Abstract
Background
Patients with schizophrenia or bipolar disorder (BPD) may be differentially sensitive to antipsychotics. This study assessed the median time to onset, duration, and rate of somnolence associated with asenapine and other antipsychotics in both indications.
Methods
Ten clinical trials (n = 4786) were analyzed as five cohorts pooled according to indication and study design.
Results
In the short-term schizophrenia cohort, the incidence of somnolence was 13.1%, 19.1%, 8.5% 5.2%, and 6.9% with asenapine, olanzapine, risperidone, haloperidol, and placebo, respectively. Median time to onset of somnolence was 2 days for asenapine and olanzapine, and 6, 3, and 7 days for risperidone, haloperidol, and placebo, respectively. Median duration was 15 days for asenapine and olanzapine, and 3, 22.5, and 4.5 days for risperidone, haloperidol, and placebo, respectively. In the long-term schizophrenia cohort, the incidence, time to onset, and duration of somnolence with asenapine and olanzapine were 18.4% versus 19.6%, 9.0 days versus 12 days, and 22 days versus 21 days, respectively. In schizophrenia with persistent negative symptoms, the incidence, median time to onset, and duration of somnolence with asenapine and olanzapine were 18.5% versus 21.1%, 9.0 days versus 7.5 days, and 25.0 days versus 41.5 days, respectively. In the monotherapy for BPD cohort, the incidence of somnolence with asenapine, olanzapine, and placebo was 23.8%, 26.4%, and 6.4%, respectively. Median time to onset and duration of somnolence with asenapine, olanzapine, and placebo were 1, 2, and 2 days, respectively, and 7, 8.5, and 5 days. In the adjunctive therapy for BPD cohort, the incidence, median time to onset, and duration of somnolence with asenapine and placebo were 24.0% versus 10.2%, 1.5 days versus 2 days, and 12.5 days versus 7 days, respectively.
Conclusion
In the short-term schizophrenia cohort, time to onset and duration of somnolence with asenapine was similar to that with olanzapine and haloperidol. Only asenapine and olanzapine had significantly higher rates of somnolence relative to placebo. The time to onset, duration, and incidence of somnolence with asenapine and olanzapine was similar in patients with long-term schizophrenia and those with BPD. Patients with BPD were more sensitive than those with schizophrenia to asenapine and olanzapine.
Introduction
Schizophrenia and bipolar disorder (BPD) are two of the most common and debilitating psychiatric disorders, causing severe impairment, and are associated with an increased risk of suicide.Citation1 Although each disorder presents with its own distinct symptom profile, antipsychotics are commonly used in both conditions. Depending on the pharmacological profile of a drug, the use of antipsychotics in patients with schizophrenia or BPD can be associated with an increased incidence of somnolence, including sedation and hypersomnia.Citation2 Antipsychotic-induced sedation can be associated with poor cognition and an increase in reported accidents, and prolonged or severe sedation could potentially impair quality of life and result in noncompliance with medication. However, sedation may be helpful under certain circumstances in the management of acute schizophrenia or mania because of its ability to regulate sleep disturbances and manage agitation. In this regard, patients with BPD have been reported to be more sensitive in general than those with schizophrenia to the sedative effects of antipsychotics.Citation2
Asenapine is a tetracyclic antipsychotic with a unique pharmacologic profile,Citation3 and is approved in the United States for the treatment of schizophrenia in adults and for the acute treatment of manic or mixed episodes associated with bipolar I disorder, either as monotherapy or as therapy adjunctive to lithium or valproate. In the European Union, asenapine is approved for the treatment of moderate to severe manic episodes associated with bipolar I disorder.Citation4 In both short-term and long-term clinical studies, sublingual asenapine was effective and generally well tolerated in patients with schizophreniaCitation5–Citation8 and in those with manic or mixed symptoms associated with bipolar I disorder.Citation9–Citation13
Asenapine is formulated as a fast dissolving and rapidly absorbed sublingual tablet, and is able to be initiated at therapeutic doses without titration. Like some antipsychotics,Citation2 somnolence and/or sedation are the most commonly reported adverse events associated with asenapine in patients with schizophrenia or BPD.Citation14 However, it remains unclear if asenapine has a similar risk of treatment-emergent somnolence compared with other antipsychotics, and if patients with schizophrenia or BPD have a similar risk, onset, and duration of treatment-emergent somnolence on asenapine. Such information would aid clinicians in the appropriate use of asenapine. Therefore, we conducted a post hoc analysis of 10 clinical trials of asenapine in the treatment of schizophrenia and BPD to compare the incidence, median time to onset, and average duration of somnolence/sedation, to determine whether there was a difference in the risk of treatment-emergent somnolence with asenapine, olanzapine, risperidone, and haloperidol relative to placebo, and to explore if there was any difference in sensitivity to the sedative effects of asenapine in these therapeutic settings.
