Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Background
Paliperidone palmitate (PP) is a long-acting injectable formulation of an atypical antipsychotic, paliperidone. Its dose can be expressed in milligram or milligram equivalents (mg eq) of active paliperidone (39, 78, 117, 156, and 234 mg of PP correspond to 25, 50, 75, 100, and 150 mg eq of paliperidone). The recommended initiation dosing regimen for PP is 150 [day 1]/100[day 8] mg eq. Labeling guidance allowed a ± 2 day window for the day 8 injection that provides more flexibility with patient scheduling and avoids missing the day 8 initiation dose. Recently, expansion of the day 8 dosing window from ±2 to ±4 days has been approved in the United States based on results obtained from the model-based simulations and review of safety data presented here.
Methods
The predicted exposure for the recommended initiation regimen of PP was compared with day 1/day 4, and day 1/day 12 dosing scenarios; each scenario was compared with the highest clinically evaluated initiation regimen (150[day 1]/150[day 8] mg eq) and to the recommended 6 mg/day oral dose of extended-release paliperidone.
Results
Simulated exposures with PP 150 mg eq on day 1 and 100 mg eq on days 4, 8, or 12 overlap considerably, with ±3 ng/mL variation in median maximum plasma concentrations. Based upon pharmacokinetic bridging/bracketing, the peak concentration with PP 150/100 mg eq [days 1/4] was lower than that with the highest initiation regimen. Exposures for PP 150 mg eq on day 1 and 100 mg eq on days 4, 8, or 12 were maintained close to those of 6 mg of paliperidone extended-release.
Conclusion
These simulations indicate that using the expanded dosing window of ±4 days has little effect on paliperidone exposure. A review of the overall pattern of treatment-emergent adverse events did not identify any new safety risks associated with the expanded dosing window.
Introduction
Poor compliance with long-term treatment is a common problem associated with all chronic medical conditions and can be particularly problematic among patients with schizophrenia. This poses major challenges in ensuring continuous antipsychotic therapy. In these patients, continuous antipsychotic therapy is essential for achieving sustained symptom control and reducing the risk of relapse and rehospitalization. Long-acting injectable antipsychotics provide a sustained release profile, resulting in therapeutic concentrations over several weeks and eliminating the need for daily dosing. Thus, long-acting injectable antipsychotic medications, which are administered by a health care professional on a regular schedule, provide uninterrupted drug delivery with a convenient dosing interval, and also offer information about the patient’s condition during known treatment adherence. Taken together, these characteristics provide greater potential for continuous effective exposure in patients treated with long-acting injectables.
Paliperidone palmitate (PP) is a once-monthly, long-acting injectable formulation of paliperidone approved in several countries for the treatment of schizophrenia in adults. The dose of PP can be expressed in mg or as mg equivalents (mg eq) of active paliperidone. Specifically, 39, 78, 117, 156, and 234 mg of PP correspond to 25, 50, 75, 100, and 150 mg eq of paliperidone.
Following intramuscular administration of two initiation doses given one week apart in the deltoid muscle, PP is administered monthly at doses ranging from 25 to 150 mg eq, in either the deltoid or gluteal muscle.Citation1 The recommended initiation regimen for PP is 150 mg eq on day 1 followed by 100 mg eq on day 8, using a 1.0 inch needle in patients < 90 kg and a 1.5 inch needle in patients ≥ 90 kg.Citation2 Based upon data from model-based pharmacokinetic simulations and clinical trials, the label provides guidance to health care providers when patients miss the day 8 initiation dose, indicating that it may be administered within ±2 days, without a clinically significant impact on the plasma concentrations of paliperidone.Citation3 Recently, an expansion of the dosing window from ±2 to ±4 days for a missed day 8 injection has been approved by the United States Food and Drug Administration (FDA); the PP label has been updated accordingly.Citation1,Citation4 We report results of the analysis, that formed the basis for the PP label update. The analysis included model-based simulations to evaluate the potential effects of the day 8 dosing window expansion on paliperidone plasma exposures and potential implications on the safety and efficacy profile.
