Abstract
Objectives:
This study aimed to investigate the role of tumor necrosis factor (TNF)-α and the neuronal nitric oxide synthase enzyme in dysregulation of indoleamine 2,3-dioxygenase (IDO) enzyme, and hence serotonin availability in chronic mild stress (CMS), an animal model of depression.
Methods:
Rats were divided into five groups: two control and CMS-exposed for 6 weeks, and another three groups exposed to CMS and administered pentoxifylline 50 mg/kg/day intraperitoneally, 7-nitroindazole 40 mg/kg/day subcutaneously, or imipramine 20 mg/kg/day intraperitoneally for the previous 3 CMS weeks. Rats were assessed for neurochemical and immunohistochemical abnormalities.
Results:
Pentoxifylline-, 7-nitroindazole-, and imipramine-treated rats showed amelioration of CMS-induced behavioral deficits that was accompanied by significant reduction in kynurenine/serotonin molar ratio and nitrates/nitrites in frontal cortex and hippocampus. In the pentoxifylline and 7-nitroindazole groups, serum TNF-α was reduced relative to the CMS group (18.54 ± 0.85 and 19.16 ± 1.54 vs 26.20 ± 1.83 pg/mL, respectively; P < 0.05). Exposure to CMS increased TNF-α and IDO immunohistochemical staining scores in both hippocampus and midbrain raphe nuclei. 7-Nitroindazole and pentoxifylline significantly (P < 0.05) reduced TNF-α immunostaining in hippocampus and raphe nuclei, with significant (P < 0.01) reduction of IDO immunostaining in raphe nuclei. Likewise, imipramine reduced TNF-α immunostaining (P < 0.05) in hippocampus.
Conclusion:
Neuronal nitric oxide synthase and TNF-α may play a concerted role in modulating IDO enzyme activity in CMS-exposed rats and provide additional evidence for possible alternative approaches to switch the neurobiological processes in depression.
Introduction
Several studies suggest that the link between stress and depression might involve disturbances in tryptophan metabolism.Citation1–Citation3 Tryptophan undergoes two major metabolic pathways: the serotonin-synthesis pathway and the kynurenine pathway. A shift in the balance of tryptophan metabolism from the serotonin towards the kynurenine pathway might be involved in the pathophysiology of depression. Indeed, kynurenine metabolites contribute to several neurobiological changes associated with depressive illness.Citation4
Not infrequently, clinical reports have revealed an increase in plasma kynurenine/tryptophan ratio, which is associated with increased activity of the cellular immune activation. Disturbed metabolism of tryptophan affects biosynthesis of neurotransmitter 5-hydroxytryptamine (serotonin), and it appears to be associated with an increased susceptibility for depression.Citation5 Indoleamine 2,3-dioxygenase (IDO) is predominantly expressed intracellularly as constitutive or inducible forms in blood monocytes and tissue macrophages, including microglial cells within the brain parenchyma.Citation6 Actually, IDO degrades tryptophan along the kynurenine pathway, so that fluctuations in its enzymatic activity can alter brain tryptophan metabolism. Enhancement of the kynurenine pathway deprives the neuronal tissue microenvironment from tryptophan and induces subsequent reduction in serotonin synthesis, and this may suggest a strong link between the kynurenine pathway, serotonin synthesis, and depression.Citation4,Citation7
The IDO enzyme is induced by proinflammatory cytokines, mainly interferon (IFN)-γ and tumor necrosis factor (TNF)-α.Citation8,Citation9 In chronic inflammatory conditions, IDO is activated, and the degree of activation matches the intensity of depressive symptoms, as in patients suffering from malignancies and those chronically treated with IFN-α.Citation10 Recently, O’Connor et alCitation11 reported that peripheral administration of lipopolysaccharide (LPS) activates IDO and culminates in a distinct depressive-like behavioral syndrome. This was measured by increased duration of immobility in both the forced-swim and tail-suspension tests. Blockade of IDO activation, either indirectly with the anti-inflammatory tetracycline derivative minocyclineCitation12 or directly with the IDO antagonist 1-methyltryptophan,Citation11 attenuated LPS-induced expression of proinflammatory cytokines (TNF-α, IL-1β), prevented the development of depressive-like behavior, and normalized the kynurenine/tryptophan ratio in the plasma and brain of LPS-treated mice. Thus, IDO might be considered a critical molecular mediator of inflammation-induced depressive-like behavior, probably through the catabolism of tryptophan along the kynurenine pathway.
On the other hand, speculations were raised on the role of neuronal nitric oxide (nNOS) in modulating depressive-like symptoms.Citation13 Yildiz et alCitation14 reported that nNOS inhibitors produce an antidepressant-like effect in the forced-swimming test in mice and rats. Furthermore, increased expression of TNF-α and nNOS has been noted in brain areas of depressed patients.Citation13,Citation15 Like nNOS inhibition, deletion of either TNF-α receptor subtypes (especially, type 2 TNF-α receptor) was reported to induce antidepressive-like effects in experimental animals.Citation16
Currently used antidepressants, which target monoamines, only produce “remission” in 30% of patients. Part of the problem is the fact that the pathophysiology of depression has not been fully elucidated, and treatments are based more on empirical data rather than on consistent pathophysiological reasoning.Citation17,Citation18 Chronic mild stress (CMS), a validated animal model of depression,Citation19 has been reported to result in long-lasting changes of neurochemical, neuroimmune, and neuroendocrinological variables that resemble the depressive state,Citation20,Citation21 hence CMS provides an interesting model to explore the pathophysiology of depression. Accordingly, the present study aims to examine the hypothesis that exposure to CMS might induce alterations in the balance between the two major tryptophan metabolic pathways – kynurenine/serotonin – in the frontal cortex and hippocampus of male Wistar rats. Special interest is directed towards using pharmacological tools for nNOS and TNF-α inhibitors (7-nitoindazole and pentoxifylline, respectively) to investigate their antidepressant-like effects and their modulation of the balance between serotonin and kynurenine in the frontal cortex and hippocampus, compared to the prototype antidepressant imipramine.