Materials and methods
This was a post hoc analysis of 10 clinical trials involving a total of 4786 patients. The data were analyzed and pooled into five cohorts according to treatment indication (cohorts 1, 2, and 3 for schizophrenia and cohorts 3 and 4 for bipolar mania). These five cohorts were also constructed based on study design, such as acute (cohort 1) versus long-term (cohorts 2 and 3), placebo-controlled (cohort 1) versus head-to-head active drug comparison (cohorts 2 and 3), fixed-dose (cohort 1) versus flexible dose (cohorts 2 and 3), and monotherapy (cohort 4) versus adjunctive therapy (cohort 5). A summary description of each of the five cohorts, including study designs, patient population, permitted concomitant medications, and major eligibility criteria is presented in . In each study, patients assigned to asenapine received the study drug at a dose of 5–10 mg twice daily. The primary results of these studies have been reported elsewhere.Citation5,Citation7–Citation9,Citation12,Citation13,Citation15
Table 1 Summary of patient groups
For the purposes of this analysis, somnolence was defined as any treatment-emergent adverse event corresponding to the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms of somnolence, sedation, or hypersomnia. The severity (maximum intensity of the event) was determined by the primary investigators of each study. If more than one event occurred, the maximum intensity was determined as the greatest of those events. The time to onset of somnolence was calculated as the number of days from the first dose of study medication to the first day of the event. Duration of somnolence was calculated as the number of days between the day of onset and the last day of the event. If an event was unresolved at the end of the study, the last day was imputed as seven days after the last dose for an adverse event or 30 days after the last dose for a serious adverse event. If there were no events by the end of the trial or at early discontinuation, the time to the event was considered right censored at the last day of the study medication.
Schizophrenia
Cohort 1: short-term schizophrenia
The short-term schizophrenia cohort consisted of 1318 patients with acute exacerbation of their positive symptoms enrolled in four 6-week monotherapy trials, including 572 patients treated with asenapine (5–10 mg twice daily), 378 treated with placebo, 59 with risperidone (3 mg twice daily), 194 with olanzapine (5–20 mg once daily), and 115 with haloperidol (4 mg twice daily).Citation5,Citation7,Citation16
Cohort 2: long-term schizophrenia and schizoaffective disorder
The long-term (52-week) schizophrenia and schizoaffective cohort consisted of 1219 patients with acute exacerbation of their positive symptoms and enrolled in a single monotherapy safety trial, including 908 asenapine-treated (5–10 mg twice daily) and 311 olanzapine-treated (5–20 mg once daily) patients.Citation8
Cohort 3: monotherapy for schizophrenia with persistent negative symptoms
The persistent negative symptoms (PNS) cohort consisted of 949 patients suffering from PNS enrolled in two 26-week monotherapy trials, including 485 asenapine-treated (5–10 mg twice daily) and 464 olanzapine-treated (5–20 mg once daily) patients.Citation15
Bipolar mania
Cohort 4: short-term monotherapy for bipolar 1 disorder
The short-term bipolar I disorder cohort consisted of 976 patients enrolled in two 3-week monotherapy trials, including 379 patients treated with asenapine (5–10 mg twice daily), 203 with placebo, and 394 with olanzapine (5–20 mg once daily).Citation9,Citation12
Cohort 5: short-term adjunctive therapy for bipolar 1 disorder
The adjunctive bipolar I disorder cohort consisted of 324 patients enrolled in a single 12-week trial, including 158 asenapine-treated (5–10 mg twice daily) patients and 166 placebo-treated patients who were administered asenapine as an adjunct to lithium or valproate.Citation13
Key outcomes
Key outcome measures were incidence, time to onset, and duration of somnolence. Somnolence as an adverse event was defined using MedDRA preferred terms and included somnolence, sedation, and hypersomnia. An additional exploratory measure was the percentage of patients who took any sedatives/hypnotics or benzodiazepines during the studies and in whom use of these medications was associated with onset of somnolence within 24 hours.