Materials and methods
Pharmacokinetic data from clinical trials for model verification
Pharmacokinetic data from two multiple-dose studies of PP in patients with schizophrenia were used to support the model-based simulation exercise. The first study was a long-term (53-week), open-label, study (ClinicalTrials.gov identification, NCT01150448) in which PP was administered with an initiation regimen of 150 mg eq on both days 1 and 8 in the deltoid muscle, and then once monthly in the deltoid or gluteal muscle.Citation5 The second study was a randomized, double-blind, double-dummy, active-controlled, 13-week study (ClinicalTrials.gov identification number, NCT00589914) in which the initiation regimen was 150 mg eq on day 1 (deltoid), and then 100 mg eq on day 8 (deltoid).Citation6 In both studies, blood samples for the pharmacokinetic analysis were collected at baseline and at regular intervals thereafter. Detailed study designs and methodologies for these studies have been described previously.Citation5,Citation6
Steady-state pharmacokinetic data for paliperidone extended-release were available for: 31 patients with schizophrenia from a randomized, double-blind, placebo-controlled study (ClinicalTrials.gov identification number, NCT00074477), in which paliperidone extended-release was administered for one week;Citation7 and 140 patients with schizophrenia who underwent an oral tolerability test with 6 days of paliperidone extended-release treatment before receiving PP in a randomized, double-blind, placebo-controlled, 13-week study (ClinicalTrials.gov identification number, NCT00590577).
Plasma concentrations of paliperidone for all pharmacokinetic samples were determined using validated liquid chromatography coupled with a tandem mass spectrometry method, with a limit of quantification of 0.1 ng/mL.Citation5–Citation9
Dataset preparation and analysis software
Dataset preparation was performed using SAS® version 9.1.3 (SAS Institute Inc, Cary, NC, USA) and S-Plus® 6.2 software (Insightful Corporation, Seattle, WA, USA). The database was prepared according to the format required by the NONMEM software. Analyses were performed using the nonlinear mixed-effect approach as implemented in NONMEM® V and VI (NONMEM users guides, Icon Development Solutions, Ellicott City, MD, USA) and visualization of results was performed using S-Plus® version 6.2.
Pharmacokinetic simulations
The simulations are based on two historical population pharmacokinetic models for PPCitation10 and paliperidone extended-release,Citation11 which have been described in detail and used for simulations in a previously published report.Citation3 Briefly, a one-compartment model with first-order elimination was used to simulate the pharmacokinetics of paliperidone after intramuscular administration of PP. The absorption submodel allowed a fraction of the dose to enter the central compartment fairly quickly via a zero-order process. After a lag time, the remaining fraction of the dose entered the systemic circulation via a first-order process that determined the shape of the pharmacokinetic profile following intramuscular injection. In addition, the pharmacokinetics of paliperidone extended-release was simulated using a two-compartment disposition model with linear elimination from the central compartment. Oral absorption was described using a sequential zero order input into a depot compartment and first-order absorption with a lag time from the depot to the central compartment. To assess further the predictive performance of the historical population models, a visual predictive checkCitation12 was performed for plasma profiles from the above-mentioned studies. Data from only the first 5 weeks from two studiesCitation5,Citation6 were utilized for the PP visual predictive check because the objective of the analysis was to assess the model’s ability to predict the pharmacokinetic profile after different PP initiation regimens.
