Abstract
No studies have compared mirtazapine with duloxetine in patients with major depressive disorder (MDD). Fifty-six patients were nonrandomly assigned to a 4-week treatment with either 15 to 45 mg/day of mirtazapine (n = 22) or 20 to 60 mg/day of duloxetine (n = 34). The primary efficacy measurements were the Hamilton Rating Scale for Depression (HRSD) and the Montgomery–Åsberg Depression 6-point Rating Scale (MADRS) scores. The second efficacy measurements were the response and remission rates of treatment. Tolerability assessments were also performed. Fifty-six patients (43 male; age, 43.6 years) were recruited. There was no significant difference in the discontinuation rate between the mirtazapine and duloxetine treatment groups (P = 0.867). Both mirtazapine and duloxetine significantly improved the HRSD and MADRS scores from baseline (P < 0.0001–0.0004). While mirtazapine was superior to duloxetine in the reduction of HRSD scores (P = 0.0421), there was no significant change in MADRS scores in terms of between-group differences (P = 0.171). While more somnolence was observed with mirtazapine (P = 0.0399), more nausea was associated with duloxetine (P = 0.0089). No serious adverse events were observed for either antidepressant. Mirtazapine and duloxetine were safe and well-tolerated treatments for Japanese patients with MDD. Double-blind controlled studies are needed to further explore the efficacy and safety of mirtazapine and duloxetine in Japanese patients with MDD.
Introduction
In July 2009 and April 2010, mirtazapine and duloxetine, respectively, were approved as antidepressants in Japan for the management of major depressive disorder (MDD). Mirtazapine is classified as a noradrenergic and specific serotonergic antidepressant (NaSSA). NaSSAs act by antagonizing various adrenergic and serotonin receptors, which typically comprise α1-adrenergic and α2-adrenergic receptors and serotonin (5-HT)2A, 5-HT2C, and 5-HT3 receptors, respectively. By blocking α2-adrenergic autoreceptors and heteroreceptors, NaSSAs enhance adrenergic and serotonergic neurotransmission, notably 5-HT1A-mediated transmission.Citation1 Unlike many common antidepressants, NaSSAs have no efficacy as serotonin-reuptake inhibitors. According to a recent meta-analysis, while mirtazapine did not show a greater efficacious response rate or remission rate compared with tricyclic antidepressants (TCAs), mirtazapine was inferior to selective serotonin-reuptake inhibitors (SSRI) in terms of their response rate.Citation2 On the other hand, duloxetine is a well-known selective serotonin and noradrenaline-reuptake inhibitor (SNRI).Citation3 According to another recent meta-analysis, there was no significant difference in the efficacy or safety between duloxetine and SSRIs.Citation3 Conversely, the Meta-Analyses of New Generation Antidepressants (MANGA) study (a multiple treatment meta-analysis and statistical technique that allows for both direct and indirect comparisons even when two of the treatments have not been directly compared) reported that mirtazapine was significantly more efficacious than duloxetine.Citation4 However, there was no direct comparison between mirtazapine and duloxetine. The antidepressant effect of both drugs is considered to be due to increased serotonin and noradrenaline in the synaptic cleft. However, as stated above, the pharmacological action of both drugs is different. Therefore, we conducted a 4-week open-label, parallel-group clinical study involving direct comparisons of mirtazapine and duloxetine focusing on the clinical profile of the onset of action and tolerability in patients with MDD in routine practical clinical settings in Japan.
Method
Subjects
The present study was conducted from November 2010 to May 2012. Patients were recruited from a cohort of patients in Fujita Health University, The Jindai Hospital, The Okehazama Hospital, and The Toyota Memorial Hospital in Japan. The patients were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (fourth edition) criteria for MDD with the consensus of at least two experienced psychiatrists on the basis of an unstructured interview and a review of all medical records. Patients with bipolar disorder were excluded. All subjects met the following inclusion criteria: (1) a score of >15Citation5 on the Hamilton Rating Scale for Depression (HRSD) 5-point scale,Citation6 (2) age of 20 to 70 years, (4) no systemic or neurologic diseases, including disturbances of hematopoiesis, (5) no history of electroconvulsive therapy within 6 months prior to study enrollment, (6) not pregnant, and (7) no dependence on any substances other than nicotine during the 5 years before enrollment. No patient was excluded from the study because of a medical condition at baseline.