Materials and methods
Animals
Adult male Wistar rats weighing 225–275 g were purchased from the National Research Centre, Giza, Egypt. Rats were acclimatized as follows: 12-hour light–dark cycle, light on at 5 am, temperature ≈25°C, and 50%–60% relative humidity. Experimental procedures were approved by the ethical committee of the National Research Centre, Giza, Egypt.
Drugs and chemicals
Pentoxifylline and imipramine hydrochloride were dissolved in saline. 7-Nitroindazole was dissolved in dimethyl sulfoxide, and volume was adjusted with saline. Kynurenine, l-tryptophan, serotonin, 5-hydroxyindoleacetic acid (5-HIAA), and 3-nitro-l-tyrosine were all purchased from Sigma-Aldrich (St Louis, MO, USA).
Study design
Male Wistar rats were divided into five groups (n = 10/group): control group, CMS group (exposed to CMS for 6 weeks and administered saline intraperitoneally for the last 3 CMS weeks), and the other three groups were exposed to CMS for 6 weeks and administered imipramine 20 mg/kg/day intraperitoneally,Citation22 7-nitroindazole 40 mg/kg/day subcutaneously,Citation23 or pentoxifylline 50 mg/kg/day intraperitoneally for the last 3 CMS weeks.Citation24
Chronic mild stress
The CMS battery consisted of exposure to a variety of mild unpredictable stressors: water and/or food deprivation, restricted food access (ie, two to three chow pellets) after food deprivation, empty water bottles after water deprivation, cage tilting, 24-hour lighting, pairing, stroboscopic light, intermittent white noise (85 dB), and cold temperature (≈10°C). Stressors were repeated each week for a total of 6 weeks, modified after Bekris et al.Citation25 A sucrose preference test (SPT; behavioral test) was used to assess the development of anhedonia. Control animals were kept in a separate room ().
Table 1 Chronic mild stress (CMS) exposure protocol
Sucrose preference test
The SPT was done as a behavioral test at the start of dark cycle, ie, at 5 pm.Citation26 After a 23-hour period of food and water deprivation, animals were presented simultaneously with two preweighed bottles: one with 2% sucrose and the other with plain water for a period of 30 minutes. Two baseline tests were carried out during the training period before the start of the CMS protocol. Then the SPT was conducted weekly in all groups. Sucrose preference was measured by calculating the proportion of sucrose consumption out of total consumption of liquid.Citation25
Body weight
Body weight was measured weekly for each rat throughout the study.
High-performance liquid chromatography
The rat brain was dissected on ice, with the rat-brain atlas of Paxinos and WatsonCitation27 as a guide. Kynurenine determinations in brain tissue homogenates were performed using a Luna high-performance liquid chromatography (HPLC) column (5 μm C18 [2] 150 × 4.60 mm; Phenomenex, Torrance, CA, USA), according to the method described by Wang and Tang,Citation28 with modifications suggested by Bellac et al.Citation29 Briefly, the mobile phase for ultraviolet detection consisted of 15 mmol/L sodium acetate/acetic acid solution containing 2.7% (v/v) acetonitrile, pH 3.6. The flow rate was set at 1.3 mL/minute. The injected sample volume was 100 μL. Peaks were detected at 365 nm wavelength. 3-Nitro-l-tyrosine was used as an internal standard.
Determinations of tryptophan, serotonin, and 5-HIAA levels in the brain-tissue homogenates were performed according to the method described by Bellac et al and Mefford.Citation29,Citation30 Briefly, the mobile phase for the electrochemical detector HP 1049A (Hewlett Packard, Palo Alto, CA, USA) consisted of acetonitrile, 100 mM acetic acid, 100 mM ammonium acetate (10:90) v/v, and 50 mg/L ethylenediaminetetraacetic acid, pH 5.1.Citation31 3-Methoxy-4-hydroxyphenethyl alcohol was used as an internal standard. Measurements were done at an electrode potential of 0.850 mV. The limit of detection for all assayed compounds was 0.05–0.10 pmol. All chemicals were purchased from Sigma-Aldrich.
Nitrate/nitrite assay
Nitric oxide metabolite (nitrates/nitrites) concentrations in frontal cortex and hippocampus homogenates were measured by nitrate/nitrite colorimetric assay kit (23479; Fluka, Buchs, Switzerland), applying the Griess assay. The procedure followed the manufacturer’s instructions. Absorbance was measured at 540 nm with a microplate reader. NO metabolites were expressed as nmol/mg tissue.
TNF-α in serum
TNF-α protein was determined using enzyme-linked immunosorbent assay (ELISA) by adding 50 μL of standard and serum samples to a 96-well plate of a commercially available rat TNF-α ELISA kit (RayBiotech, Norcross, GA, USA) according to the manufacturer’s instructions.
Immunohistochemical study
Coronal sections of the brain at the hippocampal and raphe nuclei levels were fixed in formalin (10%) and embedded in paraffin blocks. Sequential 4 μm sections were cut on charged slides. The sections were incubated with the primary antibodies (rabbit anti-IDO polyclonal antibody [EB06950; Everest Biotech, Upper Heyford, UK]) at a concentration 1:50, and the next section with primary monoclonal TNF-α antibody (J1D9; Thermo Fisher Scientific, Waltham, MA, USA) at a concentration of 1:50. The immunohistochemical technique was performed by applying the supersensitive avidin-biotin detection kit (Biogenex, CA, USA) and following the technique of Hsu and Raine.Citation32 The immunostained sections were blindly examined qualitatively and semiquantitatively. The semiquantitative immunohistochemical evaluation was done as follows: it was first graded as negative = (0), mild (1+), moderate (2+), or strong (3+) according to the intensity of the staining as well as the percentage of the stained area, then the numerical evaluation was further analyzed statistically.
