Abstract
Optimal treatment of oncologic pain is a challenge to all professionals who deal with cancer and its complications. The management of upper abdominal pain is usually difficult and it is often refractory to conservative therapies. In this context, celiac plexus neurolysis (CPN) appears to be an important and indispensable tool because it alleviates pain, gives comfort to patients and is a safe procedure. In this study, the importance of CPN is reviewed by a retrospective study of 74 patients with pain due to upper abdominal cancer. Almost all cases evaluated (94.6%) had an excellent result after CPN and the majority of side effects were transitory.
Introduction
Injections of neurolytic agents to destroy nerves and interrupt pain pathways have been used for several years.Citation1–Citation3 Celiac plexus neurolysis (CPN) is an ablative procedure of the celiac plexus (CP) that aims to destroy afferent pain transmitting fibers from abdominal viscera. It can be chemical, thermic or surgical, with the chemical method being limited to alcohol or phenol.
CPN is thought to be a safe and effective technique, indicated in patients with severe and intractable pain, in whom less aggressive maneuvers are ineffective or intolerable because of either poor physical condition or development of side effects.Citation4 The World Health Organization Cancer Pain Relief Program recommends CPN for pain relief in patients with upper abdominal cancer.Citation3–Citation6
The noteworthy adverse effects of alcohol neurolysis include regional pain, hypotension, diarrhea, hypoxemia, and acute alcoholic intoxication,Citation7,Citation8 most of them transient and controllable.
The CP is situated retroperitoneal in the upper abdomen at the level of the 12th thoracic and 1st lumbar vertebrae, anterior to the crura of the diaphragm. It surrounds the abdominal aorta, celiac and superior mesenteric arteries. The plexus is comprised of a network of nerve fibers, from both sympathetic and parasympathetic systems. It receives parasympathetic fibers from the vagus nerve and contains two large ganglia that receive sympathetic fibers from the three splanchnic nerves; the right ganglion is, on average, 0.6 cm inferior to the celiac artery, whereas the left is 0.9 cm inferior.
The neural information related to visceral pain is not carried on by sympathetic nerve fibers and these pain syndromes are not dependent on sympathetic activity within the CP. The afferent fibers that bring up visceral sensory information from the upper abdomen, including the pancreas, diaphragm, liver, spleen, stomach, bowel, proximal portion of the transverse and ascending colon, suprarenal glands, kidneys, abdominal aorta and mesentery are located within the plexus anatomical region.Citation4,Citation9 Impulses pass from the CP to the splanchnic nerves and enter the spinal cord from the 5th to the 9th thoracic segments.Citation10
Pain due to oncologic infiltration of somatic territories into the abdominal wall is not conducted through celiac plexus fibers and, therefore, cannot be treated by CP ablation.
Objective
This study aims to evaluate the importance of alcohol CPN in the treatment of visceral pain due to upper abdominal cancer.
Material and methods
We retrospectively reviewed 74 patients with upper abdominal cancer and visceral pain syndromes who were submitted to percutaneous alcohol CPN from June 1989 to March 2011. Patients were selected to the procedure while having disabling pain, refractory to optimized medication and to other nonsurgical procedures; all of them had advanced oncologic disease with life expectancy close to one year.
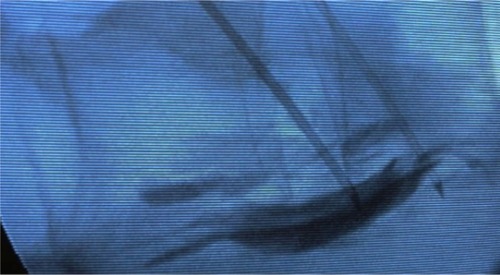
Celiac plexus neurolysis was first described by Ischia et al in 1919;Citation11 since then, several modifications have been proposed. Our practice was based on the description of percutaneous splanchnic nerve block technique: a patient under general anesthesia is positioned in the prone position and the skin is cleaned with antiseptics. Then percutaneous fluoroscopic guided bilateral puncture is performed with a needle, 7 centimeters lateral to the midline, 45 degrees in the coronal plane, and 15 degrees cephalic to the lateral portion of the L1 vertebral body. The tip of the needle is placed under lateral-lateral and antero-posterior fluoroscopic guide, 1 to 2 centimeters anterior to the L1 vertebral body. Aspiration is first performed to ensure that vascular puncture has not occurred, then 3 mL to 5 mL of contrast agent is injected to visualize by fluoroscopy the contrast diffusion to the retrocrural and retroaortic space, anterior and lateral to the superior lumbar and inferior thoracic vertebra. The injection of 20 mL to 50 mL of absolute alcohol under fluoroscopy finalizes the procedure ().
Figure 1 Fluoroscopic view of the spine in lateral position at the level of L1 with the insertion tubes in each side of the prevertebral space.
Response to treatment was evaluated up to 10 days after the procedure. Response was considered “bad” when pain persisted with no improvement, “good” when partial relief was achieved with improvement of quality of life, and “excellent” when pain was completely abolished.
