blood collection.
Laboratory tests included baseline blood glucose, platelet count, international normalized ratio, and D-dimer levels. At our stroke center, the patient received intravenous thrombolysis in the emergency department and was immediately admitted to a neurological intensive care unit after intravenous thrombolysis. Follow-up D-dimer sampling was then collected the next morning. ΔD-dimer was calculated as follow-up D-dimer minus baseline D-dimer. D-dimer level was measured by an Enzyme Linked Fluorescent Assay with a coagulation analyzer (CS-5100, Sysmex, Japan). The normal range of D-dimer concentration in our hospital laboratory is 0–550 μg/L.
Evaluation of Outcomes
Our study evaluated 2 outcomes: (1) Functional outcome at 3 months was assessed with mRS score and dichotomized into good outcome (0–2) and poor outcome (3–6). Hemorrhagic transformation was accessed according to the European Cooperative Acute Stroke Study (ECASS) II trial.Citation12 Symptomatic intracerebral hemorrhage (sICH) was defined as cerebral hemorrhagic transformation in combination with clinical deterioration in the NIHSS score by ≥4 points at 24 hours.Citation12
Statistical Analysis
Clinical characteristics were summarized as mean ± SD or median (25th-75th percentile) for quantitative variables depending on the normality of the distribution and as frequency (percentage) for categorical variables, as appropriate. Fisher exact test was used to compare the dichotomous variables between 2 groups, whereas an independent sample 2-tailed t-test or a Mann–Whitney U-test was used for the continuous variables, depending on the normality of the distribution. Associations of D-dimer parameters with poor functional outcome and sICH were determined using binary logistic regression models adjusted by baseline characteristics with a P value of <0.1 in univariate analyses, respectively. Each D-dimer parameter was entered in the binary logistic regression models alone as the potential collinearity. Receiver operating characteristics (ROC) curve analysis was used to determine predictive value. The ROC-derived optimal cutoff was determined at the maximal Youden’s Index. All statistical analyses were performed using SPSS, Version 22.0 (IBM, Armonk, New York). A P value <0.05 was considered statistically significant.
Results
Overall Characteristics
A total of 386 consecutive AIS patients received intravenous rt-PA during the study period, and of these, 325 patients were included in the final analysis after excluding patients with lack of baseline D-dimer level data (n = 59) and lost to follow-up (n = 2).
Of the included patients, the mean age was 67.9 ± 14.0 years, and 100 (30.8%) were female. The median baseline NIHSS score was 3 (interquartile range, 2–7), median time from onset to needle was 150 (interquartile range, 100–200) minutes. The median baseline D-dimer levels were 450 (interquartile range, 230–1315) μg/L and follow-up D dimer levels increased to 1800 (interquartile range, 880–3940) μg/L after intravenous rt-PA. The mean time interval from rt-PA infusion to follow-up blood collection was 9.6 (interquartile range, 5.6–14.4) hours. Among them, there were 61 (18.7%) patients with poor functional outcome and 25 (7.6%) patients with sICH.
D-Dimer Parameters and Poor Functional Outcomes
As shown in , patients with poor functional outcome were older (75.8 vs 66.1 years, P<0.001), had a higher frequency of atrial fibrillation (32.8% vs 16.3%, P=0.003), higher baseline NIHSS score (9 vs 3, P<0.001) and lower baseline platelet level (179.7 vs 195.4, P=0.024) than those with good functional outcome. The baseline D-dimer level (1170 vs 400 μg/L, P<0.001), follow-up D-dimer level (6270 vs 1380 μg/L, P<0.001) and ΔD-dimer (3260 vs 710 μg/L, P<0.001) were higher in poor functional outcome group. Binary logistic regression analyses indicated that follow-up D-dimer level (OR 1.230; 95% CI 1.119 to 1.351; P<0.001) and ΔD-dimer (OR 1.347; 95% CI 1.165 to 1.559; P<0.001) were independently associated with poor functional outcome after controlling for age, atrial fibrillation, baseline NIHSS score, onset to needle time, baseline platelet level ().