IDO was topographically expressed as a diffuse intra-cytoplasmic, while TNF-α was expressed as a diffuse intra- and extracellular staining. A semiquantitative score was calculated based on the staining intensity. The sections were examined for raphe nuclei in the brain stem, CA1 and CA3 of the hippocampus proper, granular, molecular, and polymorphic layers of dentate gyrus, and the alveus, which represent the projecting myelinated axons of the hippocampal pyramidal neurons in the cerebral hemispheres.
Statistical analysis
The results are expressed as means ± standard error of the mean. A nonparametric Kruskal–Wallis test, t-test, and one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test were performed for statistical comparisons between experimental groups. Repeated-measures ANOVAs were used for analysis in sucrose preference, followed by Bonferroni’s post hoc test. Nonparametric Kruskal–Wallis test followed by post hoc Dunn’s test were utilized for analysis of immunohistochemical staining score.
All statistical analyses were performed using the software package SPSS for Windows, version 15.0 (IBM, Armonk, NY, USA). P-values were considered significant at <0.05.
Results
Sucrose preference test
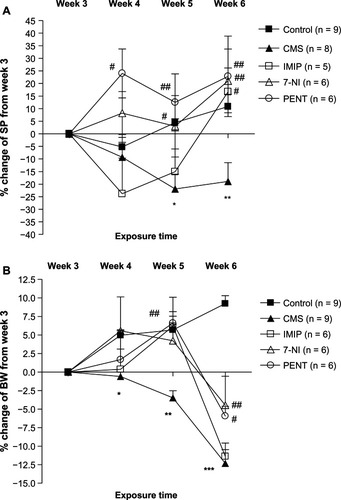
shows the effect of tested drugs on the percentage change in SP from the third week (pretreatment week). The repeated-measures ANOVA revealed significant within-subject (week) effects (F[3,27] = 2.97, P = 0.049) and between-subject (treatment) effects (F[4,29] = 5.531, P = 0.002) and a significant interaction between the factors week and treatment on percentage change of sucrose preference from the third week (F[12,87] = 2.67, P = 0.004). CMS induced significant reduction in SP in the fifth and sixth weeks (P < 0.05 and P < 0.01, respectively) in comparison to control rats. Treatment with imipramine, 7-nitroindazole, and pentoxifylline significantly (P < 0.05, P < 0.01, and P < 0.01, respectively) increased SP at the end of week 6 compared to the untreated CMS group. Interestingly, pentoxifylline significantly (P < 0.05) increased SP in week 4 and week 5 (P < 0.05 and P < 0.01, respectively), while 7-nitroindazole significantly (P < 0.05) increased SP in week 5, denoting early reversal of anhedonia by both drugs compared to the delayed reversal with imipramine.
Figure 1 Effects of chronic treatment with imipramine (IMIP), 7-nitroindazole (7-NI), and pentoxifylline (PENT) on weekly percentage changes of (A) sucrose preference (SP) and (B) body weight (BW) from the pretreatment week (week 3) in male Wistar rats exposed to chronic mild stress (CMS).
Body weight
shows the effect of tested drugs on the percentage change of body weight from the third week. The repeated-measures ANOVA revealed significant effects of the within-subjects factor, week (F[3,29] = 7.04, P = 0.004), the between-subjects factor, treatment (F[4,31] = 4.31, P = 0.023), and a significant interaction between the factors week and treatment on percentage change of body weight from the third week (F[12,93] = 6.2, P = 0.034). An overall decrease in body weight was observed in the CMS group in the fourth, fifth, and sixth weeks compared to the control group (P < 0.05, 0.01 and 0.001, respectively). Treatment with 7-nitroindazole and pentoxifylline significantly reduced the CMS-induced weight loss at the fifth and sixth weeks.
Neurochemical changes
Tryptophan and serotonin concentrations by HPLC in frontal cortex and hippocampus
CMS reduced tryptophan concentrations in frontal cortex and hippocampal homogenates, with no statistical significance, as demonstrated in ; imipramine significantly (P < 0.01) reversed this effect in the frontal cortex. However, the increase (21%) was statistically insignificant in the hippocampus (122.1 ± 3.539 vs 100.9 ± 10.61 pmol/mg tissue) in comparison to the CMS-untreated group. Neither 7-nitroindazole nor pentoxifylline induced significant changes.
Table 2 Tryptophan concentration in the frontal cortex and hippocampus (pg/mg tissue)
In rats exposed to CMS, serotonin concentrations in frontal cortex homogenates were decreased by 23% (1.374 ± 0.1174 vs 1.794 ± 0.215 pmol/mg tissue), with no statistical significance, as shown in . However, CMS induced a significant (1.365 ± 0.116 vs 2.445 ± 0.466 pmol/mg tissue, P < 0.001) decrease in serotonin concentrations in hippocampus homogenates compared to the control group. The tested drugs did not induce any significant effect on serotonin concentrations in the frontal cortex or hippocampus.
Table 3 Serotonin molar concentration in the frontal cortex and hippocampus (pmol/mg tissue)
5-HIAA concentrations by HPLC in frontal cortex and hippocampus
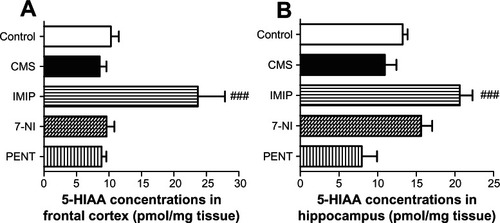
CMS decreased mean concentration of 5-HIAA in the frontal cortex (16.7%) (8.580 ± 1.031 vs 10.30 ± 1.226 pmol/mg tissue) and hippocampus (17.4%) (10.94 ± 1.49 vs 13.25 ± 0.637 pmol/mg tissue) homogenates compared to control, with no statistical significance. Imipramine induced a significant (P < 0.001) increase of 5-HIAA mean concentrations in the frontal cortex and hippocampus compared to the CMS group. 7-Nitroindazole increased 5-HIAA concentrations in the hippocampus by 43.05% (15.65 ± 1.43 vs 10.94 ± 1.49 pmol/mg tissue) compared to the CMS group; however, this effect was statistically insignificant ().