Results
Patients were aged between 25 and 81 years old, with a mean age of 56.8 years. Thirty-nine of the 74 patients were male (52.7%), and thirty-five were female (47.3%). Seventy patients (94.6%) submitted to percutaneous alcohol CPN had an excellent response to treatment and four of them (5.4%) had a good response. The patients who had an excellent response to CPN did not need opioid medication and only stayed on lower doses of this medication for a few days in order to rule out the possibility of opioid withdrawal syndrome. The group of patients who had a good response to the CPN procedure still required some opioid and/or anti-inflammatory medication, but the analgesic medication became effective in controlling pain.
The majority of patients who underwent the procedure had uncontrolled disease and most of them experienced a progression of the disease afterwards. The CPN procedure aimed to improve the quality of life by reducing pain, and did not interfere with the disease process itself. Patients in this treatment group had a poor prognosis and low survival rate at the time of initiating the procedure. Theoretically, patients who experienced an improvement in their quality of life should respond better to treatment of their primary disease, but this fact was not really observed in this investigation.
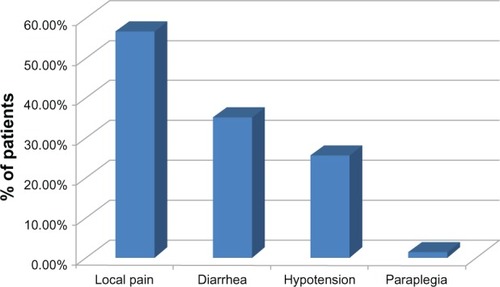
Complications related to the procedure were: transient pain in the puncture location in 42 patients (56.7%), transitory diarrhea in 26 patients (35.1%), and hypotension responsive to volume infusion in 19 patients (25.6%), see . No significant pleural effusion occurred in this study. One patient experienced permanent crural paraplegia (1.35%); this was the only severe complication in this trial. This patient was a 65-year-old with typical upper abdominal pain related to advanced pancreatic cancer. Around six months before the procedure the patient underwent local radiotherapy in order to control the cancer, using the same volume of alcohol that was used in the CPN procedure guided by fluoroscopy. The neurological examination revealed anterior spinal artery syndrome with severe neurological motor deficits, with no significant sensory loss in the same territory.
Discussion
The control of visceral pain related to abdominal cancer, especially in the upper abdomen remains a challenge. Pain control is one of the most important aspects of quality of life maintenance during the treatment of these cancer patients, who often are in an advanced stage of the disease, with a short life expectancy.Citation2,Citation10 Unfortunately, many patients have resistance to pain medication and side effects of opioids.Citation1–Citation3,Citation4
The celiac plexus has a diffuse anatomical structure and is composed of more than two ganglia, therefore regardless of the technique used, CPN may have a long-lasting benefit in 70% to 90% of patients with upper abdominal cancer,Citation8 appearing as a safe and cost-effective approach to treating visceral pain associated with cancer.Citation12,Citation13
Despite the World Health Organization Cancer Pain Relief Program recommending CPN as the most suitable intervention in a palliative setting, this procedure still carries some misconceptions by many physicians, withholding a potential improvement in the quality of life of many terminally ill patients.Citation14
Chemical ablative procedures of a nerve or a nerve group are preferred to other mechanical modalities in order to disrupt diffuse neural networks, such as the celiac plexus. Alcohol is preferred to phenol because of the perception that it leads to more complete ablation and avoids the potential mutagenic effects of phenol.Citation15
Lillemoe et alCitation2 concluded that CP alcohol neurolysis for irreversible abdominal pain from pancreatic cancer can provide significant analgesia for up to 6 months and improve survival (P < 0.0001). Wong et al,Citation10 in a prospective randomized trial proved this pain relief to be more effective than optimized analgesic therapy alone, however with no impact on life quality or survival.
Studies comparing percutaneous CPN with the use of opioids suggest that CPN results in a mild-to-moderate sustained reduction of pain in pancreatic cancer and an important decrease in opioid use, but does not eliminate the need for additional medication.Citation3,Citation4,Citation16,Citation17 Our study could also reproduce the good results found in the literature since all patients involved exhibited satisfactory control of pain.
Besides the fluoroscopic guided percutaneous celiac neurolysis, the computed tomography (CT) guided procedure can also be mentioned, also the intraoperative approach and the endoscopic ultrasound guided procedure (EUS). Yamamuro et alCitation14 state that there are no significant differences in efficacy between fluoroscopic and CT guided CPN, although the use of CT assures a correct needle tip placement.
Currently, there are few data about CPN under EUS guidance. However, the results are comparable to other conventional methods used to relieve pancreatic pain with neurolytic agent injections.Citation12,Citation19,Citation20
The transient adverse effects after alcohol CPN in this study were the same observed in correlated articles.Citation7,Citation10,Citation14,Citation21 Our one case of a severe complication (1.35%, crural paraplegia) was an unexpected event, nevertheless it is under the rate of 2% reported in the literature for neurological deficits after CPN.Citation14,Citation21 A possible hypothesis was the involvement of the Artery of Adamkiewicz either as a direct lesion or a severe arterial spasm responsible for an ischemic event in the spinal cord. This artery is the largest anastomotic segmental artery in the lumbar region responsible for the blood supply to the anterior portions of the terminal spinal cord. In 75% of people, the artery of Adamkiewicz originates on the left side of the aorta between the T8 and L1 vertebral segments.Citation22 Although, this a relatively rare event, similar cases were reported after each of the techniques: the posterior percutaneous under image guidance,Citation22,Citation24 the anterior open technique under direct viewCitation25,Citation26,Citation27 and the anterior endoscopic guided technique.Citation28
The benefits of CPN are very well known qualitatively, however quantitative studies are needed. Pain practitioners should consider the role of these blocks in adjuvant therapy for the optimal treatment of cancer pain.
