Abstract
Background
Migraine often occurs during weekends. The efficacy of frovatriptan, naproxen sodium, or no therapy for the acute or prophylactic treatment of weekend migraineurs was tested in an open-label, nonrandomized pilot study.
Methods
Twenty-eight subjects (mean age 36 ± 12 years, including 18 females) suffering from migraine without aura were followed up for six consecutive weekends. No treatment was administered during the first two weekends. On the third and fourth weekends, patients were given frovatriptan 2.5 mg and on the fifth and sixth weekends naproxen sodium 500 mg. Treatment was taken on Saturday and Sunday morning, regardless of the occurrence of migraine. Efficacy was evaluated through a diary, where patients reported the severity of migraine on a scale from 0 (no migraine) to 10 (severe migraine) and use of rescue medication.
Results
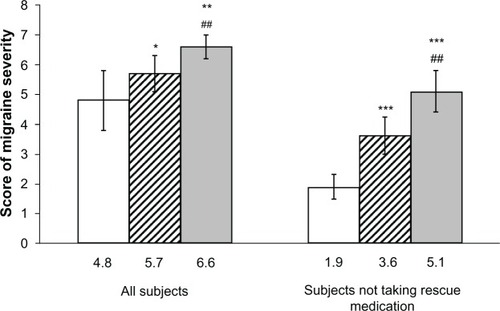
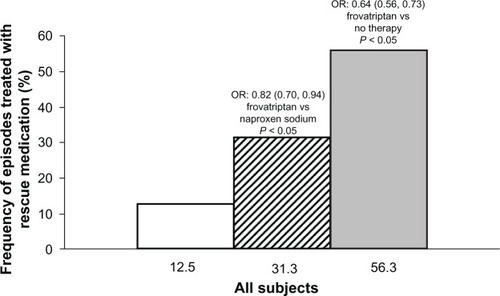
The migraine severity score was significantly lower with frovatriptan (4.8 [95% confidence interval (CI) 3.8–5.9]) than with naproxen sodium (5.7 [CI 5.1–6.4], P< 0.05 versus frovatriptan) or no therapy (6.6 [6.2–7.0], P< 0.01 versus frovatriptan). The difference in favor of frovatriptan was more striking in patients not taking rescue medication (frovatriptan, 1.9 [1.5–2.3]) versus naproxen sodium 3.6 [3.0–4.2], P< 0.001) and versus no therapy (5.1 [4.4–5.8], P< 0.001) and on the second day of treatment. The rate of use of rescue medication was significantly (P< 0.05) lower on frovatriptan (12.5%) than on naproxen sodium (31.3%) or no therapy (56.3%).
Conclusion
This pilot study provides the first evidence of the efficacy of a second-generation triptan as symptomatic or prophylactic treatment for weekend migraine.
Introduction
Migraine is a chronic, recurrent, disabling condition affecting millions of people worldwide.Citation1 Some epidemiological studies indicate that up to 20% of subjects with migraine or tension-type headache suffer from weekend attacks.Citation2–Citation4 The origin of this condition is unknown, being likely related to social or psychological factors.Citation5 Weekend headache attacks are clinically similar to those of common migraine, but with a significantly higher incidence of concomitant symptoms and a higher degree of headache severity.Citation6 Unfortunately, weekend migraine has been poorly investigated over the years and evidence of drug efficacy for treatment of this condition is lacking.
Effective first-line therapies for mild to moderate migraine are nonsteroidal anti-infammatory drugs and combination analgesics containing acetaminophen, aspirin, and caffeine. Selective serotonin 5-HT1B/1D receptor agonists and triptans are first-line therapies for moderate to severe migraine, and for mild to moderate migraine that has not responded to adequate doses of simple analgesics.Citation7,Citation8 During the past decade, there has been a rapid increase in the number of randomized studies successfully testing the efficacy and safety of triptans in several forms of migraine.Citation8,Citation9 In the present paper, we report the results of a comparative pilot study which shows the efficacy of frovatriptan versus naproxen sodium as symptomatic or prophylactic treatment for weekend migraineurs.