Figure 2 Effects of chronic mild stress (CMS) and treatments with imipramine (IMIP), 7-nitroindazole (7-NI), and pentoxifylline (PENT) on changes of 5-hydroxyindoleacetic acid (5-HIAA) concentrations (pmol/mg tissue) in frontal cortex (A) and hippocampus (B) homogenates.
Kynurenine concentrations by HPLC and kynurenine/serotonin ratio in frontal cortex and hippocampus
Exposure to CMS induced a significant (P < 0.01) increase of kynurenine mean concentration in the frontal cortex and in hippocampus homogenates compared to control. Treatment with imipramine, 7-nitroindazole, and pentoxifylline significantly decreased kynurenine mean concentration in frontal cortex homogenates (P < 0.01, P < 0.001, and P < 0.001, respectively). In the hippocampus, imipramine, 7-nitroindazole, and pentoxifylline significantly decreased kynurenine mean concentrations (P < 0.05, P < 0.01, and P < 0.01, respectively) compared to the CMS group ().
Figure 3 Effects of chronic mild stress (CMS) and treatments with imipramine (IMIP), 7-nitroindazole (7-NI), and pentoxifylline (PENT) on changes of kynurenine concentrations (pmol/mg tissue) and kynurenine/serotonin ratio in frontal cortex (A and C) and hippocampus (B and D) homogenates.
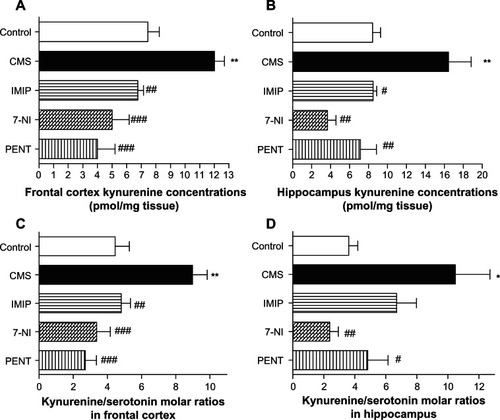
As demonstrated in , CMS induced a significant increase of kynurenine/serotonin molar ratios in the frontal cortex and hippocampus (P < 0.01 and P < 0.05, respectively) compared to the control group. Imipramine, 7-nitroindazole, and pentoxifylline treatment significantly reduced kynurenine/serotonin mean ratio in the frontal cortex (P < 0.01, P < 0.001, and P < 0.001, respectively) compared to the CMS group. A significant reduction in kynurenine/serotonin mean ratio in hippocampus homogenates was demonstrated for 7-nitroindazole and pentoxifylline (P < 0.01 and P < 0.05, respectively) compared to the CMS group. Imipramine induced a statistically insignificant reduction (6.70 ± 1.267 vs 10.48 ± 2.22) in the kynurenine/serotonin mean.
Ratio in hippocampus homogenates compared to CMS group
Nitric oxide metabolites in frontal cortex and hippocampus
demonstrates that CMS-exposed rats exhibited a significant (P < 0.001) increase in nitric oxide metabolite concentrations in frontal cortex and hippocampus homogenates in comparison to nonexposed control rats.
Figure 4 Effects of chronic mild stress (CMS) and treatments with imipramine (IMIP), 7-nitroindazole (7-NI), and pentoxifylline (PENT) on changes of nitric oxide metabolites (nitrates/nitrites, nmol/g tissue) in frontal cortex (A) and hippocampus (B) homogenates.
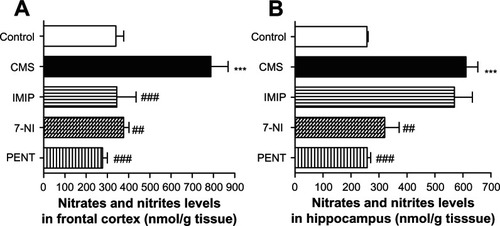
Treatment with imipramine significantly (P < 0.001) decreased nitric oxide metabolite concentrations in the frontal cortex, but was not statistically significant in the hippocampus (569.6 ± 64.15 vs 610.9 ± 42.19 nmol/g tissue). 7-Nitroindazole and pentoxifylline significantly decreased nitric oxide metabolites in the frontal cortex and hippocampus.
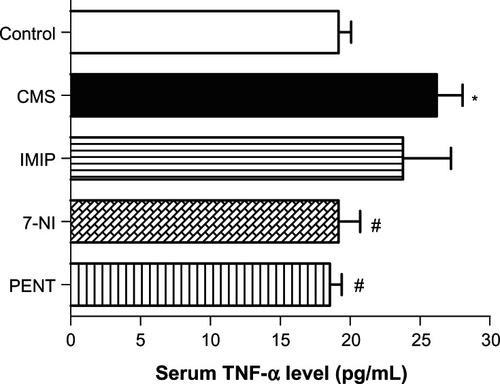
Serum TNF-α analysis
shows that CMS induced a significant (P < 0.05) increase in TNF-α mean serum concentration compared to control. Treatment with imipramine reduced mean serum TNF-α by 10%, with no statistical significance (23.38 ± 3.860 vs 26.20 ± 1.831 pg/mL) compared to the untreated CMS group. Treatments with 7-nitroindazole and pentoxifylline showed significant (P < 0.05) reduction of CMS-induced increase in mean serum TNF-α concentration.
Figure 5 Effects of chronic mild stress (CMS) and treatments with imipramine (IMIP), 7-nitroindazole (7-NI), and pentoxifylline (PENT) on serum tumor necrosis factor (TNF)-α level (pg/mL).