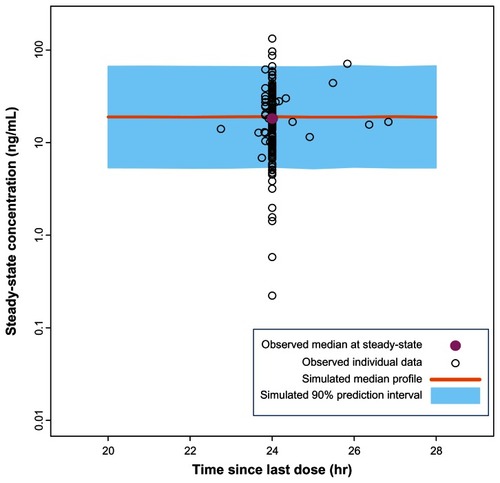
Datasets were simulated based on the fixed effect (typical parameter estimates and covariate effects) and random effect (intrapatient variability, interoccasion variability, and residual variability) estimates in the historical PP population-pharmacokinetic model.Citation10 Simulated datasets had study design features and covariates (ie, dosing information and demographics) similar to the above-mentioned studies. A visual predictive check was performed after the 5th, 50th, and 95th percentiles were calculated from the simulated profiles.Citation12 These percentiles were superimposed on the raw observed data to allow assessment of model predictability. All plasma samples with quantifiable concentrations of paliperidone, with date and time of sampling, including the dosing history, were used in the plots for the visual predictive check. Using the same methodology, a visual predictive check was also performed to assess the ability of the population pharmacokinetic model for paliperidone extended-releaseCitation11 to predict steady-state levels of paliperidone at the recommended 6 mg dose. The observed paliperidone data and demographic characteristics for the paliperidone extended-release visual predictive check were derived from the studies by Kramer et alCitation7 and Pandina et al.Citation8
Once the historical population pharmacokinetic models were verified (ie, the visual predictive checks were found to be satisfactory), these models were used for the simulation exercise. Paliperidone plasma concentrations were simulated based on final estimates of the population pharmacokinetic models for PPCitation10 and paliperidone extended-release.Citation11 For each dataset, the covariates of interest were obtained by resampling from the patient covariates (ie, the patient is the resampling unit) available in the population pharmacokinetic databaseCitation10 and the joint distribution of patient-specific characteristics was maintained. A 5000-patient Monte Carlo simulation of pharmacokinetic profiles was conducted for various scenarios, as described in . Medians and 90% prediction intervals were computed from the simulated profiles to allow visualization of results. The first set of simulations compared the recommended initiation dosage of 150 mg eq on day 1 followed by 100 mg eq on day 8, with PP initiation doses (150 mg eq and 100 mg eq) administered on day 1/day 4 and on day 1/day 12. The second set of simulations compared the administration of the second initiation dose (100 mg eq) on day 4, day 8, or day 12 with initiation regimen of 150 mg eq on both days 1 and 8. The third set of simulations compared the recommended daily dose (6 mg) of the oral formulation paliperidone extended-release with the 150/100 mg eq initiation doses administered on days 1 and 4, days 1 and 8, and days 1 and 12.
Table 1 Summary of paliperidone palmitate initiation regimen simulations
Finally, safety data during the first 5 weeks of two previous clinical studies (ClinicalTrials.gov identification numbers, NCT00590577 and NCT01150448) of PPCitation5,Citation8 were compared in order to assess the potential safety profile with an initiation regimen of 150 mg eq on day 1 and 100 mg eq on day 4.
