Abstract
Purpose
The aim of our study was to explore the relation between serum levels of non-enzymatic antioxidants, thyroid function with the risk of non-suicidal self-injury (NSSI) in depressed adolescents.
Patients and Methods
We retrospected the electronic records of 454 hospitalized patients aged 13–17 years old with a diagnosis of major depressive disorder (239 patients with NSSI and 215 subjects without NSSI), and collected their demographic and clinical information, including serum levels of total bilirubin (Tbil), uric acid (UA), free triiodothyronine (FT3), free thyroxine (FT4) and thyroid stimulating hormone (TSH).
Results
The incidence of NSSI was 52.6% among depressed adolescents aged 13–17, 57.1% in female and 38.5% in male. After using the propensity scoring method to exclude the influence of age between the two groups, it was found that patients with NSSI showed lower levels of Tbil (P=0.046) and UA (P=0.015) compared with those without NSSI. Logistic regression results showed that serum UA was associated with NSSI behavior in female patients (OR=0.995, 95% CI: 0.991–0.999, P=0.014), and TSH was associated with NSSI in male participants (OR=0.499, 95% CI: 0.267–0.932, P=0.029).
Conclusion
Female and male may have different pathological mechanisms of NSSI. NSSI is more likely to be related to antioxidant reaction in female adolescent patients, while more likely to be related to thyroid function in male depressed adolescent patients.
Introduction
Non-suicidal self-injury (NSSI) means any deliberate action that leads to destruction of tissues without any intention of committing suicide.Citation1,Citation2 The prevalence of occasional NSSI in adolescents is 19.7% and the lifetime prevalence of NSSI is 27.6%.Citation3 One occurrence of NSSI behavior is likely to indicate that the behavior will happen again.Citation4 The number of adolescents who reported participating in repetitive NSSI was approximately 7.8%.Citation3 Adolescents with major depressive disorder (MDD) exhibited greater and more stable involvement in NSSI than non-MDD adolescents.Citation4 NSSI can also cause physical injuries such as bleeding, bruising and infection, and it also increases the risk of other high-risk behaviors, including suicide.Citation5–7
NSSI is influenced by many factors, such as psychosocial,Citation8 behavioral,Citation9 neurobiological and genetic factors.Citation10 Multiple studies focused on the psychosocial factors related to NSSI, such as adverse childhood events,Citation11,Citation12 bullying and media influence.Citation13 However, investigations focusing on biological factors possibly correlating to NSSI are few, as the mechanism of NSSI is still unclear. Neurobiological studies have suggested that the hypothalamic-pituitary-adrenal (HPA) axisCitation14,Citation15 and endogenous opioidsCitation16 probably participated the pathogenesis of NSSI.
Oxidative stress is important in clinical neuroscience, the brain is especially vulnerable to oxidative stress for its enormous consumption of oxygen, moderate antioxidant defense system and rich in lipid cosmetics.Citation17 The imbalance between reactive oxygen species and antioxidant defense leads to relaxation of brain function and abnormal neuronal signaling.Citation18 Oxidative stress played a key role in normal brain function and the pathogenesis of neuropsychiatric diseases such as depression,Citation19 bipolar disorderCitation20,Citation21 and Alzheimer’s disease.Citation22,Citation23 Previous studies have shown that serum bilirubinCitation24 and uric acid (UA)Citation25–27 could be involved in the pathophysiology process of depression by regulating oxidative stress. In addition, one study indicated patients with suicide attempts had higher levels of nitrogen oxides (products of nitrates and nitrites), lipid hydroperoxides (biomarkers of oxidative damage to lipids / lipid peroxidation) and lower TRAP (biomarkers of total antioxidant defense system) than those who had no history of suicide attempts.Citation28 Bartoli et al attempted to examine whether the level of UA associated with specific clinical and behavioral features in patients with mood disorders, though no behavioral/clinical features were found to be associated with changes in blood uric acid.Citation29
It is well established knowledge that the thyroid hormone plays a role in neuronal differentiation, migration, myelination and synaptic formation during brain development.Citation30 The link between thyroid function and MDD has long been recognized. Most of the existing studies have reported that the prevalence and incidence of hypothyroidism in patients with depression are higher than those within the general population.Citation31 The dysregulated levels of thyroid stimulating hormone (TSH), free triiodothyronine (FT3), and free thyroxine (FT4), even within the normal range, correlated with the severity of depression.Citation32,Citation33 Suicide attempts were associated with reduced activity in the hypothalamic-pituitary-thyroid (HPT) axis in depressed patients. Lack of TSH response to TRH can be a risk factor for suicideCitation34. The serum FT4 levels in individuals with depression or who had attempted suicide were lower than those who had not attempted suicide, while there was no significant variation in TSH and FT3 levels.Citation35 Mouri et al study focusing on thyrotropin receptor knockout murine models demonstrated phenotypes within murines such as hyperactivity, impulsiveness and increase in aggression.Citation36 It is generally considered that NSSI behaviour is impulsive.Citation37 However, the relationships between thyroid hormone and NSSI behavior in depressive patients have not been reported yet.