Subgroup analyses
A subgroup analysis of patients in the short-term schizophrenia and short-term bipolar 1 disorder monotherapy cohorts (cohorts 1 and 4, respectively) was evaluated according to baseline Positive and Negative Syndrome Scale (PANSS) anxiety score G2 <4 or ≥4, PANSS depression score G6 <4 or ≥4, and PANSS Marder Factor (MF) anxiety/depression score <16 or ≥16. The BPD subgroups were evaluated using a Montgomery-Åsberg Depression Rating Scale (MADRS) total score <20 or ≥20 and a Clinical Global Impressions of Bipolar Disorder severity of depression score <4 or ≥4.
Statistical analysis
All post hoc analyses were conducted in patients who received at least one dose of active agent or placebo for each trial. Data were pooled across doses in each cohort for asenapine, active controls (risperidone, olanzapine, and haloperidol), and placebo. The number needed to treat to harm (NNH) for an event of somnolence for each active group relative to the placebo group was calculated in cohorts 1, 4, and 5, and relative to the olanzapine group in cohorts 2 and 3 when differences in the incidence rates between the treatment groups were statistically significant. The 95% confidence intervals (CIs) were based on differences in treatment rate using the Miettinen and Nurminen method.Citation17 Median time to onset and duration of somnolence were calculated based on patients who experienced treatment-emergent somnolence/sedation events. P values to test differences in distribution of time to onset of somnolence were calculated using the log-rank test.
Results
Schizophrenia
Cohort 1: short-term schizophrenia risk for somnolence
In the short-term schizophrenia cohort, the incidence of somnolence () with all doses of asenapine was 13.1%, 6.9% with placebo, 8.5% with risperidone, 5.2% with haloperidol, and 19.1% with olanzapine. The NNH (95% CI) for asenapine and olanzapine relative to placebo was 16 (10, 43) and 8 (5, 15), respectively. The difference between risperidone or haloperidol and placebo was not significant. No patients discontinued because of somnolence/sedation in the placebo, risperidone, or haloperidol groups, but one patient (0.2%) receiving asenapine and two patients (1.0%) receiving olanzapine discontinued due to somnolence. One event of severe somnolence was reported in the asenapine group. Moderate events of somnolence with placebo, asenapine, risperidone, olanzapine, and haloperidol were experienced by five (1.3%), 13 (2.3%), two (3.4%), 13 (6.7%), and one (0.87%) patients, respectively (). All other events of somnolence with each treatment were mild.
Figure 1 Frequency of somnolence events by intensity.
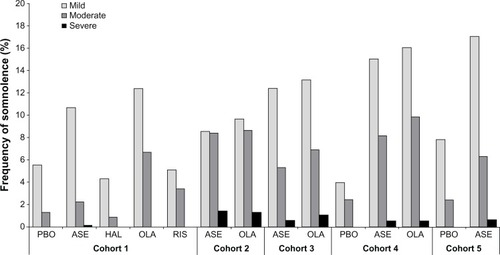
Table 2 Somnolence in short-term schizophrenia cohort
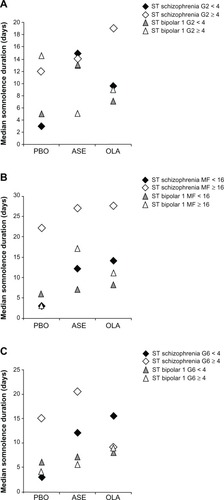
As shown in , in patients with less anxiety at baseline (PANSS G2 <4), only olanzapine had a significantly increased risk for somnolence, with a NNH of 8 [95% CI (5, 21)]. In contrast, in patients with elevated baseline anxiety (PANSS G2 ≥4), both asenapine and olanzapine were associated with a significantly increased risk of somnolence, with an NNH of 11 [95% CI (6, 39)] and 8 [95% CI (5, 29)], respectively. In patients with less severe depression at baseline (PANSS MF anxiety/depression <16 or PANSS G6 <4), both asenapine and olanzapine had a significantly increased risk of somnolence, but in patients with more severe depression (PANSS MF anxiety/depression ≥ 16 or PANSS G6 ≥4), none of the active treatments increased the risk of somnolence.