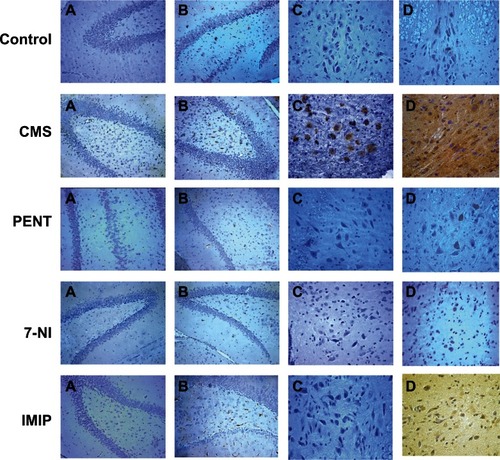
Immunohistochemical examination
Qualitative assessment
In the control group, immunohistochemical staining of the hippocampus demonstrated negative IDO and mild positive TNF-α staining, as shown in CA1, CA3, and the dentate gyrus. CMS-exposed rats exhibited strong positive IDO staining in the alveus, although this was negative in the hippocampus proper, and strong positive TNF-α staining in CA1, CA3, dentate gyrus, and alveus. In pentoxifylline- and 7-nitroindazole-treated rats, there was negative IDO staining and mild TNF-α staining in CA1, CA3, and the dentate gyrus. Imipramine-treated rats demonstrated negative IDO and moderate positive TNF-α staining in CA1, CA3, and the dentate gyrus. As regards midbrain raphe nuclei, immunohistochemical staining demonstrated negative IDO and mild positive TNF-α staining in the control, pentoxifylline, and 7-nitroindazole groups, while the imipramine group showed negative IDO and moderate positive TNF-α. In CMS the mid brain raphe nuclei demonstrated strong positivity with IDO and TNF-α staining which are approximately equal in their staining degree. Consecutive sections showed topographic proximity between IDO and TNF-α staining ().
Figure 6 (A–D) Effects of chronic mild stress (CMS) and treatments with imipramine (IMIP), 7-nitroindazole (7-NI), and pentoxifylline (PENT) on indoleamine 2,3-dioxygenase (IDO) and tumor necrosis factor (TNF)-α immunohistochemical staining of hippocampus and raphe nuclei in comparison to control group (n = 5/group). (A) hippocampus proper stained with IDO × 200; (B) hippocampus proper stained with TNF-α × 200; (C) raphe nuclei stained with IDO × 400; (D) raphe nuclei stained with TNF-α × 400.
Semiquantitative assessment
shows that exposure to CMS induced significant increase in TNF-α immunohistochemical staining score in both the hippocampus and raphe nuclei compared to control group (P < 0.05 and P < 0.01, respectively). At the same time, the CMS group showed increase in IDO immunohistochemical staining in both the hippocampus and raphe nuclei, which was significant only in raphe nuclei (P < 0.001) compared to the control group. Both 7-nitroindazole and pentoxifylline significantly (P < 0.05) reduced TNF-α immunostaining in the hippocampus and raphe nuclei, and this effect was accompanied by reduction of IDO staining in both areas. Nevertheless, this effect was only significant (P < 0.01) in raphe nuclei. Likewise, imipramine reduced TNF-α and IDO immunostaining in the hippocampus and raphe nuclei; however, its effects were statistically insignificant, except in hippocampus TNF-α immunostaining (P < 0.05).
Table 4 Effects of chronic mild stress (CMS) and treatments with imipramine (IMIP), 7-nitroindazole (7-NI), and pentoxifylline (PENT) on indoleamine 2,3-dioxygenase (IDO) and tumor necrosis factor (TNF)-α immunohistochemical staining semiquantitative score of hippocampus and raphe nuclei in comparison to control group
Discussion
In this study, rats exposed to CMS developed depressive-like behaviors. This was evidenced by anhedonia in SPT, which was associated with neurochemical changes in the serum, hippocampal and frontal cortical molar concentrations of serotonin, kynurenine, NO metabolites and TNF-α, in addition to changes occur in their immunostaining.
The neurochemical changes induced by CMS in this work have been reported individually by other investigators. Indeed, the CMS-induced depressive-like behavior that was associated with increased hippocampal TNF-α levelCitation33 and nNOS expression.Citation34 At the same time, a meta-analysis reported significantly higher concentrations of TNF-α in depressed subjects,Citation35 and a postmortem examination of hippocampi of patients with major depression showed more expression of nNOS.Citation13 Ito et alCitation36 reported an increase in plasma kynurenine in healthy subjects exposed to physical stress, while Miura et alCitation1 suggested that proinflammatory cytokines stimulated the IDO enzyme under stress, promoting the kynurenine pathway.
Chronic treatment with pentoxifylline or 7-nitroindazole displayed antidepressant-like activity (reversed anhedonia in SPT) that was comparable to imipramine effect. Moreover, early reversal of CMS-induced anhedonia was observed with pentoxifylline and 7-nitroindazole suggesting a faster antidepressant onset compared to imipramine. Concurrently, all tested drugs reduced kynurenine, the kynurenine/serotonin ratio, and NO metabolites in the frontal cortex and hippocampus, besides reduction in serum TNF-α level and immunohistochemical staining of TNF-α and IDO in the hippocampus and raphe nuclei. Moreover, imipramine and 7-nitroindazole increased hippocampal 5-HIAA concentrations, denoting an increase in extracellular serotonin level, yet the effect of the later was statistically insignificant.