Each clinician explained the study purpose and design to patients eligible for this study, and informed consent was obtained from each patient before participating in the study. The trial was conducted in accordance with good clinical practices and the Helsinki Declaration. This study was approved by the ethics committees at Fujita Health University and the Jindai, Toyota Memorial, and Okehazama hospitals.
Procedures and evaluation of psychopathology, tolerability, and safety
This study was open-label and active-controlled. Patients were nonrandomly assigned to one of two treatment groups. Mirtazapine or duloxetine was taken once a day for 4 weeks. Mirtazapine and duloxetine were initiated at daily doses of 15 (at bedtime) and 20 mg/day (after breakfast), respectively. After 1 week, the dose was flexibly altered by each doctor based on the patient’s systemic clinical conditions and side effects at each visit (weeks 1, 2, and 4). The doses of mirtazapine and duloxetine were gradually increased to maximums of 45 and 60 mg, respectively, depending on the patient’s condition. The authorized usual daily doses of mirtazapine and duloxetine for patients with MDD in Japan are 15 to 45 mg and 20 to 60 mg, respectively. Therefore, these doses were used for this study. Patients with severe insomnia were prescribed zolpidem or brotizolam, but no other psychotropic drugs were permitted during the study. Zolpidem and brotizolam were used within the Japanese authorized dose ranges, ie, 5 to 10 mg for zolpidem and 0.25 mg for brotizolam at bedtime.
Patients were assessed for depressive symptoms with the HRSD and the Montgomery–Åsberg Depression 6-point Rating Scale (MADRS)Citation7 as primary efficacy measurements at baseline and at 1, 2, and 4 weeks. The second efficacy measurements were the treatment response rate and the remission rate. A clinical response was defined as a decrease of >50% in the baseline HRSD score within 4 weeks,Citation8 and clinical remission was defined as an HRSD score of <7 at 4 weeks.Citation8,Citation9 Patients’ systemic subjective clinical conditions and side effects were monitored by full physical examination including vital signs and general questioning regarding adverse events at each visit (weeks 1, 2, and 4). The clinical characteristics of the patients in this study, classified according to these definitions, are shown in .
Table 1 Participant characteristics at baseline
Statistical analysis
An intent-to-treat analysis was performed using the last-observation-carried-forward method. Subjects with at least two measurement points were entered into the study analyses. Each participant’s last observation was taken as the endpoint. The percentage change in the HRSD and MADRS scores from baseline to endpoint was not significantly different from a normal distribution according to the Shapiro–Wilk test. The paired Student’s t test was used to assess the significance of the change in psychopathology scores from baseline to endpoint in each treatment group. To determine which antidepressant was more effective for the psychopathology of the patients with MDD, we used linear regression to analyze the relationship between each antidepressant and changes in the final HRSD and/or MADRS total scores. For both mirtazapine and duloxetine, significant differences with HRSD or MADRS scores at baseline were detected (). Analyses for comparison between the treatment groups were conducted using the mixed-model repeated measures (MMRM) approach. For the MMRM analyses, changes from baseline at all postbaseline times were the dependent variables. Independent variables included treatment group, week, HRSD or MADRAS score at baseline, age at recruitment, sex, and the treatment-group-by-week interaction. The error matrix was unstructured. The analyses of the response and remission rates were performed using Fisher’s exact test. All statistical tests were carried out via JMP (JMP 5.0. 1 J; SAS Japan Inc, Tokyo, Japan), and all P values of <0.05 were considered statistically significant.