The median time to onset of somnolence () was significantly shorter with asenapine (2.0 days; P<0.01) and olanzapine (2.0 days; P<0.0001) compared with placebo (7.0 days). The median times to onset with risperidone (6.0 days) and haloperidol (3.0 days) were not significantly different from placebo. The median duration of somnolence () with asenapine was numerically longer (15 days) than with placebo (4.5 days) or risperidone (3 days), similar to olanzapine (15 days), and numerically less than haloperidol (22.5 days). Duration of somnolence was longer in patients in the olanzapine group with elevated baseline anxiety (PANSS G2 ≥4, ). In the asenapine and olanzapine groups, the median duration of somnolence was prolonged in the subgroup of patients with PANSS MF anxiety/depression ≥16 ().
Figure 2 Distribution of time to first occurrence of somnolence in (A) cohort 1, (B) cohort 2, (C) cohort 3, (D) cohort 4, and (E) cohort 5.
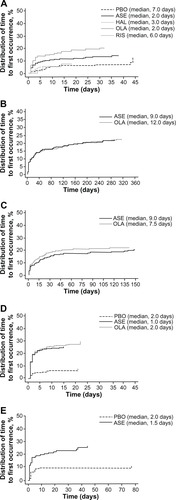
Figure 3 Median duration of somnolence by antipsychotic agent in patients with (A) schizophrenia and (B) bipolar disorder.
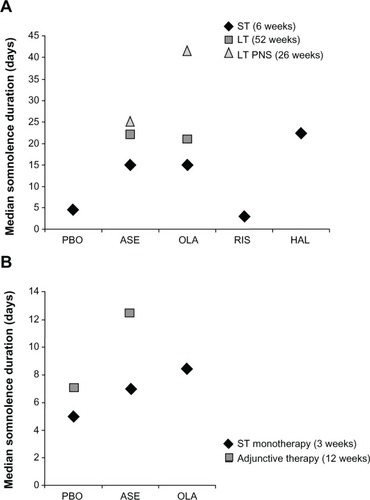
Figure 4 Median duration of somnolence in short-term studies of patients with schizophrenia or bipolar 1 disorder with baseline (A) anxiety (PANSS G2 <4 or ≥4), (B) anxiety/depression (PANSS MF anxiety/depression < 16 or ≥16), and (C) depression (PANSS G6 < 4 or ≥4).
Cohort 1: short-term schizophrenia rates of sedative/hypnotic use
In the short-term schizophrenia cohort, 23.5%–64.4% of patients across all treatment groups used sedatives/hypnotics (). Significantly more patients treated with risperidone received sedatives/hypnotics compared with those on placebo, with a difference in rate of 21.8 (95% CI [8.1, 34.0]). In contrast, significantly fewer patients treated with haloperidol received sedatives/hypnotics than those treated with placebo, with a difference in rate of −19.1 (95% CI [−27.7, −9.3]). Benzodiazepines were used by 52.2%–83.1% of patients in this cohort during treatment with the study drug. The likelihood of treatment-emergent somnolence occurring with any treatment within 24 hours of receiving hypnotics/benzodiazepines was relatively low (placebo 0.3%, asenapine 2.3%, other antipsychotics 0%–1.7%).
Table 3 Sedative/hypnotic use in short-term schizophrenia cohort
Similarly, in patients with less severe baseline anxiety (PANSS G2 <4) or symptoms of depression (PANSS G6 <4 or PANSS MF <16), significantly more treated with risperidone but significantly fewer treated with haloperidol received sedatives/hypnotics than those treated with placebo (). No significant change was observed in the use of sedatives/hypnotics by patients in these groups who were administered asenapine. In contrast, with the exception of those treated with risperidone, significantly fewer patients with severe baseline anxiety (PANSS G2 ≥4) treated with other antipsychotics received sedatives/hypnotics than those treated with placebo. In patients with worse baseline anxiety (PANSS G2 ≥4), use of benzodiazepines ranged from 65.6% to 90.6% across all groups. In patients with less severe baseline anxiety (PANSS G2 <4), use of benzodiazepines ranged from 43.6% to 74.1%.
In patients with worse symptoms of depression at baseline (PANSS MF ≥16), use of sedatives/hypnotics ranged from 37.5% to 71.4%. Use of benzodiazepines ranged from 70.4% to 87.5% across all groups. In patients with baseline PANSS G6 ≥4, use of sedatives/hypnotics ranged from 40.9% to 63.2% and benzodiazepines from 70.1% to 89.5% across all treatment groups. In patients with less severe depression at baseline, use of sedatives/hypnotics was 20.4%–64.7% with PANSS MF anxiety/depression <16 and 19.4%–65.0% with PANSS G6 <4. Use of benzodiazepines was 50.0%–82.4% in patients with baseline PANSS MF anxiety/depression <16 and 46.2%–80.0% in those with PANSS G6 <4.