At present, a close relationship between antioxidant level and thyroid function had been reported.Citation38 Thyroid hormones regulate cellular oxidative stress through mitochondrial oxygen consumption. Changes in thyroid hormone levels lead to subtle variations in the degree of antioxidant enzyme presence, resulting in an imbalance for the removal of reactive oxygen species. From one perspective, hyperthyroidism can lead to increased oxidative metabolism and reduced glutathione peroxidase in rats, thus leading to increased lipid peroxidation level.Citation39 Following another perspective, hypothyroidism is related to increased reactive oxygen species production, enhanced oxidative stress, and then enhance lipid peroxidation.Citation40
In view of the lack of studies on biochemical and hormone levels related to NSSI, the present study investigated biological correlations between clinical biochemical indicators and NSSI, to explore the potential pathophysiological mechanisms associated with the occurrence and development of NSSI. Total bilirubin (Tbil) and UA are important endogenous antioxidants in the body,Citation41,Citation42 that can reflect the antioxidant capacity of individuals. FT3, FT4 and TSH are the main indicators reflecting thyroid function. Previous studies have confirmed that females are more likely to engage in NSSI behaviors than males.Citation43 Moreover, there are differences in the levels of antioxidants and thyroid function between males and females.Citation44–46 Therefore, we speculate that there are gender differences in the association between antioxidant stress system and thyroid function and adolescent depression. The results of this study are expected to enhance our understanding of the relationship between NSSI behavior and serum indicators of oxidative and thyroid hormone levels in adolescent depression, thereby improving the treatment of NSSI behavior associated with adolescent depression.
Materials and Methods
Patient Study Groups
We conducted a retrospective study that included 454 patients with MDD, with individual age ranging from 13 to 17 years old. All participants admitted to hospital between February 2016 and June 2022 from the In-patient Department of Psychiatry, University Town Hospital Affiliated to Chongqing Medical University. The diagnosis of MDD was determined in accordance with the International Statistical Classification of Diseases and Related Health Problems - 10th Revision (ICD-10) for depression by two experienced psychiatrists. Moreover, the 9-item Patient Health Questionnaire (PHQ-9) score of all patients was ≥10. Patients were excluded for the following reasons: co-diagnosis with kidney disease (chronic renal failure, nephritis, etc.), liver disease, allergic diseases (asthma), metabolic diseases (metabolic syndrome, dyslipidemia, gout, etc.), hypertension, thyroid dysfunction and other physical diseases, co-diagnosis for schizophrenia spectrum bipolar disorder, psychoactive substance use disorder and suicide attempts (self-harm behavior with the purpose of ending one’s life) history. All patients were diagnosed by two senior doctors.
This current study was conducted according to the Helsinki Declaration. In addition, our study was approved by the Ethics Committee of University Town Hospital Affiliated to Chongqing Medical University (LL-202161). As our study was a retrospective study, we had obtained permission from the Ethics Committee of University Town Hospital Affiliated to Chongqing Medical University to waive informed consent. All patient information included was strictly confidential.
Data Collection
The customized electronic report questionnaire was used for data collection. We collected sociodemographic and clinical information in the database of the Case-data Management Platform, including demographic characteristics, lifestyle and personal history. The database recorded the basic information, disease progress and treatment process for all patients. Data quality control was conducted by two trained researchers, and independently confirmed by two researchers. The calculation formula for Body mass index (BMI) is as follows: BMI = weight(kg)/height(m)2.
PHQ-9 was used to assess the severity of depressive symptoms.Citation47 The PHQ-9 consisted of 9 items, all of which were rated on a 4-point scale (0: not present; 3: almost every day). 7-item Generalized Anxiety Disorder scale (GAD-7) was used to assess the severity of anxiety symptoms.Citation48 The GAD-7 consisted of 7 items, all of which were rated on a 4-point scale (0: not present; 3: almost every day).