Results
Visual predictive check
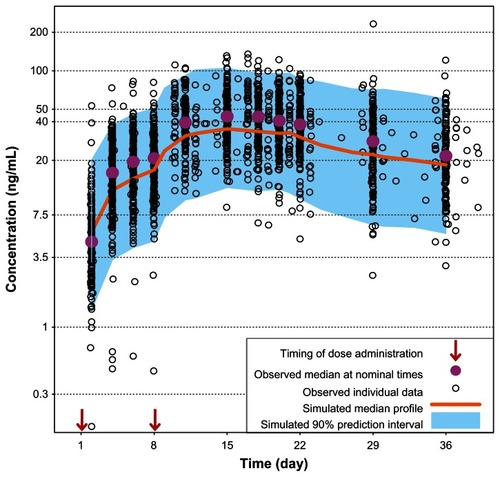
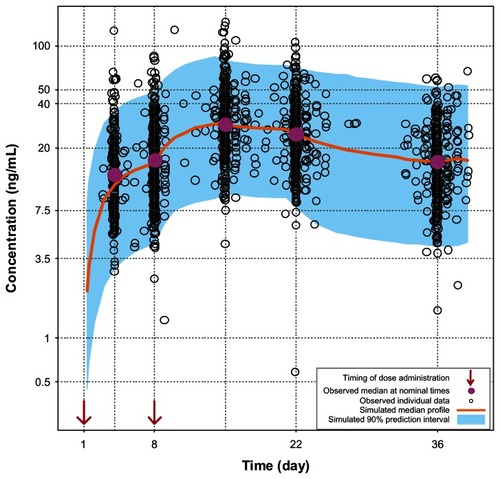
Pharmacokinetic profiles obtained during the first 5 weeks from 407 participants who received the initiation regimen of 150 mg eq on day 1 and 100 mg eq on day 8,Citation6 were compared with the simulated profiles in order to verify the PP population pharmacokinetic model. Similarly, the pharmacokinetic data from 199 participants who received the initiation regimen of 150 mg eq on days 1 and 8Citation5 were also compared with the simulated profiles. The results of these comparisons showed that the simulated profiles obtained using the PP population pharmacokinetic model were similar to the pharmacokinetic profiles obtained in two PP clinical studies ( and ). It should be noted that the observed concentrations are slightly higher than the simulated values in ; however the difference is likely to be clinically irrelevant. Moreover, the simulated curves for 150 mg eq/150 mg eq initiation (see next section) will represent conservative predictions since the actual concentrations will be higher than the model-based predictions at the higher doses. In addition, the PK profiles for 150 mg eq/100 mg eq and 150 mg eq/150 mg eq day 1/day 8 from study NCT00590577 have been recently published and these profiles were compared with the model based predictions.Citation3 For study NCT00590577 the model accurately predicted the exposure for both the lower and higher initiation regimens. Thus, the small discrepancy in predictions for NCT01150448 could in part be due to a study-related effect. Finally, the paliperidone extended-release data from previous studiesCitation7,Citation8 show considerable similarity with the population pharmacokinetic simulation for paliperidone extended-release at steady-state for the 6 mg dose (). These comparisons add confidence to the population pharmacokinetic models and support their use as simulation tools for assessing the day 8 ± 4 day dose window for PP.
Figure 1 Comparison of model-based projections versus observed data for the initial 5 weeks of treatment with PP 150 mg eq on day 1 followed by 100 mg eq on day 8. Abbreviations: mg eq, milligram equivalents; PP, paliperidone palmitate.
Simulation exercises
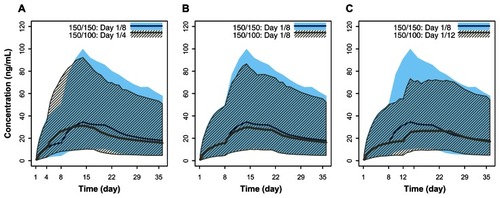
When comparing the effects of different days for the second initiation dosing, the comparisons were quantitatively characterized () in addition to their visualization. Simulated plasma concentration profiles of PP with a second initiation dose administered on day 8 ± 4 days versus the recommended day1/day 8 regimen showed considerable overlap, with a ±3 ng/mL variation in median maximum plasma concentrations (). These minor differences suggest that PP 150 mg eq administered on day 1 followed by 100 mg eq administered ±4 days around the recommended day 8 dose has minimal effects on the pharmacokinetic profile. Furthermore, the concentrations for the day 1/day 4 regimen exhibited a minor excursion that exceeded the exposure window for the recommended day 1/day 8 regimen by approximately one day around the peak plasma concentration (Cmax), which is probably clinically irrelevant.
Figure 4 Pharmacokinetic simulations of the PP with second initiation dose (day 8) window expanded to ±4 days. (A) PP 150 mg eq on day 1 and 100 mg eq on day 8 versus 150 mg eq on day 1and 100 mg eq on day 4. (B) PP 150 mg eq on day 1 and 100 mg eq on day 8 versus 150 mg eq on day 1 and 100 mg eq on day 12.