Cohort 2: long-term schizophrenia and schizoaffective disorder
In the cohort with long-term (52-week) schizophrenia and schizoaffective disorder, the incidence of somnolence with asenapine was 18.5% and 19.6% with olanzapine, without any significant difference between the two groups. Thirteen patients (1.43%) treated with asenapine and four (1.29%) treated with olanzapine experienced severe somnolence (). Nine patients (1.0%) receiving asenapine and one receiving olanzapine (0.3%) discontinued as a result of somnolence/sedation. No statistically significant difference was observed for median time to onset of somnolence between asenapine and olanzapine (9.0 versus 12.0 days, respectively; P = 0.8007, ). The median duration of somnolence with asenapine was similar to that with olanzapine (22.0 versus 21.0 days, respectively, ). Of those patients treated with asenapine and olanzapine, 25.8% and 16.1%, respectively, used sedatives or hypnotics, and 49.3% and 43.4% used benzodiazepines. The incidence of somnolence occurring within 24 hours of receiving benzodiazepines was 0.3% with asenapine and 1.3% with olanzapine.
Cohort 3: schizophrenia with PNS
The incidence of somnolence was similar for asenapine (18.4%) and olanzapine (21.1%) in the cohort with schizophrenia and PNS. The majority of somnolence events were mild or moderate, with three patients (0.62%) treated with asenapine and five patients (1.08%) treated with olanzapine experiencing severe somnolence (). Five patients (1.0%) in the asenapine group and three (0.7%) in the olanzapine group discontinued as a result of somnolence/sedation. No statistically significant difference in median time to onset of somnolence was observed between asenapine and olanzapine (9.0 days versus 7.5 days, respectively; P=0.2152, ). Median duration of somnolence () with asenapine was numerically shorter than with olanzapine (25.0 days versus 41.5 days, respectively).
In the cohort with schizophrenia and PNS, 15.9% and 11.9% of patients treated with asenapine and olanzapine, respectively, used sedatives/hypnotics, and 28.3% and 20.9% used benzodiazepines. The incidence of somnolence within 24 hours of receiving benzodiazepines was low (asenapine 0.4%, olanzapine 0.2%).
Bipolar 1 mania
Cohort 4: risk of somnolence with 3-week monotherapy
In the 3-week bipolar I monotherapy cohort, the incidence of somnolence in the asenapine and olanzapine groups was significantly higher than that in the placebo group, with a NNH of 6 (95% CI [4, 9]) and 5.0 (95% CI [4, 7]), respectively (). No patient in the placebo group, one (0.3%) in the asenapine group, and two (0.5%) in the olanzapine group discontinued because of somnolence/sedation. In both active arms, patients with lower (PANSS G2 <4) or higher (PANSS G2 ≥4) baseline anxiety had significantly higher rates of somnolence than in the placebo group, although the NNH was smaller in those with worse anxiety. Further, patients with less or more severe symptoms of depression (PANSS G6 < or ≥4) had significantly higher rates of somnolence with both active arms than with placebo. Severe somnolence was experienced by two patients (0.53%) treated with asenapine, two patients (0.51 %) treated with olanzapine, and none with placebo ().
Table 4 Incidence of somnolence in short-term bipolar 1 monotherapy and adjunctive cohorts
No statistically significant difference in median time to onset of somnolence () was observed with asenapine (1.0 days; P=0.0780) or olanzapine (2.0 days; P=0.4375), compared with placebo (2.0 days). The median duration of somnolence () with asenapine (7 days) was longer than with placebo (5 days), but shorter than with olanzapine (8.5 days). A numerical decrease in duration of somnolence was observed in patients with PANSS G2 ≥4 receiving asenapine (). No further clear differences were observed in duration of somnolence for the patient subgroups with either lower or elevated anxiety or depression, as measured by PANSS MF anxiety/depression and PANSS G6 depression scores ( and ). In addition, no clear differences were observed in median duration of somnolence in patients with fewer or more symptoms of depression as measured by MADRS <20 or ≥ 20 (placebo 7 versus 4 days, asenapine 8 versus 4 days, olanzapine 8 versus 11 days) or CGI-BD-S <4 or ≥4 (placebo, 7 days versus 3 days; asenapine, 10.5 days versus 5 days; olanzapine, 7 days versus 14 days).