The behavioral findings (anhedonia) in the present study agree with Bah et al,Citation37 who detected that pentoxifylline administration significantly reversed the depressive-like behavior seen after myocardial infarction in rats. Likewise, the behavioral effect of 7-nitroindazole was previously investigated by Yildiz et alCitation14 and Joca and Guimarães,Citation38 who reported that nNOS inhibitors produced an antidepressant-like activity in the forced-swimming test. Additionally, Zhou et alCitation39 reported that 7-nitroindazole significantly reversed the nNOS-derived NO suppression of hippocampal neurogenesis in depression. Interestingly, Ulak et alCitation40 reported that nNOS inhibition potentiated the behavioral effects of some antidepressants – imipramine, citalopram, and fluoxetine – in rats; however, the exact mechanism was not clear.
One mechanism was suggested by Zhou et al,Citation34 who reported that CMS induced hippocampal nNOS overexpression via activating mineralocorticoid receptors. In turn, hippocampal nNOS-derived NO significantly downregulated local glucocorticoid receptor expression, and consequently nNOS inhibition reversed the CMS-induced depressive behaviors via preserving hippocampal glucocorticoid receptors.
Another possible mechanism of 7-nitroinidazole antidepressant effect can be explained by the reported increase in extracellular levels of serotoninCitation41 and decrease in tyrosine hydroxylase expression in the hippocampus of rat treated with 7-nitroinidazole.Citation42 Indeed, NO has a bimodal effect in IDO function. High micromolar concentrations of the NO donors decreased IDO activity, while low micromolar concentrations increased IDO activity in IFN-stimulated monocytic cells.Citation43 The iNOS isoform generates much larger quantities of NO (nanomolar range) than constitutive NOS, like nNOS isoforms (picomolar range).Citation44 In CMS, the iNOS activity may be attenuated by high glucocorticoid level in the hippocampus,Citation45 while nNOS activity increases,Citation34 resulting in low NO concentrations that stimulate IDO activity, leading to a reduction in hippocampus serotonin and an elevation in kynurenine concentrations. This in turn might explain the reduction in kynurenine concentrations and kynurenine/serotonin ratio with nNOS inhibition in the present study.
The findings with pentoxifylline are suggestive of an involvement of TNF-α in the pathophysiology of depression and are supportive of the “inflammatory hypothesis of depression.”Citation46 A possible mechanism for the involvement of TNF-α in depression is thought to be through its ability to affect tryptophan metabolism. Many lines of evidence suggest that the underlying link between stress and depression might involve a shift of tryptophan metabolism from serotonin towards the kynurenine pathwayCitation1 that ultimately produces quinolinic acid, which has neurotoxic properties contributing to several neurobiological changes associated with depressive illness.Citation4
TNF-α is reported to induce the activity of the IDO enzyme. At the same time, an association between serum TNF-α and brain kynurenine concentrations was reported by O’Connor et al,Citation11 who demonstrated that induction of the IDO enzyme was associated with increased central TNF-α, and elevated kynurenine was shown to induce degenerative changes in the hippocampus, which might be involved in depression.Citation2,Citation3 Indeed, Zhu et alCitation47 have shown that treatment with IL-1 or TNF-α is associated with increased activity of serotonin transporters and decrease in its synaptic availability. Immune activation has also been shown to decrease tryptophan availability.Citation10,Citation48,Citation49 Targeting the kynurenine pathway by pentoxifylline could potentially reduce TNF-α, with loss of its inducing effect on the IDO enzyme consequently reducing conversion of tryptophan to kynurenine, with its prodegenerative effects on hippocampus tissue.
In the present study, immunoreactivities of both TNF-α and IDO were found to be in close proximity, providing a topographic possibility for TNF-α to exert its proposed effects on IDO. In the immunohistochemistry assays, IDO was negative and TNF-α was mildly positive in control brain sections (both the hippocampal and raphe sections), however, it was strongly positive for TNF-α in the hippocampus of rats exposed to CMS. In the raphe, strong positive reactions were found for both TNF-α and IDO. Raphe regions are the areas of projecting serotonin fibers to the hippocampus. Most probably, the expression of IDO at neurons, microglia, and astrocytes at the raphe regions may compete or deprive the serotonin neurons from their tryptophan resources, by shifting the tryptophan towards the kynurenine pathway.Citation7,Citation50
Increased TNF-α expression in the hippocampus, and specifically at the dentate gyrus, in rats exposed to CMS, as shown in the present study, may point to a role for cytokines in the neurobiology of depression, other than compromising the serotonin pathway. TNF-α may affect neurogenesis in the hippocampusCitation51 and compromise the expression of BDNF;Citation52 both conditions are reportedly associated with depression.Citation53
Interestingly, nNOS inhibition is associated with decreased TNF-α expression in mice exposed to inflammatory pain,Citation54 and local application of both nNOS inhibitor and TNF-α antiserum induced neuroprotection and improved functional outcome following spinal cord injury.Citation55 The tendency of pentoxifylline to reduce NO metabolites was also noted in other studies. Schwartz et alCitation56 demonstrated that the production of NO by glial cells induced by Streptococcus pneumoniae was inhibited by pentoxifylline. Furthermore, Beshay et alCitation57 reported that pentoxifylline suppressed NO production of LPS/IFN gamma-stimulated macrophages. These studies, as well as the findings of the present study, highlight the relevance of the role of nNOS and TNF-α in neurodegeneration.
In the present work, the antidepressant effects of imipramine were associated with marked elevation of 5-HIAA and reduction in kynurenine, the kynurenine/serotonin ratio, and NO metabolites in the frontal cortex and hippocampus. Imipramine did not significantly decrease serum TNF-α level; however, it could significantly reduce the TNF-α immune-staining in the hippocampus proper. These findings are in agreement with recent studies by Abbas et al and Hsu et al,Citation58,Citation59 who demonstrated that imipramine treatment increased 5-HIAA in the hippocampus of forced-swim-stressed rats and mice, respectively. Furthermore, Kubera et alCitation60 showed that chronic treatment with imipramine reduced immune activation in rats subjected to CMS. Additionally, imipramine inhibited the production of TNF-α in whole human bloodCitation61 and microglia and astrocyte cultures.Citation62
The early reversal of CMS-induced anhedonia by 7-nitroindazole and pentoxifylline in the present work may be explained by their direct inhibition of nNOS and TNF-α. In contrast, imipramine may affect TNF-α and nNOS later in the process, through the long-term effects on serotonin. Recently, Zhang et alCitation63 reported that the 5-HT1 AR-selective agonist 8-OH-DPAT and fluoxetine downregulated hippocampal nNOS expression. The limitations of this study were that direct enzyme activities of NO (inducible and neuronal) and IDO in the frontal cortex and hippocampus were not measured. Furthermore, TNF-α concentrations were measured in serum, not in the studied brain regions.