All personnel involved in blood collection had undergone standardized training. Blood collection was carried out according to standard specifications and procedures. All blood samples were collected between 06:30–07:00 after overnight fasting. Blood samples were sent to the laboratory for testing within 30 minutes after collection. UA and Tbil were measured by a Mindray BS-800® automatic biochemistry analyzer [mindray, made in Shenzhen, China]. Thyroid functions, including FT3, FT4 and TSH, were measured by the Roche® automatic electrochemical luminescence immune analyzer [Roche, made in Basel, Switzerland]. All tests were conducted by skilled doctors in the laboratory department according to standardized procedures. Finally, 454 antioxidant index data and 397 thyroid function data were collected.
Non-Suicidal Self-Injury Evaluation
In the present study, NSSI refers to behaviour involving self-injury with no suicidal intent.Citation1 Participants who had repeatedly committed self-harm in the year prior to admission were assigned to the “self-harm group” (MDD/NSSI), if they never did not assign to the “non-self-harm group” (MDD/non-NSSI).
Statistical Analysis
SPSS 26.0 ® was used for statistical analysis. The propensity scoring method (PSM) was used to eliminate age differences between the two groups. Enumeration data was expressed in percentiles (%) and measurement data was expressed in medians (IQR) or mean ± standard deviation (SD). Shapiro–Wilk test was used to conduct normality analysis. Difference between two groups of measurement data was determined by Mann–Whitney U-test or independent t test. Difference between two groups of counting data was assessed by Chi-square tests. Spearman correlation analysis was used to assess the correlation between thyroid function and antioxidant factors. Logistic regression was used to explore the relationship between antioxidant factor, thyroid function and NSSI. NSSI was as dependent variable, Tbil, UA, FT3, FT4, TSH as independent variables, respectively. The factors that may affect NSSI were controlled as covariates, and the variable selection adapted the enter method. Statistical significance was defined as p<0.05.
Results
Characteristics of Adolescent Non-Suicide Self-Injury Behavior
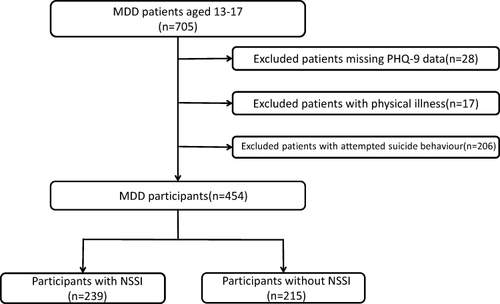
We identified 705 MDD patients aged 13–17 years old. We excluded 28 patients for lacked PHQ-9 score. We excluded 17 patients because they had physical diseases, such as hypertension, asthma, urticaria, thyroid disease, etc. In addition, we excluded 206 patients who had attempted suicide. Finally, a total of 454 MDD patients aged 13–17 years were included, including 239 patients with NSSI and 215 patients without NSSI (see ). lists the demographic data and clinical variations between MDD/NSSI group and MDD/non-NSSI group. Univariate analysis elucidated differences in age (P<0.001), gender (P=0.001), age of onset (P<0.001), antidepressant use (P<0.001), PHQ-9 score (P<0.001) and GAD-7 score (P=0.003) in the two groups. However, there was no significant difference in BMI (P=0.190), history of smoking (P=0.971), family history of mental illness (P=0.533) or duration of illness (P=0.252) between the two groups.
Table 1 Demographic and Clinical Characteristics of the Participants
The incidence of NSSI in adolescents with depression was 52.6%. There were 345 female MDD patients, 197 of whom had NSSI behaviour, and the incidence of NSSI in female adolescents with depression was 57.1%. In addition, there were 109 male MDD patients, 42 of whom had NSSI behavior and the incidence of NSSI in male adolescents with depression was 38.5%. The incidence of NSSI in female patients was significantly higher than that in male patients in this study (χ²=11.457, P=0.001) (See Figure S1).
As age was one of the main factors affecting non suicidal self injury, PSM was conducted between the two groups using age as a covariate. After PSM, there were 158 patients in each group. Differences were found only in gender (P=0.047), antidepressant use (P<0.001), PHQ-9 scores (P<0.001), and GAD-7 scores (P=0.011) between the two groups after PSM ().
In addition, considering the discrepancy in the incidence of NSSI between male and female patients, we conducted a stratified analysis based on the data after PSM.