Cohort 4: rate of sedative/hypnotic use with 3-week monotherapy
In the 3-week bipolar I monotherapy cohort, 35.1%–37.0% of patients across all treatment groups used sedatives/hypnotics, and 53.8%–63.6% used benzodiazepines. There were no significant differences between patients treated with antipsychotics and placebo with regard to rates of using sedatives/hypnotics (). However, patients with less severe baseline anxiety or depression tended to use fewer sedatives/hypnotics across the three treatment arms compared with those with worse anxiety and depression.
Table 5 Sedative/hypnotic use in bipolar mania studies
Cohort 5: 12-week adjunctive therapy in bipolar 1 mania
In the bipolar I adjunctive therapy cohort, the rate of somnolence was significantly higher with asenapine (24.1%) than with placebo (10.2%), with a NNH of 7 (95% CI [5, 18], ). One patient in each group (0.6%) discontinued due to somnolence/sedation. One patient treated with asenapine and none treated with placebo experienced severe somnolence (). Compared with placebo, no statistically significant difference in median time to onset of somnolence was observed with asenapine (2.0 days and 1.5 days, respectively; P=0.6697, ). The median duration of somnolence with asenapine was longer than with placebo (12.5 days versus 7.0 days, ). During the study period, 41.6% with asenapine and 43.0% with placebo used sedatives/hypnotics, and 54.8 and 53.2% used benzodiazepines. The incidence of somnolence within 24 hours of receiving benzodiazepines was low (placebo, 1.8%; asenapine, 4.4%).
Discussion
Somnolence and sedation are common side effects of antipsychotic medications prescribed for schizophrenia or BPD,Citation2 and may result in unintentional injury,Citation18 impaired functioning, and noncompliance with treatment. Asenapine has been shown to be associated with somnolence/sedation in patients with schizophrenia and bipolar I disorder.Citation11,Citation14 However, to the authors’ knowledge, this is the first report to examine treatment-emergent somnolence with asenapine and other antipsychotics in the treatment of schizophrenia and BPD systematically using incidence rates, time to onset, duration, severity of somnolence, and use of sedatives/hypnotics. This may also be the first study using the PANSS and MADRS to explore the relationship between treatment-emergent somnolence and the baseline severity of anxiety and depression.
The results of this study confirm that somnolence/sedation occurs with asenapine in a limited number (13%–24%) of patients with schizophrenia or BPD early in the course of treatment, when it is of limited duration and generally of mild to moderate intensity. More importantly, treatment-emergent somnolence with asenapine was not associated with an increased risk of discontinuation of treatment, which is consistent with findings for other antipsychotics in the treatment of schizophrenia and BPD.Citation2 Our results for treatment-emergent somnolence with olanzapine, risperidone, and haloperidol () were also consistent with those of a previous study by Gao et al,Citation2 in which olanzapine, but not risperidone or haloperidol, had an increased risk for somnolence relative to placebo in the treatment of schizophrenia.
Treatment-emergent somnolence associated with asenapine was more similar to that with olanzapine than with other antipsychotics with respect to time to onset and duration of somnolence ( and ). However, the risk of somnolence with asenapine was less than that for olanzapine, as refected by an NNH of 16 for asenapine and 8 for olanzapine (). Both olanzapine and asenapine bind to a broad range of receptor subtypes, including H1 receptors.Citation3,Citation19 H1 antagonists are well known to be associated with sedation,Citation20 and the difference in risk of somnolence could be a result of the greater affinity of olanzapine for H1 receptors compared with asenapine. However, the risk of somnolence in bipolar I mania was similar (NNH 6 for asenapine; 5 for olanzapine).
An increased risk of somnolence in patients with bipolar mania compared with those having schizophrenia has been reported previously for other antipsychotics, including risperidone, quetiapine, aripiprazole, and ziprasidone, but not for olanzapine.Citation2 In the post hoc analyses described here, the greater sensitivity to somnolence associated with asenapine of patients with bipolar mania compared to those with schizophrenia (NNH 16 for schizophrenia; 6 for bipolar mania) was similar to that previously reported for aripiprazole (NNH 14 for schizophrenia; 8 for bipolar mania).