Abbreviations: PP, paliperidone palmitate; mg eq, milligram equivalents.
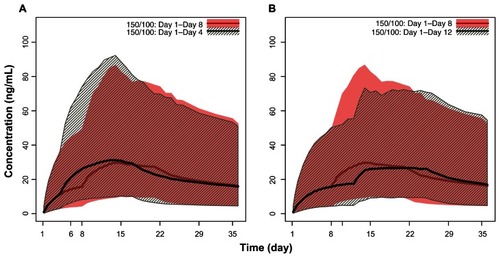
Table 2 Quantitative comparison of simulated peak exposures with paliperidone palmitate initiation regimens
Administration of the second initiation dose of PP (100 mg eq) at day 4, day 8, or day 12 produced plasma exposures that did not exceed the maximum exposure observed with the initiation regimen of 150 mg eq on day 1 and 150 mg eq on day 8 (). The peak concentration from the PP 150 mg eq on day 1 and 100 mg eq on day 4 administration was below the paliperidone exposure observed with the initiation regimen of 150 mg eq on days 1 and 8 (). Based upon the pharmacokinetic bridging or a bracketing approach, exposure of paliperidone with 150 mg eq on day 1 and 100 mg eq on day 4 falls within the exposure range for the recommended initiation regimen of 150 mg eq on day 1 and 100 mg eq on day 8 and the highest initiation regimen of 150 mg eq on both days 1 and 8. This indicates that the proposed flexible dosing around the recommended day 8 injection would not pose a safety concern during the initiation phase.
Figure 5 Pharmacokinetic simulations for PP doses 150 mg eq and 100 mg eq with day 1/day 4, day 1/day 8, and day 1/day 12 initiation regimens compared with the highest initiation regimen (150 mg eq/day 1 followed by 150 mg eq/day 8). (A) PP 150 mg eq on both days 1 and 8 versus 150 mg eq on day 1 and 100 mg eq on day 4. (B) PP 150 mg eq on both days 1 and 8 versus 150 mg eq on day 1 and 100 mg eq on day 8. (C) PP 150 mg eq on both days 1 and 8 versus 150 mg eq on day 1 and 100 mg eq on day 12.
Abbreviations: PP, paliperidone palmitate; mg eq, milligram equivalents.
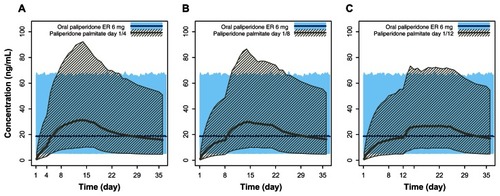
Finally, comparison of paliperidone exposures from multiple daily doses of 6 mg of paliperidone extended-release (recommended oral dose) with PP 150 mg eq administered on day 1 followed by 100 mg eq on day 4, day 8, and day 12 showed that the plasma concentrations of paliperidone were only slightly higher than the desired paliperidone extended-release 6 mg exposure during the month after the second initiation dose (). This indicates that the proposed flexible dosing around the recommended day 8 injection would not pose an efficacy concern during the initiation phase.
Figure 6 Pharmacokinetic simulations for PP doses 150 mg eq and 100 mg eq with day 1/day 4, day 1/day 8, and day 1/day 12 initiation regimens compared with the recommended daily dose of paliperidone ER (6 mg). (A) Steady-state 6 mg paliperidone ER versus PP 150 mg eq on day 1 and 100 mg eq on day 4. (B) Steady-state 6 mg paliperidone ER versus PP 150 mg eq on day 1 and 100 mg eq on day 8. (C) Steady-state 6 mg paliperidone ER versus PP 150 mg eq on day 1 and 100 mg eq on day 12.
Abbreviations: PP, paliperidone palmitate; ER, extended-release; mg eq, milligram equivalents.
Safety
Review of safety data from the clinical studies that evaluated the highest initiation regimen of PP (150 mg eq on day 1 and 150 mg eq on day 8) indicated that the highest initiation regimen was well tolerated with a safety profile similar to that observed with the recommended initiation regimen (). No new or clinically significant treatment-emergent adverse events were noted with the highest initiation regimen.