In female participants, univariate analysis showed that there were differences in anti-depressant use (P=0.022), PHQ-9 score (P<0.001) and GAD-7 score (P=0.006) between the MDD/NSSI group and the MDD/non-NSSI group (See Table S1). In male MDD patients, univariate analysis elucidated that there was no significant differences in age, BMI, smoking, family history of mental illness, age of onset, duration of illness, anti-depressant use, PHQ-9 scores and GAD-7 scores between the two groups(P>0.05) (See Table S2).
The Relationship Between Antioxidant System and Thyroid Function and Non-Suicide Self-Injury Behavior in the Total Sample
Compared to MDD/non-NSSI group, the levels of serum Tbil (P=0.046) and UA (P=0.015) in the MDD/NSSI group were lower. However, there were no significant differences in the levels of FT3 (P=0.116), FT4 (P=0.213) and TSH (P=0.416) between the MDD/NSSI and MDD/non-NSSI group (see ).
Relativity analyses were employed to evaluate the relationship between antioxidant factor and thyroid function in patients. Datasets from total sample revealed a significant positive correlation between serum Tbil level and FT3 (r=0.127, P=0.034) and FT4 (r=0.364, P<0.001) levels. Serum UA was positively correlated with FT3 (r=0.155, P=0.010) and FT4 (r=0.170, P=0.005) levels. In MDD/non-NSSI group, serum Tbil was positively associated with FT4 (r=0.341, P<0.001). In MDD/NSSI group, serum Tbil was positively associated with FT4 (r=0.366, P<0.001). Serum UA was positively associated with FT3 (r=0.208, P=0.016) and FT4 (r=0.181, P=0.036)(See Table S3).
Binary logistic regression analysis was used to evaluate whether the antioxidant factors and thyroid function were associated with NSSI. It was found that serum UA (OR=0.997, 95% CI: 0.994–0.999, P=0.017) was associated with NSSI in patients with MDD. After adjusted gender, antidepressant use, PHQ-9 and GAD-7, no correlation was found between antioxidant factor, thyroid function and NSSI (Table S4).
The Relationship Between Antioxidant System and Thyroid Function and Non-Suicide Self-Injury Behavior in Female Participants
Compared to MDD/non-NSSI group, the levels of UA (P=0.026) in the MDD/NSSI group were significantly lower. However, the levels of Tbil (P=0.237), FT3 (P=0.179), FT4 (P=0.434) and TSH (P=0.755) did not significantly differ within the MDD/NSSI and the MDD/non-NSSI groups (See Figure S2).
Binary logistic regression analysis highlighted that serum UA was associated with NSSI in female patients with MDD (OR=0.996, 95% CI: 0.992–1, P=0.028). After adjusted antidepressant use, PHQ-9 and GAD-7, serum UA (OR=0.995, 95% CI: 0.991–0.999, P=0.014) was negatively associated with NSSI in female patients ().
Table 2 Binary Logistic Regression of Related Factors of NSSI in Female MDD Patients
The Relationship Between Antioxidant System and Thyroid Function and Non-Suicide Self-Injury Behavior in Male Participants
Compared to MDD/non-NSSI group, the levels of TSH (P=0.019) in the MDD/NSSI group was significantly lower. The serum levels of Tbil (P=0.509), UA (P=0.719), FT3 (P=0.791) and FT4 (P=0.519) did not show statistical significance within the MDD/NSSI and MDD/non-NSS group (See Figure S3).
Binary logistic regression analysis highlighted that the level of TSH was associated with NSSI in male patients with MDD (OR=0.499, 95% CI: 0.267–0.932, P=0.029) (see ).
Table 3 Binary Logistic Regression of Related Factors of NSSI in Male MDD Patients
Discussion
This is the first research to examine the relationship between serum non-enzymatic antioxidant levels, thyroid hormone and NSSI behavior in adolescents with MDD. We found that the incidence of NSSI behavior was 52.6% in depressive adolescents aged 13–17 years old. Among them, the female NSSI incidence is 57.1%, and the incidence of male NSSI is 38.5%. According to our findings, serum Tbil and UA levels in MDD participants with NSSI were significantly lower than MDD participants without NSSI. Controlling for confounding variables, these factors were not related to NSSI. In female participants, serum UA was associated with NSSI. In addition, serum TSH correlated with NSSI in male participants.