Hypnotics, sedatives, and benzodiazepines were widely used throughout the studies and it is possible that a degree of somnolence may be associated with the use of these sedating drugs. However, the incidence of somnolence did not appear to be related directly to the use of hypnotics or benzodiazepines, because the risk of developing somnolence within 24 hours of administering these drugs was very low. This suggests that the increased occurrence of somnolence with asenapine and olanzapine may be a “true” effect of these two medications. In addition, the finding that more patients treated with risperidone used sedatives/hypnotics seems consistent with the less “sedating” pharmacological profile of this agent ().
It is well known that patients with schizophrenia or BPD often have inadequate sleep and sleep disturbances.Citation21,Citation22 Under these circumstances, antipsychotic-related somnolence may actually prove beneficial in alleviating these symptoms as well as agitation. However, the finding that patients with higher baseline anxiety were more likely to experience somnolence suggests that clinicians may need to focus attention on this group of patients to prevent discontinuation because of somnolence/sedation. Further, patients with schizophrenia and worse baseline anxiety (G2 ≥4) were less likely to receive sedatives/hypnotics with each of the antipsychotics relative to those on placebo. These data further support that the treatment-emergent somnolence was not related to use of sedatives/hypnotics. Importantly, these data expand upon the previous finding by Gao et al that patients with generalized anxiety disorder were more sensitive to antipsychotics than patients with schizophrenia, BPD, or major depressive disorder.Citation23
Patients with schizophrenia or BPD with less severe depression at baseline tended to be at higher risk of treatment-emergent somnolence with asenapine or olanzapine relative to placebo than those with more severe depression at baseline. The findings of this post hoc analysis will need to be confirmed by further research, in view of the variation in size of the underlying databases for each of the compounds studied.
Limitations
The results of this study are limited by the original study designs, including the methods used to collect data and intervals between study visits. For instance, visit schedules for cohort 1 were at day 4 and weeks 1, 2, 3, 4, 5, and 6 whereas those for cohort 2 were at weeks 1, 2, 3, 4, 6, and 8, and then monthly. The median time to onset of somnolence with asenapine and olanzapine in cohort 1 was 2 days for both drugs, whereas the median time to onset of somnolence with asenapine and olanzapine in cohort 2 was 9 days and 12 days, respectively. The difference in time to onset of somnolence between cohorts 1 and 2 could be due to lack of assessment at day 4 in cohort 2 or different dosing schedules being used in cohorts 1 and 2. Given that the median time to onset of somnolence with asenapine and olanzapine in cohort 2 was longer than 7 days, it is more likely that the longer median time to onset in cohort 2 was due to the flexible-dosing schedule used. In cohort 1, the dosing schedule was fixed, which might be a reason for the shorter median time to onset of somnolence. The flexible-dosing schedule in cohort 2 is similar to that used in routine clinical care. Therefore, the findings from cohort 2 might be more clinically relevant.
These results could be confounded further by uncontrolled factors related to sleep-wake cycles in the original studies, such as consumption of caffeine, alcohol, nicotine, prescribed and nonprescribed drugs, quality and quantity of night-time sleep, medical conditions such as thyroid disease, and other sleep disturbances. Further, we counted the frequency of somnolence as an adverse event using MedDRA preferred terms, including somnolence, sedation, and hypersomnia. If there was more than one event reported, that with maximal severity was chosen. Clearly, this method not only ignored the severity of somnolence, but also may have obscured its prevalence.
Conclusion
The findings of this post hoc analysis confirm that treatment-emergent somnolence can occur in patients with schizophrenia or BPD receiving treatment with asenapine. In the cohort of patients with short-term schizophrenia, the pattern of time to onset and duration of somnolence with asenapine was similar to those for olanzapine and haloperidol compared with risperidone, but only asenapine and olanzapine had significantly higher rates of somnolence compared with placebo. In the cohort with long-term schizophrenia or BPD, the patterns of time to onset, duration, and incidence of somnolence for asenapine and olanzapine were similar. This study confirms our previous finding that patients with bipolar mania are more sensitive than those with schizophrenia to the sedative effects of antipsychotics. Our data suggest that although olanzapine has the highest rate of somnolence, clinicians should be aware that somnolence can occur with asenapine when used in the treatment of patients with schizophrenia or BPD. A lower dose or slower titration may be necessary in patients with BPD. On the other hand, the early onset and limited duration of treatment-emergent somnolence associated with asenapine may be useful in certain clinical situations, such as agitation and/or insomnia.