Table 3 Treatment-emergent adverse events reported in ≥5% of patients in any dose arm, by clinical trial
The overall incidence rates and specific reports of treatment-emergent adverse events were generally similar across the two initiation regimens (PP 150 mg eq on both days 1 and 8 versus 150 mg eq on day 1 and 100 mg eq on day 8). Headache, insomnia, akathisia, anxiety, schizophrenia, diarrhea, injection site pain, fatigue, nasopharyngitis, and tachycardia were reported in ≥5% of patients in any treatment group ().
Overall, there were very few serious treatment-emergent adverse events observed in study NCT00590577,Citation8 and were limited to psychiatric events that were likely because of inadequate treatment of the underlying disease. No safety signal was detected for the 150/150 mg eq treatment group on the basis of these findings. Likewise, the pattern of serious treatment-emergent adverse events in the study NCT01150448Citation5 was consistent with worsening of the underlying disease state.Citation5 Neither the nature nor the frequency of serious treatment-emergent adverse events in the study NCT01150448 suggested a new safety signal for patients treated with PP 150 mg eq on both day 1 and day 8.
Discussion
The previously recommended initiation regimen of PP supported administration of a second initiation dose within 2 days of the recommended day 8 injection (days 6 through 10) when the scheduled day 8 dose could not be administered on time. This previously suggested a ±2 day window around the recommended day 8 injection did not have a clinically significant impact on plasma paliperidone concentrations.Citation3 The modeling results presented here provide support for a more flexible initiation schedule with a ±4 day dosing window for administration of the second dose, ie, the second injection can occur between day 4 and day 12, to avoid a missed day 8 injection and offer a more convenient dosing schedule for stable patients (approved by the FDA in August 2012).Citation4 This increased flexibility in initiation is also valuable clinically, because increasing restrictions on length of hospital stay for acute treatment of psychosis make it difficult to complete initiation of PP in many inpatient settings. Furthermore, once discharged from the hospital, follow-up visits may not be scheduled until several weeks after the discharge. Such gaps in treatment for patients whose symptoms are not fully stabilized may lead to clinical decompensation and rapid rehospitalization.
It has been shown that gaps in medication of as few as 1–10 days can double the likelihood (odds ratio 1.98) of hospitalization due to relapseCitation13 and lead to increased suicide attempt rates.Citation14 Relapses are associated with higher costs for inpatient services as well as for outpatient services and medications.Citation15 Because of the close links between psychotic relapse and noncompliance with antipsychotic medication, it is important to identify ways to improve adherence. Any method that can potentially reduce the need for follow-up after hospital discharge may ultimately lead to lower relapse rates over time. The greater flexibility for providing an earlier second initiation dose should help bridge these problems in continuity of care for unstable, recently relapsed patients and so support continuous effective antipsychotic exposure to this patient population.
The objectives of this analysis were two-fold. We surmised that injection of the second dose on day 4 is likely to produce a higher Cmax. In order to evaluate the potential safety issues, we needed to determine whether this initial higher exposure would still be lower than the exposure observed with the previously tested highest initiation regimen of PP (150 mg eq on both days 1 and 8).Citation5,Citation8 On the other hand, the initiation regimen of 150 mg eq on day 1 with the 100 mg eq injection extended to day 12 may result in a lower paliperidone concentration, which may fall below the therapeutic range, resulting in decreased efficacy. Thus, a simulation exercise was carried out to assess whether expansion of the dose window to avoid a missed dose would likely pose either an efficacy or safety concern.