Results
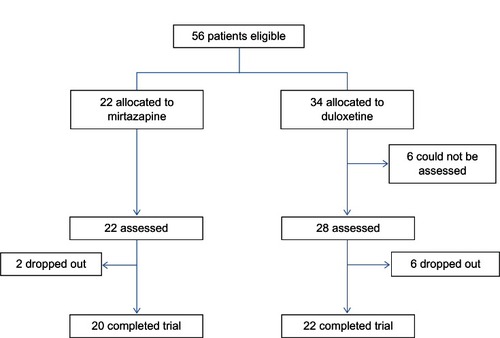
Patient demographics and distributions are presented in . Fifty-six patients (43 male; age, 43.6 years) were recruited for inclusion in the trial. There were significant differences in the HRSD and MADRS scores at baseline and the number of hospitalizations at baseline between the two treatment groups (). Six of 23 patients (26.1%) in the mirtazapine group and nine of 33 patients (28.1%) in the duloxetine group were dropped from the study because of any cause (). One of 22 patients (4.6%) in the mirtazapine group and three of 34 patients (8.8%) in the duloxetine group were dropped from the study because of inefficacy. Four of 22 patients (18.2%) in the mirtazapine group and four of 34 patients (11.8%) in the duloxetine group were dropped from the study because of side effects. However, there were no significant differences in the discontinuation rate due to any cause (P = 0.867), inefficacy (P = 0.485), and side effects (P = 0.662) between the groups. The mean mirtazapine and duloxetine doses at 4 weeks were 27 ± 10.4 and 37.6 ± 8.79 mg/day, respectively. Eleven patients (50.0%) in the mirtazapine group and 26 patients (76.5%) in the duloxetine group were taking sleeping pills during the trial (P = 0.0495).
Both mirtazapine and duloxetine significantly improved the HRSD and MADRS scores from baseline. While mirtazapine was superior to duloxetine in the reduction of HRSD scores (), there was no significant change in MADRS scores in terms of between-group differences (, ). In addition, there were no significant differences in the response rate (mirtazapine, 59.1%; duloxetine, 28.6%) or remission rate (mirtazapine, 31.8%; duloxetine, 10.7%) between the two groups ().
Figure 2 Change from baseline to weeks 1, 2, and 4 in the total scores of the Hamilton Rating Scale for Depression.
Abbreviation: MMRM, mixed-models repeated measures.
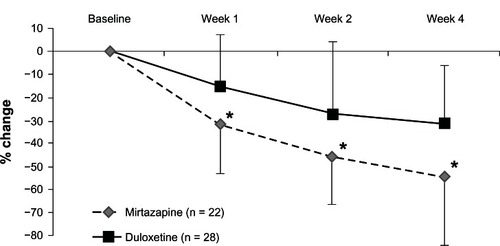
Figure 3 Change from baseline to weeks 1, 2, and 4 in the total scores of the Montgomery–Åsberg Depression Rating Scale.
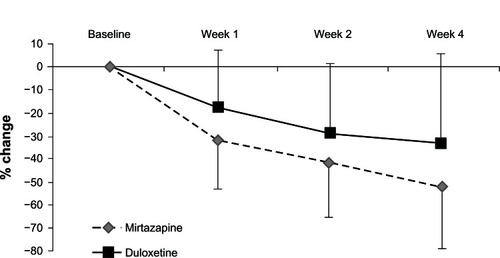
Table 2 Efficacy: change from baseline to weeks 1, 2, and 4 in the total scores of Hamilton Rating Scale for depression and Montgomery– Åsberg Depression Rating Scale, and response and remission rates
While more somnolence was observed with mirtazapine (P = 0.0399), more nausea was associated with duloxetine (P = 0.0089). We did not find any other differences in side effects, including insomnia, dry mouth, weight gain, and dizziness, between the treatment groups. No serious adverse events were observed for either medication.
Discussion
This is the first comparison between mirtazapine and duloxetine to focus on efficacy and safety in patients with MDD. Both antidepressants were beneficial and relatively well tolerated in patients with MDD in Japan. Unfortunately, there were significant differences in the HRSD and MADRS scores at baseline. Therefore, we controlled for these confounding factors by evaluating the change in HRSD and MADRS scores from baseline using MMRM. Although there was a difference in the number of patients who discontinued the trial between the two treatment groups, mirtazapine seemed to be more acceptable. While mirtazapine was superior to duloxetine in terms of the reduction in HRSD scores (), there was no significant change in MADRS scores in terms of between-group differences (). Because three of the 17 items of the HRSD concern sleep disturbances and contribute to up to 11.5% of the total score, it has been hypothesized that the HRSD may favor sedating antidepressant drugs (eg, mirtazapine).