Materials and methods
Study population
The study included subjects of male or female gender, age 19–65 years, with a current history of migraine without aura, the occurrence of which was limited to weekend or nonworking days. Migraine was defined according to the criteria reported in the 2nd Edition of the International Headache Classification of the International Headache Society.Citation10
A patient could not be enrolled in the study in the event of: uncontrolled hypertension; ischemic heart disease; cardiac arrhythmias or symptomatic Wolff-Parkinson-White syndrome; previous stroke or transient ischemic attack; severe liver or renal impairment; any other severe or disabling medical condition; a history of alcohol, analgesic, or psychotropic drug abuse; known hypersensitivity to study drugs; previously demonstrated inadequate response to treatment with drugs of the same class as those employed in the study; current use of propranolol or ergotamine (and its derivatives) as a prophylactic agent; current use or use in the previous 2 weeks of monoamine oxidase inhibitors; use of either test medication to treat any one of the last three episodes of migraine; and other headaches lasting for more than 6 days. Pregnant women and breast-feeding mothers were also excluded, while women with childbearing potential but not practicing an effective method of birth control were to be submitted to a pregnancy test, if clinically indicated. Written informed consent was obtained from all patients prior to their inclusion in the study. The study was approved by the independent institutional review board of the study center.
Study design
This was a single-center, nonrandomized, open-label pilot study. Subjects were followed up for 6 consecutive weekends. During the first 2 weekends, no treatment was administered. This period in the absence of treatment served as the control. On the third and fourth weekends, patients were asked to take one 2.5 mg dose of frovatriptan on Saturday and another on Sunday morning, regardless of the occurrence of migraine. On the fifth and sixth weekend, patients were asked to take a 500 mg dose of naproxen sodium in the same manner. All patients were allowed to take no more than one daily dose of a rescue medication, in the afternoon, at least 6 hours after morning awakening. Alternative rescue medication could not include triptans, naproxen sodium, propranolol, or contain ergotamine or its derivatives.
Efficacy was evaluated through a migraine diary, where patients had to report the severity of migraine on a scale ranging between 0 (no migraine) and 10 (severe migraine) and use of rescue medication. The diary was delivered to the patient upon enrolment. It had to be completed at the end of each weekend and returned to the investigator at study end.
The study involved one screening (baseline) and one final visit. At the initial visit, after signing written informed consent, subjects provided a medical, treatment, and migraine history. A physical and neurological examination and pregnancy test (if appropriate) were performed. Blood pressure and heart rate were measured in all subjects. At the end of the visit, the study drugs and the migraine diary were given to the patient. Unused drugs and diaries were returned by the patient to the investigator at the final visit, which occurred 6 weeks after enrolment.
Data analysis
Analysis was done by considering each treatment period as a separate study group (frovatriptan, naproxen sodium, and control with no therapy). For each group or period, the average score of migraine severity (and 95% confidence interval) was computed. Differences between treatment periods were evaluated using a generalized estimating equations (GEE) model. The GEE model belongs to a class of semiparametric regression techniques used to estimate the parameters of a generalized linear model with a possible unknown correlation between outcomes.Citation11,Citation12 The factors included in the model were gender, treatment, use of rescue medication, and weekend day. Interactions between these factors were tested in the model. The average score was estimated using age as a covariate. Analysis was applied to the whole study population and separately to the subgroups taking or not taking rescue medication. The data were analyzed for each weekend day (Saturday and Sunday) and for the two days pooled together. Statistical analysis was performed using the SPSS version 17.0 (IBM Corporation, Armonk, NY). The P value refers to the statistical significance of between-treatment difference. The level of statistical significance was kept at 0.05 throughout the study.
Results
A total of 28 subjects were enrolled and completed the study. The average age of the study population was 36 ± 12 years (median 33 years, range 19–60 years), with 18 of 28 subjects (64.3%) being female. Other baseline subject characteristics are summarized in .
Table 1 Demographic and migraine history characteristics of the 28 patients at baseline
Results of multivariate analysis (GEE model) showed a significant overall treatment effect (P < 0.001), and a significant interaction between treatment*gender (P = 0.035), treatment*weekend day (P = 0.002), treatment*rescue medication (P < 0.001), treatment*age (P = 0.003), and treatment*rescue medication*weekend day (P < 0.001). These results confirm the assumption of our model, namely, that the headache score depends significantly on type of treatment, use of rescue medication, weekend day, and age.