Conclusion
Upper abdominal cancer causes severe pain in most patients and is often difficult to treat. Palliation of pain in these cases often requires a multidisciplinary approach, with options including oral analgesics, chemotherapy, radiotherapy, psychotherapy, nonsurgical procedures and CPN.
Timely interventional cancer pain therapies complement conventional pain management by reducing the need for high-dose opioid therapy and its associated toxicity.
In a seriously impaired cancer patient scenario CPN appears as an effective procedure because it relieves visceral pain, generally with no serious adverse effects and excellent results. This positive impact on quality of life after CPN is a central aspect of this procedure among other palliative care therapeutic options.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrownDLMooreDCThe use of neurolytic plexus block for pancreatic cancer: anatomy and techniqueJ Pain Symptom Manage198832062093192964
- LillemoeKDCameronJLKaufmanHSChemical splancnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trialAnn Surg19932174474577683868
- KawamataMIshitaniKIshikawaKComparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer painPain1996645976028783327
- PolatiEFincoGGottinLProspective randomized double-blind trial of neurolytic celiac plexus block in patients with pancreatic cancerBr J Surg1998851992019501815
- LankischPGNatural course of chronic pancreatitisPancreatology2001131412120264
- CaraceniAPortenoyRKPain management in patients with pancreatic carcinomaCancer1996786396538681303
- DaviesDDIncidence of major complications of neurolytic coeliac plexus blockJ R Soc Med1993862642668505748
- MooreJCAdlerDGCeliac plexus neurolysis for pain relief in pancreatic cancerJ Support Oncol200973889019507454
- PlancarteRVelasquezRPattRNeurolytic blocks of the sympathetic axisPrattRBPainPhiladelphiaLippincott Williams & Wilkins1993377
- WongGYSchroederDRCarnsPEEffect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trialJAMA20042911092109914996778
- IschiaSPolatiEFincoGGottinLCeliac block for the treatment of pancreatic painCurr Rev Pain20004212713310998724
- LevyMJTopazianMDWiersemaMJInitial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct ganglia neurolysis and blockAm J Gastroenterol200810319810317970834
- GressFSchmittCShermanSA prospective randomized comparison of endoscopic ultrasound and computed tomography-guided celiac plexus block for managing chronic pancreatitis painAm J Gastroenterol19999490090510201454
- YamamuroMKusakaKKatoMTakahashiMCeliac plexus block in cancer pain managementTohoku J Exp Med2000192111811128864
- WangYCLeeWHChenWYFuYMHistopathological examination of chemo-sympathectomy in catsNeurol Res200022442042410874694
- MercadanteSCeliac plexus block versus analgesics in pancreatic cancer painPain1993521871928455966
- YanBMMyersRPNeurolitic celiac plexus block for pain control in unresectable pancreatic cancerAM J Gastroenterol200710243043817100960
- IwataKYasudaIEnyaMPredictive factors for pain relief after ultrasound-guided celiac plexus neurolysisDig Endosc201123214014521429019
- WiersemaMUWiersemaLMEndosonography-guided celiac plexus neurolysisGastrointest Endosc1996446566628979053
- SakamotoHKitanoMKomakiTEndoscopic ultrasound-guided neurolysis in pancreatic câncerPancreatology201111Suppl 2S52S58
- EisenbergECarrDBChamlersTCNeurolytic celiac plexus block for treatment of cancer pain: a meta-analysisAnesth Analg19958022902957818115
- LazorthesGGouazeAZadehJOArterial vascularization of the spinal cord. Recent studies of the anastomotic substitution pathwaysJ Neurosurg197135325326222046635
- TakedaJNamaiHFukushimaKAnterior spinal artery syndrome after left celiac plexus blockAnesth Analg19968311781798659731
- HayakawaJKobayashiOMurayamaHParaplegia after intraoperative celiac plexus blockAnesth Analg19978424474489024045
- KinoshitaHDendaSShimojiKOhtakeMShiraiYParaplegia following coeliac plexus block by anterior approach under direct visionMasui19964510124412468937021
- AbdallaEKSchellSRParaplegia following intraoperative celiac plexus injectionJ Gastrointest Surg19993666867110554376
- KumarATripathiSSDharDBhattacharyaAA case of reversible paraparesis following celiac plexus blockReg Anesth Pain Med2001261757811172517
- FujiiLClainJEMorrisJMLevyMJAnterior spinal cord infarction with permanent paralysis following endoscopic ultrasound celiac plexus neurolysisEndoscopy201244E265E26622814912