This study found that the incidence of NSSI in adolescents with depression was 52.6%, which is lower than that observed in other studies (about 70%) reporting the incidence of NSSI in inpatient samples.Citation49,Citation50 This may be because our focus was on NSSI behavior, and patients who had previously attempted suicide were excluded. Co-occurrence of NSSI and previous attempted suicide is highly likely.Citation51 In addition, in the present research, we found that NSSI associated with adolescent depression was more common in females rather than in males. A meta analysis including 116 articles revealed that a higher prevalence of NSSI in females,Citation43 but some studies believed there was no difference between the females and males.Citation52 The reasons for the inconsistency of the results could be related to the selected sample size, sample source and the inconsistency in the assessment of NSSI. Girls who self-injure were more likely to be hospitalized than boys because their injuries were sometimes taken more seriously than boys’.Citation43
Animal studies have revealed that psychomotor stimulants such as methamphetamineCitation53 and amphetamineCitation54 can cause intense dopamine release in mice, inducing the production of reactive oxygen species and reactive nitrogen, leading to oxidative stress and ultimately resulting in long-term neuronal damage and NSSI. However, dopamine receptor antagonistsCitation55 and free radical scavenging agentsCitation53 could significantly reduce methamphetamine-induced NSSI. Therefore, we speculated that due to the low antioxidant capacity of the body, reactions associated with oxidative stress may lead to the production of many harmful substances that cause nerve dysfunction, ultimately increasing the incidence of NSSI. Unfortunately, although our study found differences in Tbil and UA levels between the MDD/NSSI group and MDD/non-NSSI group, regression analysis did not show any association between them and NSSI after adjusting for confounding factors.
Our study investigated the correlation between thyroid hormone and non-enzymatic antioxidant serum levels, where we elucidated that UA is correlated to FT3 and FT4, together with Tbil being correlated to FT3 and FT4 in depressed patients. Oxidative stress is closely related to thyroid hormone serum level, as the former can affect thyroid hormone synthesis. The synthesis of thyroid hormones requires hydrogen peroxide (H2O2) as the substrate, so the production of reactive oxygen species (ROS) is a physiological requirement of thyroid hormones.Citation56,Citation57 Chao et al found a linear correlation between uric acid levels and FT3 and FT4 through the presence of normal thyroid function.Citation58 It is speculated that thyroid hormone can dysregulate cytokine levels produced by oxidative stress and inflammation. Dysregulated thyroid hormone levels lead to variations in the production of related cytokines and enzyme levels, and ultimately affect uric acid levels. The results of this investigation were consistent with previous studies.
UA is an important antioxidant factor, which can well reflect the antioxidant capacity. According to the results of our study, the serum level of UA is associated with NSSI in female adolescent MDD patients. In addition, our previous study has shown that lower serum uric acid levels have a significant relationship with female’s suicide risk.Citation59 Suicide and self-injury have common characteristics in some aspects. Therefore, we speculate that UA, an important antioxidant factor, may participate in the pathological mechanism of female suicide and self-injury at the same time. However, we did not observe this relationship in males. On the one hand, the relatively small proportion of male participants may be one of the reasons. On the other hand, differences in sex hormones between female and male may explain a part of this discrepancy. The level of UA in females is lower than that in males, because the sex hormone estrogen reduces the level of UA by increasing its excretion of UA and reducing the production simultaneously,44 but testosterone has been proven to grow the level of UA through increasing its renal reabsorption.Citation60 Individuals who have low level of UA may have disappeared some of the antioxidant protection from the UA. Furthermore, a research found that the treatment for UA can improve clinical prognosis in female stroke patients.Citation61 All of these suggest that serum level of UA might be an important clinical indicator of antioxidant stress in females.
The present study also found that NSSI behavior was related to TSH level in male patients with MDD, although we did not find this association in females with MDD. One possible reason is that men and women have different levels of TSH. Researches reported that the serum TSH level in males was lower than females.Citation45,Citation46 This gender difference in TSH levels may be due to males being more blunted in response to TRH stimulation.Citation62 In addition, a study reported that the level of TSH is negatively correlated with male depression.Citation63 To sum up, serum TSH level may be an important clinical indicator related to male NSSI behaviour.