Table 1 Comparison of Characteristics Between Patients with Different Outcomes
Table 2 Multivariate Regression Analysis for Prediction of Symptomatic Intracranial Hemorrhage and Poor Functional Outcome in Patients with Intravenous Thrombolysis
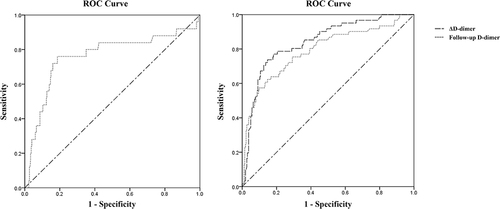
shows the diagnostic parameters including AUCs, cut-off values, sensitivity, specificity at the maximal Youden’s Index of follow-up D-dimer level and ΔD-dimer in predicting poor functional outcome. Based on the ROC curve, the optimal cut-off value of follow-up D-dimer level and ΔD-dimer as an indicator for diagnosis of poor functional outcome was projected to be 3838 μg/L and 2190 μg/L, which yielded a sensitivity and a specificity of 62.3%, 84.5% and 73.8%, 85.2%, respectively. The ROC curves of follow-up D-dimer level and ΔD-dimer in predicting poor functional outcome are shown in .
Table 3 Predictive Value of Different D-Dimer Parameters for Symptomatic Intracranial Hemorrhage and Poor Functional Outcome
D-Dimer Parameters and Symptomatic Intracerebral Hemorrhage
As summarized in , patients with sICH were older (76.1 vs 67.3 years, P=0.002), had lower BMI (22.2 vs 23.9 kg/m2, P=0.019), higher baseline NIHSS score (12 vs 3, P<0.001). The time interval from rt-PA infusion to blood collection was longer in patients with sICH (14.4 vs 9.2 hours, P=0.044) than those without sICH. In addition, the baseline D-dimer level (1120 vs 430 μg/L, P=0.001), follow-up D-dimer level (6270 vs 1610 μg/L, P<0.001) and ΔD-dimer (3200 vs 820 μg/L, P<0.001) were higher in patients with sICH. After adjustment for age, BMI, atrial fibrillation, hyperlipidemia, baseline NIHSS score and time interval from rt-PA infusion to blood collection, the results indicated that follow-up D-dimer level (OR 1.095; 95% CI 1.009 to 1.188; P=0.030) were independently related to sICH ().
The ROC curves of follow-up D-dimer level in predicting sICH are shown in . According to ROC analysis, the optimal cut-off value of follow-up D-dimer level for predicting sICH was 4185 μg/L, and the areas under the curve (AUC) were 0.760. The sensitivity and specificity for this cutoff value were 76.0% and 81.3%, respectively ().
Discussion
In the current study, our findings suggest that follow-up D-dimer level and post-thrombolytic D-dimer elevation, measured by ΔD-dimer, were associated with a higher likelihood of poor functional outcome. Moreover, follow-up D-dimer level was consistently related to an increased risk of sICH in AIS patients following intravenous rt-PA.
Intravenous rt-PA activates the fibrinolytic system and converts the plasminogen into the proteolytic enzyme plasmin.Citation13 Plasmin splits both fibrinogen and fibrin into degradation products, which results in lysis of the clot.Citation14 D-dimer, a soluble fibrin degradation final product, is derived from the cross-linked fibrin network as it undergoes plasmin-mediated degradation.Citation15 Consequently, the levels of D-dimer are significantly increased after the application of rt-PA. Hsu et al found that D-dimer level within the 24 hours after stroke onset is an independent predictor of poor outcome and sICH in AIS patients receiving intravenous rt-PA. Our study also found that post-thrombolytic elevated D-dimer level was an independent risk factor of sICH after treatment with intravenous rt-PA, which aligned with the results of the previous study. Although the underlying mechanisms of post-thrombolytic elevated D-dimer level and sICH have not been elucidated, several possible explanations might account for that. Post-thrombolytic D-dimer elevation may reflect intricate disorders of fibrinolytic and hemostatic functions, which might lead to inhibition of hemostatic function and hypocoagulable state of the blood system, making it easier to trigger massive hemorrhage.Citation8,Citation16 Furthermore, elevated D-dimer level was reported to stimulate the immune system and potentially boost the proinflammatory cytokine cascade, such as IL-1, IL-6 and TNF-α.Citation17,Citation18 Activated inflammation may contribute to the pathological alteration in ischemic brain tissue. For instance, previous animal studies in mice have reported that the increase in inflammatory mediators is usually correlated with blood-brain barrier (BBB) disruption which may lead to hemorrhagic transformation.Citation19
Moreover, we found that follow-up D-dimer level and post-thrombolytic D-dimer elevation, measured by ΔD-dimer, were associated with poor functional outcome at 3 months. A growing body of evidence demonstrated that an elevated level of D-dimer is associated with increased risk of poor outcome in AIS patients.Citation20–22 In addition to the possible explanations mentioned above. A previous study by Park et al found that a high D-dimer level has a positive correlation with infarct volume, therefore elevated D-dimer levels may predict a worse outcome through worsening the extent of damage to the ischemic brain tissue.Citation23 Risk factors for hemorrhagic transformation after AIS include larger infarct volume,Citation24 it might, to some extent, explain why sICH would be prone to occur more frequently in patients with elevated D-dimer level, especially in the setting of coagulation disorders after intravenous thrombolysis.