The highest initiation regimen of PP (150/150 mg eq) previously studied has been found to be generally well tolerated and has a safety profile similar to the currently recommended initiation regimen (150/100 mg eq, ). A comparison of the 150/100 mg eq and 150/150 mg eq groups indicated that the frequency, but not type, of treatment-emergent adverse events was numerically increased with the dosing regimen of 150 mg eq on both days 1 and day 8 compared with the recommended dosing regimen of 150 mg eq on day 1 and 100 mg eq on day 8. This suggests a dose-dependent relationship for overall frequency of treatment-emergent adverse events and also that advancing the day 8 injection to day 4 may be associated with a small increase in risk for developing treatment-emergent adverse events from previously identified categories in some patients. However, a review of the overall pattern of treatment-emergent adverse events did not identify a new or important increased risk that would be anticipated with the expanded dosing window. Although indirect evidence, the tolerability profile associated with the highest initiation regimen (150/150) supports the safety of the initiation regimen (150/100) with the second dose administered on day 4. This is because the simulated paliperidone Cmax with an initiation regimen of 150 mg eq on days 1 and 8 was higher than that predicted with an initiation regimen of 150 mg eq on day 1 and 100 mg eq on day 4.
It should be noted that some patients may be prone to tachycardia, extrapyramidal symptoms, orthostasis, or somnolence and sedation around the time of the highest plasma concentration. In these cases, it is prudent for the physician to be vigilant for occurrence of these symptoms after the second initiation dose on day 4. In addition, the initiation doses should be reduced to 100 mg eq and 75 mg eq (from 150 mg eq and 100 mg eq) in patients with mild renal impairment because systemic exposure is increased in these patients due to reduced drug excretion.
In the open-label study (NCT01150448Citation5), nasopharyngitis and tachycardia were reported in 8% of patients, which was higher than the incidence rate for these same adverse events reported in the other double-blind study (NCT00590577,Citation8 see ). To understand these findings, a separate pooled analysis of data from the few previous double-blind, placebo-controlled studies was conducted. The results of this pooled analysis showed that the incidence of nasopharyngitis in the PP-treated patients was 27/1293 (2%) and 8/510 (2%) in the placebo-treated patients. A similar pooled analysis of data from two double-blind, placebo-controlled studies (NCT00147173,Citation16 NCT00101634Citation17) showed that the incidence of tachycardia was 2/611 (<1%) in PP-treated patients and 1/262 (<1%) in placebo-treated patients. Given the variability associated with spontaneous reporting of adverse events in clinical studies, the incidence rates noted above (8%) do not appear to be unusual.
Comparison of paliperidone exposure using the recommended daily dose of paliperidone extended-release 6 mg with the initiation regimens of 150 mg eq on day 1 and 100 mg eq on day 4, day 8, or day 12 showed that the plasma concentrations of paliperidone achieved with the three different initiation regimens were similar to those obtained with 6 mg of paliperidone extended-release. Thus, it is unlikely that using an expanded dose window around the day 8 injection (ie, day 4, day 12) would affect the efficacy of PP if the patient’s condition is clinically well managed.
Population-based pharmacokinetic models allow characterization of drug absorption and disposition in patients, and this understanding can support the selection or justification of dosing strategies for inclusion in the drug’s labeling information. Thus, one of the objectives of the current analysis was to use model-based simulations to avoid additional clinical trials with alternative dosing regimens that would be more convenient, and equally safe and effective. This objective was achieved with the current analysis and the proposed dosing regimen was accepted by the FDA and led to a label update.
The findings of simulation exercises should be interpreted in perspective because they are based on pharmacokinetic bridging and safety considerations. Due to the relatively small sample size, generalizability of the safety findings reported here to a general population receiving paliperidone palmitate remains limited, and currently no clinical studies have been specifically conducted to examine the effect of this expansion. The population pharmacokinetic model used for the simulation exercises assumes complete patient adherence in the simulated virtual patients. However, continuous effective exposure is highly likely to be increased with PP because adherence is required monthly rather than daily with this product. Furthermore, knowledge of adherence is certain with an injectable medication that is provided by a health care professional. Additionally, if any dose is missed out it can be quickly identified and addressed within a window that is much larger than that available for oral medications with a short half-life.