The limitations of the current study should be noted. Firstly, this is a retrospective study, therefore no causal deductions can be made. Secondly, the results of this study was limited by the recall bias of NSSI over the past 12 months. Future research on the relative changes of NSSI can overcome this issue by using techniques such as ecological momentary assessment.Citation64 Thirdly, only peripheral blood antioxidant system was analyzed in this study. Although peripheral blood can reflect the antioxidant capacity of the central system to a certain extent, this contribution is still limited since the central and peripheral antioxidant systems are not completely consistent. Forthly, only TSH/FT3/FT4 was detected, thyroxine-binding globulin (TBG) was not detected. In further studies, we can detect the level of TBG and conduct further research. Fifthly, in essence the etiology of NSSI behavior is multi-factorial in nature, encompassing complex interactions between psychological, environmental and biological factors. Consequently, it is difficult to unravel fully the knowledge concerning the development of NSSI behavior by investigating one isolated factor - future studies need to integrate multi-factorial analyses. Sixthly, only MDD patients were analyzed in this study and lack of data for healthy controls. In future studies, it is essential to add the normal control group and conduct a cohort study to confirm our findings.
Conclusion
Collectively, we found that the incidence of NSSI was higher in female adolescents with depression than that in male adolescents with depression. Serum UA is associated with female NSSI behavior, and serum TSH is associated with male NSSI behavior. This suggests that there are complex pathophysiological mechanisms in adolescents’ NSSI behavior, which may be related to gender. Females may be involved in antioxidant defense system disorders, and males may be involved in thyroid dysfunction. In future clinical practice and scientific research, we should pay attention to the different effects of gender differences in NSSI behavior. In future study, we need a large sample of prospective research design to further prove our findings. It is also necessary to conduct in-depth basic research to explore the exact pathological mechanism of NSSI in different genders.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Thanks to all those who participated in this study.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Additional information
Funding
References
- Zetterqvist M. The DSM-5 diagnosis of nonsuicidal self-injury disorder: a review of the empirical literature. Child Adolesc Psychiatr Ment Health. 2015;9:31. doi:10.1186/s13034-015-0062-7
- Bl Z, Ym X, Jh Z, Xj L. Non-suicidal self-injury in Chinese heroin-dependent patients receiving methadone maintenance treatment: prevalence and associated factors. Drug Alcohol Depend. 2018;185:189. doi:10.1016/j.drugalcdep.2017.12.014
- Brunner R, Kaess M, Parzer P, et al. Life-time prevalence and psychosocial correlates of adolescent direct self-injurious behavior: a comparative study of findings in 11 European countries. J Child Psychol Psychiatr. 2014;55(4):337–348. doi:10.1111/jcpp.12166
- Barrocas AL, Giletta M, Hankin BL, Prinstein MJ, Abela JRZ. Nonsuicidal self-injury in adolescence: longitudinal course, trajectories, and intrapersonal predictors. J Abnorm Child Psychol. 2015;43(2):369–380. doi:10.1007/s10802-014-9895-4
- Ribeiro JD, Franklin JC, Fox KR, et al. Self-injurious thoughts and behaviors as risk factors for future suicide ideation, attempts, and death: a meta-analysis of longitudinal studies. Psychol Med. 2016;46(2):225–236. doi:10.1017/S0033291715001804
- Andover MS, Pepper CM, Ryabchenko KA, Orrico EG, Gibb BE. Self-mutilation and symptoms of depression, anxiety, and borderline personality disorder. Suicide Life-Threatening Behav. 2005;35(5):581–591. doi:10.1521/suli.2005.35.5.581
- Bl Z, Ym X, Wx X, Wc C, L J. Prevalence of deliberate self-harm among Chinese patients with heroin dependence: a meta-analysis. Frontiers in Psychiatry. 2018;9:1.