The major difference between our study and previous studies is that we include the extent of D-dimer elevation after intravenous thrombolysis as an observation index, not simply D-dimer levels. Notably, we found that the post-thrombolytic D-dimer elevation, measured by ΔD-dimer, was also related to poor functional outcome at 3 months. In addition, ΔD-dimer has a better predictive value for poor functional outcome than the follow-up DD level. D-dimer is a fibrin degradation final product, indicating the presence of clot lysis indirectly. Theoretically, the level of D-dimer increased at 2 hours after intravenous thrombolysis and returned to the baseline level at 24 hours.Citation25 In the present study, we assume that the sustained elevation of D-dimer following intravenous thrombolysis might indicate a greater baseline thrombus burden or resistance to the endogenous fibrinolytic system, weakening the thrombolytic effect of rt-PA and ultimately resulting in poor functional outcome. Another possible explanation might be that the occluded vessels could be recanalized under the work of rt-PA. The large thrombus burden at baseline may result in a large infarct volume, which is susceptible to severe hemorrhagic transformation. In this regard, it is important to monitor the dynamic changes in D-dimer level after intravenous thrombolysis.
Limitations include the study being conducted with a small sample size and retrospective design, which might have the potential of selection bias. Second, due to the lack of a standardized protocol for blood collection after intravenous thrombolysis at our center, the follow-up D-dimer levels were only measured the following morning after intravenous thrombolysis. However, it would be better to explore the correlation between post-thrombolytic D-dimer parameters and outcomes by fixing a time for blood collection and monitoring the serial change in D-dimer levels. Due to the relatively wide time range for observing dynamic changes in D-dimer levels, prospective studies with large samples and a standardized protocol are needed to validate the results in the future. Third, we only assessed the relationship between post-thrombolytic D-dimer parameters and outcomes in AIS patients with intravenous thrombolysis. Therefore, the conclusion cannot be simply extended to patients following endovascular thrombectomy (EVT). Finally, the established associations in this study may be impacted by other baseline characteristics that are not measured, which are worthy of further investigation.
Conclusion
Our study provides preliminary data indicating that elevated follow-up D-dimer levels are associated with sICH and poor functional outcome in AIS patients following intravenous rt-PA. Besides, post-thrombolytic D-dimer elevation, measured by ΔD-dimer, was a better predictive biomarker for long-term outcome at 3 months. Further prospective multicenter studies with a larger sample size need to be conducted to ascertain the causality.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Warner JJ, Harrington RA, Sacco RL, Elkind MSV. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke. Stroke. 2019;50:3331–3332. doi:10.1161/STROKEAHA.119.027708
- Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European cooperative acute stroke study (ecass). JAMA. 1995;274:1017–1025.
- Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ecass ii). Second European-Australasian acute stroke study investigators. Lancet. 1998;352:1245–1251. doi:10.1016/S0140-6736(98)08020-9
- Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (sits-most): an observational study. Lancet. 2007;369:275–282. doi:10.1016/S0140-6736(07)60149-4
- Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24:1–10. doi:10.1159/000103110
- Shrestha S, Poudel RS, Thapa LJ, Khatiwada D. Intravenous thrombolysis and risk factors for ischemic stroke. JNMA J Nepal Med Assoc. 2014;52:745–750. doi:10.31729/jnma.2615
- Jilani TN, Siddiqui AH. Tissue Plasminogen Activator. Treasure Island (FL): Statpearls; 2022.