As shown in , the average score for migraine severity was significantly lower with frovatriptan than with either naproxen sodium or no treatment in all subjects. Pain severity was also lower in the subgroup of patients not taking rescue medication, with more striking differences between frovatriptan-treated and either naproxen sodium-treated or untreated attacks.
Figure 1 Average score (and 95% confidence interval) of migraine severity in all subjects and in those not taking rescue medication.
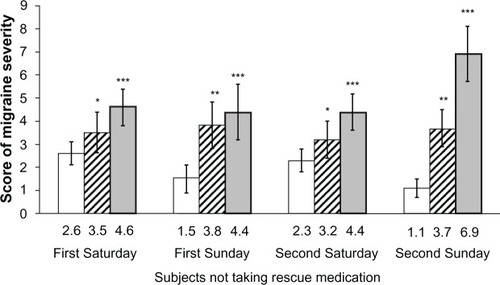
During each weekend day (Saturday or Sunday) the pain relief effect of frovatriptan was always significantly greater than with naproxen sodium or no therapy, and particularly so for subjects not taking rescue medication (). Interestingly, in frovatriptan-treated episodes, the drug was significantly (P , 0.001) more effective on the second day (Sunday) than on the first day of intake (Saturday).
Figure 2 Average score (and 95% confidence interval) of migraine severity in subjects not taking rescue medication, according to weekend day.
Overall, 14 of 112 episodes (12.5%) treated with frovatriptan needed rescue medication. This frequency was significantly lower than that observed with naproxen sodium (35/112, 31.3%) or no therapy (63/112, 56.3%). The chance of needing rescue medication was 18% less with frovatriptan than with naproxen sodium (odds ratio based on GEE analysis, 0.82 [95% confidence interval 0.70–0.94)] and 36% less with frovatriptan than with no therapy (odds ratio 0.64 [95% confidence interval 0.56–0.73], ).
Figure 3 Frequency (%) of episodes treated with rescue medication during treatment with frovatriptan (open bar), naproxen sodium (dashed bar) or during no therapy (full bar).
Discussion
In our small, open-label study, either symptomatic or prophylactic treatment of weekend migraine with frovatriptan was more effective in controlling pain severity than no treatment or treatment with naproxen sodium. The beneficial effect of frovatriptan was evident in all subjects and in those not requiring rescue medication.
Frovatriptan was more efficient on the second day of intake (Sunday), a finding which might be attributed to the cumulative effects of taking a second dose and to the pharmacokinetics of the drug.
Frovatriptan has a slower onset of action than other triptans and nonsteroidal anti-infammatory drugs. It also has a relative long half-life and a high potency of agonistic action on the 5-HT1B/1D receptor, possibly enabling a reduction in use of rescue medication and in risk of recurrence.Citation8,Citation13–Citation15 Frovatriptan might be indicated for these features as miniprophylaxis to prevent migraines in individuals for whom known triggers cannot be avoided.Citation16 This pilot study suggests that the pharmacological features of frovatriptan match well with its clinical efficacy in a particular form of migraine, such as that occurring during weekend days.
This is the first study evaluating the efficacy of pharmacological treatment in weekend migraine, and specifically of a triptan. It is also one of the first studies directly comparing frovatriptan and naproxen sodium in migraineurs. A previous study from our group assessed the daily incidence and severity of migraine, demonstrating that short-term prophylaxis of menstrual migraine over 6 days with frovatriptan is more effective than that based on naproxen sodium or transdermal oestrogen.Citation17
Another study evaluated the efficacy and tolerability of acute treatment of migraine using a combination of sumatriptan 50 mg (encapsulated) and naproxen sodium 500 mg administered concurrently in the acute treatment of migraine with respect to monotherapy.Citation18 Combination treatment resulted in significantly superior pain relief and pain-free response and lower rates of recurrence as compared with monotherapy of either sumatriptan 50 mg or naproxen sodium 500 mg. No differences were observed in the efficacy of the two monotherapies.