Disclosure
KG was a consultant for Schering-Plough, has received grant support from AstraZeneca and NARSAD, and was on an advisory board for Schering-Plough and a speaker’s bureau for Pfizer. MM and AS are shareholders and/or full-time employees of Merck. PC and JZ were full time employees of Merck at the time of the research. Medical writing assistance was provided by Karen Pemberton PhD of PPSI (a PAREXEL company) and was funded by Merck.
References
- MeyerRESalzmanCYoungstromEASuicidality and risk of suicide – definition, drug safety concerns, and a necessary target for drug development: a consensus statementJ Clin Psychiatry2010718e1e2120797373
- GaoKGanocySJGajwaniPMuzinaDJKempDECalabreseJRA review of sensitivity and tolerability of antipsychotics in patients with bipolar disorder or schizophrenia: focus on somnolenceJ Clin Psychiatry200869230230918211129
- ShahidMWalkerGBZornSHWongEHAsenapine: a novel psychopharmacologic agent with a unique human receptor signatureJ Psychopharmacol2009231657318308814
- GerritsMGde GreefRDogteromPPeetersPAValproate reduces the glucuronidation of asenapine without affecting asenapine plasma concentrationsJ Clin Pharmacol201252575776521628604
- KaneJMCohenMZhaoJAlphsLPanagidesJEfficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophreniaJ Clin Psychopharmacol201030210611520520283
- KaneJMMackleMSnow-AdamiLZhaoJSzegediAPanagidesJA randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatmentJ Clin Psychiatry201172334935521367356
- PotkinSGCohenMPanagidesJEfficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trialJ Clin Psychiatry200768101492150017960962
- SchoemakerJNaberDVrijlandPPanagidesJEmsleyRLong-term assessment of asenapine versus olanzapine in patients with schizophrenia or schizoaffective disorderPharmacopsychiatry201043413814620205074
- McIntyreRSCohenMZhaoJAlphsLMacekTAPanagidesJA 3-week, randomized, placebo-controlled trial of asenapine in the treatment of acute mania in bipolar mania and mixed statesBipolar Disord200911767368619839993
- McIntyreRSCohenMZhaoJAlphsLMacekTAPanagidesJAsenapine versus olanzapine in acute mania: a double-blind extension studyBipolar Disord200911881582619832806
- McIntyreRSCohenMZhaoJAlphsLMacekTAPanagidesJAsenapine for long-term treatment of bipolar disorder: a double-blind 40-week extension studyJ Affect Disord2010126335836520537396
- McIntyreRSCohenMZhaoJAlphsLMacekTAPanagidesJAsenapine in the treatment of acute mania in bipolar I disorder: a randomized, double-blind, placebo-controlled trialJ Affect Disord20101221–2273820096936
- SzegediACalabreseJRStetLMackleMZhaoJPanagidesJAsenapine as adjunctive treatment for acute mania associated with bipolar disorder: results of a 12-week core study and 40-week extensionJ Clin Psychopharmacol2012321465522198448
- CitromeLAsenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychoticInt J Clin Pract200963121762178419840150
- BuchananRWPanagidesJZhaoJAsenapine versus olanzapine in people with persistent negative symptoms of schizophreniaJ Clin Psychopharmacol2012321364522198451
- SzegediAVerweijPvan DuijnhovenWMackleMCazorlaPFennemaHMeta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychoticsJ Clin Psychiatry201273121533154023290326
- MiettinenONurminenMComparative analysis of two ratesStat Med1985422132264023479
- SaidQGuttermanEMKimMSFirthSDWhiteheadRBrixnerDSomnolence effects of antipsychotic medications and the risk of unintentional injuryPharmacoepidemiol Drug Saf200817435436418314925
- BymasterFPCalligaroDOFalconeJFRadioreceptor binding profile of the atypical antipsychotic olanzapineNeuropsychopharmacology199614287968822531
- NicholsonANPascoePATurnerCSedation and histamine H1-receptor antagonism: studies in man with the enantiomers of chlorpheniramine and dimethindeneBr J Pharmacol199110412702761686208
- HarveyAGSchmidtDAScarnaASemlerCNGoodwinGMSleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problemsAm J Psychiatry20051621505715625201
- PalmeseLBDeGeorgePCRatliffJCInsomnia is frequent in schizophrenia and associated with night eating and obesitySchizophr Res20111331–323824321856129
- GaoKKempDEFeinENumber needed to treat to harm for discontinuation due to adverse events in the treatment of bipolar depression, major depressive disorder, and generalized anxiety disorder with atypical antipsychoticsJ Clin Psychiatry20117281063107121034695