- Zhou Z, Liang Y, Zhang X, et al. Plasma d-dimer concentrations and risk of intracerebral hemorrhage: a systematic review and meta-analysis. Front Neurol. 2018;9:1114. doi:10.3389/fneur.2018.01114
- Rowe CA, Bolitho JS, Jane A, et al. Rapid detection of d-dimer using a fiber optic biosensor. Thromb Haemost. 1998;79:94–98. doi:10.1055/s-0037-1614227
- Hsu PJ, Chen CH, Yeh SJ, Tsai LK, Tang SC, Jeng JS. High plasma d-dimer indicates unfavorable outcome of acute ischemic stroke patients receiving intravenous thrombolysis. Cerebrovasc Dis. 2016;42:117–121. doi:10.1159/000445037
- National Institute of Neurological D, Stroke rt PASSG. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi:10.1056/NEJM199512143332401
- Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian acute stroke study (ecass ii). Stroke. 2001;32:438–441. doi:10.1161/01.STR.32.2.438
- Murray V, Norrving B, Sandercock PA, Terent A, Wardlaw JM, Wester P. The molecular basis of thrombolysis and its clinical application in stroke. J Intern Med. 2010;267:191–208. doi:10.1111/j.1365-2796.2009.02205.x
- Collen D, Lijnen HR. Thrombolytic agents. Thromb Haemost. 2005;93:627–630. doi:10.1160/TH04-11-0724
- Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: d-dimer. J Am Coll Cardiol. 2017;70:2411–2420. doi:10.1016/j.jacc.2017.09.024
- Di Castelnuovo A, Agnoli C, de Curtis A, et al. Elevated levels of d-dimers increase the risk of ischaemic and haemorrhagic stroke. Findings from the EPICOR study. Thromb Haemost. 2014;112:941–946. doi:10.1160/th14-04-0297
- Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121:1262–1268. doi:10.1378/chest.121.4.1262
- Robson SC, Shephard EG, Kirsch RE. Fibrin degradation product d-dimer induces the synthesis and release of biologically active il-1 beta, il-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol. 1994;86:322–326. doi:10.1111/j.1365-2141.1994.tb04733.x
- Choi JI, Ha SK, Lim DJ, Kim SD, Kim SH. S100ss, matrix metalloproteinase-9, d-dimer, and heat shock protein 70 are serologic biomarkers of acute cerebral infarction in a mouse model of transient MCA occlusion. J Korean Neurosurg Soc. 2018;61:548–558. doi:10.3340/jkns.2017.0200
- Yao T, Tian BL, Li G, et al. Elevated plasma d-dimer levels are associated with short-term poor outcome in patients with acute ischemic stroke: a prospective, observational study. BMC Neurol. 2019;19:175. doi:10.1186/s12883-019-1386-3
- Zhang J, Liu L, Tao J, et al. Prognostic role of early d-dimer level in patients with acute ischemic stroke. PLoS One. 2019;14:e0211458. doi:10.1371/journal.pone.0211458
- Yang XY, Gao S, Ding J, Chen Y, Zhou XS, Wang JE. Plasma d-dimer predicts short-term poor outcome after acute ischemic stroke. PLoS One. 2014;9:e89756. doi:10.1371/journal.pone.0089756
- Park YW, Koh EJ, Choi HY. Correlation between serum d-dimer level and volume in acute ischemic stroke. J Korean Neurosurg Soc. 2011;50:89–94. doi:10.3340/jkns.2011.50.2.89
- Desilles JP, Syvannarath V, Ollivier V, et al. Exacerbation of thromboinflammation by hyperglycemia precipitates cerebral infarct growth and hemorrhagic transformation. Stroke. 2017;48:1932–1940. doi:10.1161/STROKEAHA.117.017080
- Sun X, Berthiller J, Derex L, Trouillas P, Diallo L, Hanss M. Post-thrombolysis haemostasis changes after rt-pa treatment in acute cerebral infarct. Correlations with cardioembolic aetiology and outcome. J Neurol Sci. 2015;349:77–83. doi:10.1016/j.jns.2014.12.029