- Isohookana R, Riala K, Hakko H, Räsänen P. Adverse childhood experiences and suicidal behavior of adolescent psychiatric inpatients. Eur Child Adolesc Psychiatry. 2013;22(1):13–22. doi:10.1007/s00787-012-0311-8
- Lockwood J, Daley D, Townsend E, Sayal K. Impulsivity and self-harm in adolescence: a systematic review. Eur Child Adolesc Psychiatry. 2017;26(4):387–402. doi:10.1007/s00787-016-0915-5
- Hankin BL, Barrocas AL, Young JF, Haberstick B, Smolen A. 5-HTTLPR×interpersonal stress interaction and nonsuicidal self-injury in general community sample of youth. Psychiatry Res. 2015;225(3):609–612. doi:10.1016/j.psychres.2014.11.037
- Muehlenkamp JJ, Kerr PL, Bradley AR, Adams Larsen M. Abuse subtypes and nonsuicidal self-injury: preliminary evidence of complex emotion regulation patterns. J Nerv Ment Dis. 2010;198(4):258–263. doi:10.1097/NMD.0b013e3181d612ab
- Yates TM, Carlson EA, Egeland B. A prospective study of child maltreatment and self-injurious behavior in a community sample. Dev Psychopathol. 2008;20(2):651–671. doi:10.1017/S0954579408000321
- Lewis SP, Mahdy JC, Michal NJ, Arbuthnott AE. Googling Self-injury: the state of health information obtained through online searches for self-injury. JAMA Pediatr. 2014;168(5):443–449. doi:10.1001/jamapediatrics.2014.187
- Beauchaine TP, Crowell SE, Hsiao RC. Post-dexamethasone cortisol, self-inflicted injury, and suicidal ideation among depressed adolescent girls. J Abnorm Child Psychol. 2015;43(4):619–632. doi:10.1007/s10802-014-9933-2
- Kaess M, Hille M, Parzer P, Maser-Gluth C, Resch F, Brunner R. Alterations in the neuroendocrinological stress response to acute psychosocial stress in adolescents engaging in nonsuicidal self-injury. Psychoneuroendocrinology. 2012;37(1):157–161. doi:10.1016/j.psyneuen.2011.05.009
- Kirtley OJ, O’Carroll RE, O’Connor RC. The role of endogenous opioids in non-suicidal self-injurious behavior: methodological challenges. Neurosci Biobehav Rev. 2015;48:186–189. doi:10.1016/j.neubiorev.2014.11.007
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876. doi:10.1017/S1461145707008401
- Stefanatos R, Sanz A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018;592(5):743–758. doi:10.1002/1873-3468.12902
- Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25(7):1270–1276. doi:10.1016/j.drudis.2020.05.001
- Bortolasci CC, Voigt C, Turner A, et al. Interleukin-6 and total antioxidant capacity levels following N-acetylcysteine and a combination nutraceutical intervention in a randomised controlled trial for bipolar disorder. Acta Neuropsychiatr. 2020;32(6):313–320. doi:10.1017/neu.2020.25
- Jiménez-Fernández S, Gurpegui M, Garrote-Rojas D, Gutiérrez-Rojas L, Carretero MD, Correll CU. Oxidative stress parameters and antioxidants in patients with bipolar disorder: results from a meta-analysis comparing patients, including stratification by polarity and euthymic status, with healthy controls. Bipolar Disord. 2021;23(2):117–129. doi:10.1111/bdi.12980
- Kim JH, Meng HW, He MT, Choi JM, Lee D, Cho EJ. Krill oil attenuates cognitive impairment by the regulation of oxidative stress and neuronal apoptosis in an amyloid β-induced alzheimer’s disease mouse model. Molecules. 2020;25(17):3942. doi:10.3390/molecules25173942
- Saleem U, Sabir S, Niazi SG, Naeem M, Ahmad B. Role of Oxidative Stress and Antioxidant Defense Biomarkers in Neurodegenerative Diseases. Crit Rev Eukaryot Gene Expr. 2020;30(4):311–322. doi:10.1615/CritRevEukaryotGeneExpr.2020029202
- Peng YF, Xiang Y, Wei YS. The significance of routine biochemical markers in patients with major depressive disorder. Sci Rep. 2016;6:34402. doi:10.1038/srep34402
- Wen S, Cheng M, Wang H, et al. Serum uric acid levels and the clinical characteristics of depression. Clin Biochem. 2012;45(1–2):49–53. doi:10.1016/j.clinbiochem.2011.10.010
- Meng X, Huang X, Deng W, Li J, Li T. Serum uric acid a depression biomarker. PLoS One. 2020;15(3):e0229626. doi:10.1371/journal.pone.0229626
- Chaudhari K, Khanzode S, Khanzode S, Dakhale G, Saoji A, Sarode S. Clinical correlation of alteration of endogenous antioxidant-uric acid level in major depressive disorder. Indian J Clin Biochem. 2010;25(1):77–81. doi:10.1007/s12291-010-0016-z
- Vargas HO, Nunes SOV, Pizzo de Castro M, et al. Oxidative stress and lowered total antioxidant status are associated with a history of suicide attempts. J Affect Disord. 2013;150(3):923–930. doi:10.1016/j.jad.2013.05.016