The percentage of remitters, particularly in the duloxetine subgroup, was surprisingly low compared with that in other studies. In the Phase III study of duloxetine, the change in the HRSD score in the annexation analysis was −10.2 after 6 weeks of administration. On the other hand, after 4 weeks of administration, we believe that it was lower (−7.5) because the administration period was shorter. However, mirtazapine seems to be superior to duloxetine in terms of the response rate. A possible reason is that the antidepressant effect of mirtazapine may be faster than that of duloxetine ( and ).
Mirtazapine led to significantly greater somnolence than did duloxetine. The number of patients who took mirtazapine with sleeping pills was also marginally less than that in the duloxetine group. However, the reason for this was unclear. Likewise, a recent meta-analysis showed that mirtazapine caused more frequent sleepiness/drowsiness/somnolence compared with SSRIs, but not compared with TCAs and SNRIs.Citation2 Nausea occurred significantly more often with duloxetine than with mirtazapine. Another meta-analysis reported that nausea was the side effect most frequently associated with discontinuation of duloxetine treatment.Citation3 Clinicians must pay particular attention to physical findings such as drowsiness and nausea in these patients.
Compared with other similar studies,Citation2,Citation3 both antidepressants were beneficial and relatively well tolerated in patients with MDD in Japan. Although it is well known that the prevalence of MDD in females is higher than that in males, the prevalence in males was higher than that in females in the present study. The reason for this is unclear. These results may be confounded by the following limitations. One of the main limitations of this study is the small sample size. Another limitation of the present study is that it did not include a placebo control arm and was not randomized to ensure that patients and prescribers were blinded to the medication assignment. Finally, sleeping pills were allowed during the study. Although we controlled for several confounding factors using multiple regression analysis, we cannot exclude confounding effects that sleeping pills may have had on the psychopathology ratings in those patients. Mirtazapine and duloxetine were beneficial and relatively well tolerated in patients with MDD. Given the small sample size and lack of a placebo, double-blind, randomized controlled clinical trials should be conducted to further explore the utility of mirtazapine and duloxetine in patients with MDD.
Role of funding source
None. This study was conducted within clinical practices. No pharmaceutical industry provided the drugs to the patients.
Acknowledgments
We thank Ms Kitajima T, Ms Ino K, Ms Fukasawa T, Ms Okuda M, Ms Matsudaira Y, Ms Nobata R, Ms Ieda H, Ms Tanooka S, Dr Mekata T, Ms Goto S, Ms Nishizawa E, Ms Ando Y, Dr Okumura T, Ms Matsumoto Y, Ms Tani M, Ms Isogai S, Ms Niwa M, Ms Kato Y, Mr Tanaka N, Ms Miyako M, Ms Shibata E, Ms Hayakawa N, and Ms Adachi A for their technical support.
Disclosure
Drs Kishi and Iwata declare that they have no direct conflict of interest or grant support that is directly related to the content of the study. Dr Kishi has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer. Dr Iwata has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer. Drs Nagao, Funahashi, Hirano, and Fujita have nothing to disclose.
References
- CroomKFPerryCMPloskerGLMirtazapine: a review of its use in major depression and other psychiatric disordersCNS Drugs200923542745219453203
- WatanabeNOmoriIMNakagawaAMirtazapine versus other antidepressive agents for depressionCochrane Database Syst Rev201112CD00652822161405
- GartlehnerGThalerKHansenRAGaynesBNThe general and comparative efficacy and safety of duloxetine in major depressive disorder: a systematic review and meta-analysisDrug Saf200932121159117319916583
- CiprianiAFurukawaTASalantiGComparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysisLancet2009373966574675819185342
- CooksonJGilaberteIDesaiahDKajdaszDKTreatment benefits of duloxetine in major depressive disorder as assessed by number needed to treatInt Clin Psychopharmacol200621526727316877897
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry196023566214399272
- MontgomerySAAsbergMA new depression scale designed to be sensitive to changeBr J Psychiatry1979134382389444788
- KishiTKitajimaTIkedaMCLOCK may predict the response to fluvoxamine treatment in Japanese major depressive disorder patientsNeuromolecular Med2009112535719347611
- ThaseMEEntsuahARRudolphRLRemission rates during treatment with venlafaxine or selective serotonin reuptake inhibitorsBr J Psychiatry200117823424111230034