In conclusion, the findings of this study add further evidence that disturbance in brain monoaminergic function is not the sole mechanism underlying depression. It highlights the role played by the immune system and TNF-α in the pathophysiology of depression. It also elucidates the intricate relationship between TNF-α, nNOS, and tryptophan metabolism (with its two limbs: kynurenine and serotonin) and their association with depression, which might be relevant to speculating about management strategies for depression. Finally, pentoxifylline and nNOS antagonists may prove to be a useful adjunct to other antidepressants.
Disclosure
Authors declare that they have no conflict of interest. Bassim MSA Mohamed received a research grant from National Research Centre (NRC) for partial support of the present work.
References
- MiuraHOzakiNSawadaMIsobeKOhtaTNagatsuTA link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depressionStress20081119820918465467
- FurtadoCPMallerJJFitzgeraldPBA magnetic resonance imaging study of the entorhinal cortex in treatment-resistant depressionPsychiatry Res200816313314218511243
- RimolLMHatbergCBNesvågRCortical thickness and subcortical volumes in schizophrenia and bipolar disorderBiol Psychiatry201068415020609836
- MyintAMKimYKVerkerkRScharpéSSteinbuschHLeonardBKynurenine pathway in major depression: evidence of impaired neuroprotectionJ Affect Disord20079814315116952400
- WidnerBLaichASperner-UnterwegerBLedochowskiMFuchsDNeopterin production, tryptophan degradation, and mental depression – what is the link?Brain Behav Immun20021659059512401473
- GuilleminGJSmytheGTakikawaOBrewBJExpression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neuronsGlia200549152315390107
- MyintAMKimYKCytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depressionMed Hypotheses20036151952514592780
- PopovAAbdullahZWickenhauserCIndoleamine 2,3-dioxyge-nase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infectionJ Clin Invest20061163160317017111046
- FujigakiHSaitoKFujigakiSThe signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokinesJ Biochem200613965566216672265
- CapuronLGumnickJFMusselmanDLNeurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensionsNeuropsychopharmacology20022664365211927189
- O’ConnorJCLawsonMAAndréCLipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in miceMol Psychiatry20081451152218195714
- HenryCJHuangYWynneAMinocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedoniaJ Neuroinflammation200813515
- OliveiraRMGuimarãesFSDeakinJFExpression of neuronal nitric oxide synthase in the hippocampal formation in affective disordersBraz J Med Biol Res20084133334118392456
- YildizFErdenBFUlakGUtkanTGacarNAntidepressant-like effect of 7-nitroindazole in the forced swimming test in ratsPsychopharmacol (Berl)20001494144
- TonelliLHStillerJRujescuDElevated cytokine expression in the orbitofrontal cortex of victims of suicideActa Psychiatr Scand200811719820618081924
- SimenBBDumanCHSimenAADumanRSTNFα signaling in depression and anxiety: behavioral consequences of individual receptor targetingBiol Psychiatry20065977578516458261
- McNallyLBhagwagarZHannestadJInflammation, glutamate, and glia in depression: a literature reviewCNS Spectr20081350151018567974
- NierenbergAALeonACPriceLHSheltonRCTrivediMHCrisis of confidence: antidepressant risk versus benefitJ Clin Psychiatry201172e1121450147
- WillnerPValidity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluationPsychopharmacology (Berl)19971343193299452163
- KuberaMMaesMHolanVBasta-KaimARomanAShaniJProlonged desipramine treatment increases the production of interleukin-10, an anti-inflammatory cytokine, in C57BL/6 mice subjected to the chronic mild stress model of depressionJ Affect Disord20016317117811246093
- WillnerPChronic mild stress (CMS) revisited: consistency and behavioral-neurobiological concordance in the effects of CMSNeuropsychobiology2005529011016037678
- KimSHHanJSeogDHAntidepressant effect of chaihushugan-san extract and its constituents in rat models of depressionLife Sci2005761297130615642599
- CavasMNavarroJFEffects of selective neuronal nitric oxide synthase inhibition on sleep and wakefulness in the ratProg Neuropsychopharmacol Biol Psychiatry200630566716023276
- YagmurluABolekenMEErtoyDOzsanMGokcoraIHDindarHPreventive effect of pentoxifylline on renal scarring in rat model of pyelonephritisUrology2003611037104112736043
- BekrisSAntoniouKDaskasSPapadopoulou-DaifotiZBehavioural and neurochemical effects induced by chronic mild stress applied to two different rat strainsBehav Brain Res2005161455915904709
- D’AquilaPMonleonSBorsiniFBrainPWillnerPAnti-anhedonic actions of the novel serotonergic agent flibanserin, a potential rapidly-acting antidepressantEur J Pharmacol19973401211329537806
- PaxinosGWatsonCThe Rat Brain in Stereotaxic Coordinates2nd edSan DiegoAcademic Press1986
- WangRTangASimultaneous determination of kynurenine and tryptophan in serum by high performance liquid chromatographySe Pu200624140143 Chinese.16830460
- BellacCLCoimbraRSChristenSLeibSLPneumococcal meningitis causes accumulation of neurotoxic kynurenine metabolites in brain regions prone to injuryNeurobiol Dis20062439540216956766
- MeffordINApplication of high performance liquid chromatography with electrochemical detection to neurochemical analysis: measurement of catecholamines, serotonin and metabolites in rat brainJ Neurosci Methods198132072246163932