Our study suggests that frovatriptan is an effective agent when used as intermittent prophylactic therapy, as previously shown in studies including women with menstrual migraineCitation17,Citation19–Citation21 and in patients with long-duration or recurrent migraine attacks.Citation22
Some limitations of our study deserve to be discussed, and are mainly related to its design and pilot nature. The study sample population might have been too small. The treatment effect was not assessed in a double-blind, randomized fashion, and a crossover sequence was not applied. Traditional headache end points, such as pain relief, pain freedom, sustained pain freedom, and recurrence, were not investigated. Drug safety was also not evaluated. However, to the authors’ knowledge, this study is the one and only pharmacological study carried out so far in a very specific form of migraine, ie, weekend migraine. Hopefully, as a precursor, it will open the way to larger, well-designed, randomized studies in a similar population of migraineurs.
In conclusion, the results of our open-label, nonrandomized pilot study provide early evidence that a second-generation triptan used as symptomatic or prophylactic treatment of weekend migraine is more effective than naproxen sodium in terms of attenuation of headache severity. Further large, double-blind, randomized, placebo-controlled studies are needed to establish definitively the efficacy of frovatriptan versus other standard treatments in this subpopulation of migraineurs.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiptonRBChronic migraine, classification, differential diagnosis, and epidemiologyHeadache201151Suppl 2778321770929
- CuginiPRomitADiPalma LGiacovazzoMCommon migraine as a weekly and seasonal headacheChronobiol Int199074674692097080
- CouturierEGHeringRSteinerTJWeekend attacks in migraine patients: caused by caffeine withdrawal?Cephalalgia199212991001576651
- TorelliPColognoDManzoniGCWeekend headache: a retrospective study in migraine without aura and episodic tension-type headacheHeadache199939112015613189
- TorelliPColognoDManzoniGCWeekend headache: a possible role of work and life-styleHeadache19993939840811279917
- NatteroGDe LorenzoCBialeLAllaisGTorreEAnconaMPsychological aspects of weekend headache sufferers in comparison with migraine patientsHeadache19892993992708043
- GilmoreBMichaelMTreatment of acute migraine headacheAm Fam Physician20118327128021302868
- LoderETriptan therapy in migraineN Engl J Med2010363637020592298
- JohnstonMMRapoportAMTriptans for the management of migraineDrugs2010701505151820687618
- Headache Classification Subcommittee of the International Headache SocietyThe International Classification of Headache Disorders2nd edCephalalgia200424Suppl 1916014979299
- ZegerSLLiangKYLongitudinal data analysis for discrete and continuous outcomesBiometrics1986421211303719049
- LiangKYZegerSLLongitudinal data analysis using generalized lienar modelsBiometrika1986731322
- NegroALionettoLCasollaBLalaNSimmacoMMartellettiPPharmacokinetic evaluation of frovatriptanExpert Opin Drug Metab Toxicol201171449145821929465
- PiniLABertolottiMTrentiTVitaleGDisposition of naproxen after oral administration during and between migraine attacksHeadache1993331911948496057
- ZhouDZhangQLuWXiaQWeiSSingle- and multiple-dose pharmacokinetic comparison of a sustained-release tablet and conventional tablets of naproxen in healthy volunteersJ Clin Pharmacol1998386256299702847
- KelmanLReview of frovatriptan in the treatment of migraineNeuropsychiatr Dis Treat20084495418728819
- GuidottiMMauriMBarrilàCGuidottiFBelloniCFrovatriptan versus transdermal oestrogens or naproxen sodium for the prophylaxis of menstrual migraineJ Headache Pain2007828328817955167
- SmithTRSunshineAStarkSRLittlefieldDESpruillSEAlexanderWJSumatriptan and naproxen sodium for the acute treatment of migraineHeadache20054598399116109111
- BalbisiEAFrovatriptan: a review of pharmacology, pharmacokinetics and clinical potential in the treatment of menstrual migraineTher Clin Risk Manag2006230330818360605
- BrandesJLPooleAKallelaMShort-term frovatriptan for the prevention of diffcult-to-treat menstrual migraine attacksCephalalgia2009291133114819811503
- SilbersteinSDBernerTTobinJXiangQCampbellJCScheduled short-term prevention with frovatriptan for migraine occurring exclusively in association with menstruationHeadache2009491283129719751371
- KelmanLHarperSQHuXCampbellJCTreatment response and tolerability of frovatriptan in patients reporting short- or long-duration migraines at baselineCurr Med Res Opin2010262097210420642390