Conclusion
An expansion of the day 8 dose window for PP to ± 4 days appears to have minimal effect on plasma paliperidone concentrations, as indicated by model-based simulations, and it will likely have a limited impact on the overall tolerability or efficacy of PP in patients for whom use of the expanded window is necessary to avoid a missed dose. This wider dosing window supports more practical clinical initiation of PP in the hospital setting, particularly in those situations where the duration of hospital stay will be short.
Disclosure
This research was funded by Janssen Research and Development, LLC. MNS, IN, and SG are employees of Janssen Research and Development, LLC, USA. BR is an employee of Janssen Research and Development, LLC, a Division of Janssen Pharmaceutica NV, Belgium. JKS and LA are employees of Janssen Scientific Affairs, LLC, USA. All of these companies are Johnson and Johnson companies. Sarika Shirke (SIRO Clinpharm Pvt Ltd) provided writing assistance and Wendy P Battisti (Janssen Research and Development, LLC) provided additional editorial support for this manuscript. This work was presented as a poster at the 24th Annual US Psychiatric and Mental Health Congress, November 7–10, 2011, Las Vegas, NV, USA.
References
- Invega®Sustenna®prescribing information2012 Available from: http://www.invegasustenna.com/important-product-informationAccessed November 8, 2012
- GopalSGassmann-MayerCPalumboJSamtaniMNShiwachRAlphsLPractical guidance for dosing and switching paliperidone palmitate treatment in patients with schizophreniaCurr Med Res Opin20102637738720001492
- SamtaniMNGopalSGassmann-MayerCAlphsLaPalumboJDosing and switching strategies for paliperidone palmitate based on population pharmacokinetic modelling and clinical trial dataCNS Drugs20112582984521936586
- Food and Drug Administration, Department of Health and Human ServicesSupplement Approval2012 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/022264Orig1s005ltr.pdfAccessed December 10, 2012
- CoppolaDLiuYGopalSA one-year prospective study of the safety, tolerability and pharmacokinetics of the highest available dose of paliperidone palmitate in patients with schizophreniaBMC Psychiatry2012122622455454
- PandinaGLaneRGopalSA double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophreniaProg Neuropsychopharmacol Biol Psychiatry20113521822621092748
- KramerMLitmanRHoughDPaliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety studyInt J Neuropsychopharmacol20101363564719941696
- PandinaGJLindenmayerJPLullJA randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophreniaJ Clin Psychopharmacol20103023524420473057
- De MeulderMRemmerieBMde VriesRValidated LC-MS/MS methods for the determination of risperidone and the enantiomers of 9-hydroxyrisperidone in human plasma and urineJ Chromatogr B Analyt Technol Biomed Life Sci2008870816
- SamtaniMNVermeulenAStuyckensKPopulation pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychoticClin Pharmacokinet20094858560019725593
- CirincioneBRedmanMFiedler-KelleyJLudwigEVermeulenAPopulation pharmacokinetics of paliperidone ER in healthy subjects and patients with schizophreniaPaper presented at the Annual Meeting of the American Society for Clinical Pharmacology and TherapeuticsMarch 21–24, 2007Anaheim, CA
- DuffullSBAaronsLDevelopment of a sequential linked pharmacokinetic and pharmacodynamic simulation model for ivabradine in healthy volunteersEur J Pharm Sci20001027528410838017
- WeidenPJKCGroggALocklearJPartial compliance and risk of rehospitalization among California Medicaid patients with schizophreniaPsychiatr Serv20045588689115292538
- HeringsRMErkensJAIncreased suicide attempt rate among patients interrupting use of atypical antipsychoticsPharmacoepidemiol Drug Saf20031242342412899119
- Ascher-SvanumHZhuBFariesDEThe cost of relapse and the predictors of relapse in the treatment of schizophreniaBMC Psychiatry201010220059765
- GopalSHoughDXuHEfficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response studyInt Clin Psychopharmacol20102524725620389255
- NasrallahHAGopalSGassmann-MayerCA controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophreniaNeuropsychopharmacology2010352072208220555312