- MeffordINChangPKleinDCNamboodiriMASugdenDBarchasJReciprocal day/night relationship between serotonin oxidation and N-acetylation products in the rat pineal glandEndocrinology1983113158215866194975
- HsuSRaineLProtein, avidin and biotin in immunohistochemistryJ Histochem Cytochem198129134913536172466
- GrippoAJFrancisJBeltzTGFelderRBJohnsonAKNeuroendocrine and cytokine profile of chronic mild stress-induced anhedoniaPhysiol Behav20058469770615885245
- ZhouQGZhuLJChenCHippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptorJ Neurosci201125317579759021613472
- DowlatiYHerrmannNSwardfagerWA meta-analysis of cytokines in major depressionBiol Psychiatry201016744645720015486
- ItoYSaitoKMarutaKKynurenine concentration of serum was increased by exerciseAdv Exp Med Biol199946771772210721124
- BahTMKaloustianSRousseauGGodboutRPretreatment with pentoxifylline has antidepressant-like effects in a rat model of acute myocardial infarctionBehav Pharmacol20112277978421971020
- JocaSRGuimarãesFSInhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effectsPsychopharmacology (Berl)200618529830516518647
- ZhouQGHuYHuaYNeuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesisJ Neurochem20071031843185417854383
- UlakGMutluOAkarFYKomsuog luFITanyeriPErdenBFNeuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole augment the effects of antidepressants acting via serotonergic system in the forced swimming test in ratsPharmacol Biochem Behav20089056356818565575
- WegenerGVolkeVRosenbergREndogenous nitric oxide decreases hippocampal levels of serotonin and dopamine in vivoBr J Pharmacol200013057558010821785
- PrzedborskiSJackson-LewisVYokoyamaRShibataTDawsonVLDawsonTMRole of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicityProc Natl Acad Sci U S A199693456545718643444
- LópezASAlegreEDíazAMuguetaCGonzálezABimodal effect of nitric oxide in the enzymatic activity of indoleamine 2,3-dioxygenase in human monocytic cellsImmunol Lett200610616317116797727
- AldertonWKCooperCEKnowlesRGNitric oxide synthases: structure, function and inhibitionBiochem J200135759361511463332
- WalkerGPfeilschifterJKunzDMechanisms of suppression of inducible nitric-oxide synthase (iNOS) expression in interferon (IFN)-gamma-stimulated RAW 264.7 cells by dexamethasone. Evidence for glucocorticoid-induced degradation of iNOS protein by calpain as a key step in post-transcriptional regulationJ Biol Chem199727216679166879195984
- SmithRSThe macrophage theory of depressionMed Hypotheses1991352983061943879
- ZhuCBBlakelyRDHewlettWAThe proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transportersNeuropsychopharmacology2006312121213116452991
- SchiepersOJWichersMCMaesMCytokines and major depressionProg Neuropsychopharmacol Biol Psychiatry200529637638
- Catena-Dell’OssoMBellantuonoCConsoliGBaroniSRotellaFMarazzitiDInflammatory and neurodegenerative pathways in depression: a new avenue for antidepressant developmentCurrent Med Chem201118245255
- LeonardBEThe concept of depression as a dysfunction of the immune systemCurr Immunol Rev2010620521221170282
- SeguinJABrennanJManganoEHayleySProinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administrationNeuropsychiatr Dis Treat2009551419557094
- LiSWangCWangWDongHHouPTangYChronic mild stress impairs cognition in mice: from brain homeostasis to behaviourLife Sci20088293494218402983
- AnismanHMeraliZHayleySNeurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegeneration disordersProg Neurobiol20088517418346832
- ChenYBoettgerMKReifASchmittAUçeylerNSommerCNitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in miceMol Pain201061320193086
- SharmaHSA combination of tumor necrosis factor-alpha and neuronal nitric oxide synthase antibodies applied topically over the traumatized spinal cord enhances neuroprotection and functional recovery in the ratAnn N Y Acad Sci2010119917518520633123
- SchwartzDEngelhardDGallilyRMatothIBrennerTGlial cells production of inflammatory mediators induced by Streptococcus pneumoniae: inhibition by pentoxifylline, low-molecular-weight heparin and dexamethasoneJ Neurol Sci199815513229562317
- BeshayECrozeFPrud’hommeGJThe phosphodiesterase inhibitors pentoxifylline and rolipram suppress macrophage activation and nitric oxide production in vitro and in vivoClin Immunol20019827227911161985
- AbbasGNaqviSDarAComparison of monoamine reuptake inhibitors for the immobility time and serotonin levels in the hippocampus and plasma of sub-chronically forced swim stressed ratsPak J Pharm Sci20122544144522459475
- HsuLCKoYJChengHYAntidepressant-like activity of the ethanolic extract from Uncaria lanosa Wallich var. appendiculata Ridsd in the forced swimming test and in the tail suspension test in miceEvid Based Complement Alternat Med2012201249730222567032
- KuberaMSymbirtsevABasta-KaimAEffect of chronic treatment with imipramine on interleukin 1 and interleukin 2 production by splenocytes obtained from rats subjected to a chronic mild stress model of depressionPol J Pharmacol1996485035069112692
- KuberaMKenisGBosmansESuppressive effect of TRH and imipramine on human interferon-gamma and interleukin-10 production in vitroPol J Pharmacol2000248148611334244
- HwangJZhengLTOckJInhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressantsNeuropharmacology20085582683418639562
- ZhangJHuangXYeMNeuronal nitric oxide synthase alteration accounts for the role of 5-HT1 A receptor in modulating anxiety-related behaviorJ Neurosci2010302433244120164327