- Bartoli F, Crocamo C, Bava M, et al. Testing the association of serum uric acid levels with behavioral and clinical characteristics in subjects with major affective disorders: a cross-sectional study. Psychiatry Res. 2018;269:118–123. doi:10.1016/j.psychres.2018.08.039
- Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3(3):249–259. doi:10.1038/ncpendmet0424
- Wu EL, Chien IC, Lin CH, Chou YJ, Chou P. Increased risk of hypothyroidism and hyperthyroidism in patients with major depressive disorder: a population-based study. J Psychosom Res. 2013;74(3):233–237. doi:10.1016/j.jpsychores.2012.12.016
- Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20(10):1101–1114. doi:10.1111/j.1365-2826.2008.01774.x
- Feng G, Kang C, Yuan J, et al. Neuroendocrine abnormalities associated with untreated first episode patients with major depressive disorder and bipolar disorder. Psychoneuroendocrinology. 2019;107:119–123. doi:10.1016/j.psyneuen.2019.05.013
- Duval F, Mokrani MC, Erb A, Gonzalez Opera F, Calleja C, Paris V. Relationship between chronobiological thyrotropin and prolactin responses to protirelin (TRH) and suicidal behavior in depressed patients. Psychoneuroendocrinology. 2017;85:100–109. doi:10.1016/j.psyneuen.2017.07.488
- Peng R, Dai W, Li Y. Low serum free thyroxine level is correlated with lipid profile in depressive patients with suicide attempt. Psychiatry Res. 2018;266:111–115. doi:10.1016/j.psychres.2018.05.059
- Mouri A, Hoshino Y, Narusawa S, et al. Thyrotoropin receptor knockout changes monoaminergic neuronal system and produces methylphenidate-sensitive emotional and cognitive dysfunction. Psychoneuroendocrinology. 2014;48:147–161. doi:10.1016/j.psyneuen.2014.05.021
- Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339–363. doi:10.1146/annurev.clinpsy.121208.131258
- Poncin S, Gérard AC, Boucquey M, et al. Oxidative stress in the thyroid gland: from harmlessness to hazard depending on the iodine content. Endocrinology. 2008;149(1):424–433. 1. doi:10.1210/en.2007-0951
- Asayama K, Dobashi K, Hayashibe H, Megata Y, Kato K. Lipid peroxidation and free radical scavengers in thyroid dysfunction in the rat: a possible mechanism of injury to heart and skeletal muscle in hyperthyroidism. Endocrinology. 1987;121(6):2112–2118. doi:10.1210/endo-121-6-2112
- Resch U, Helsel G, Tatzber F, Sinzinger H. Antioxidant Status in Thyroid Dysfunction. Walter de Gruyter. 2002;40(11):1132–1134. doi:10.1515/CCLM.2002.198
- Lu Z, Wen T, Wang Y, Kan W, Xun G. Peripheral non-enzymatic antioxidants in patients with schizophrenia: a case-control study. BMC Psychiatry. 2020;20(1):241. doi:10.1186/s12888-020-02635-8
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–6862. doi:10.1073/pnas.78.11.6858
- Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55–64. doi:10.1016/j.cpr.2015.02.009
- Chu X, Zhang Y, Deng C, Zeng X. Estrogen and hyperuricemia. Chin J Clin Immunol All. 2019;13(05):400–405. In Chinese.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): national Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. doi:10.1210/jcem.87.2.8182
- Zhou Y, Ma Y, Wu Q, et al. Comparison of thyroid hormone levels between patients with major depressive disorder and healthy individuals in China. Front Psychiatry. 2021;12:750749. 9. doi:10.3389/fpsyt.2021.750749
- L W, Bl Z, Hf C. Prevalence of depressive symptoms among Chinese university students amid the COVID-19 pandemic: a systematic review and meta-analysis. Epidemiol Psychiat Sci. 2021;30.
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi:10.1001/archinte.166.10.1092
- Thomassin K, Guérin Marion C, Venasse M, Shaffer A. Specific coping strategies moderate the link between emotion expression deficits and nonsuicidal self-injury in an inpatient sample of adolescents. Child Adolesc Psychiatr Ment Health. 2017;11(1):21. doi:10.1186/s13034-017-0158-3
- Millon EM, Alqueza KL, Kamath RA, et al. Non-suicidal self-injurious thoughts and behaviors among adolescent inpatients. Child Psychiatry Hum Dev. 2024;55(1):48–59. doi:10.1007/s10578-022-01380-1
- Ye Z, Xiong F, Li W. A meta-analysis of co-occurrence of non-suicidal self-injury and suicide attempt: implications for clinical intervention and future diagnosis. Front Psychiatry. 2022;13:976217. doi:10.3389/fpsyt.2022.976217
- Cerutti R, Manca M, Presaghi F, Gratz KL. Prevalence and clinical correlates of deliberate self-harm among a community sample of Italian adolescents. J Adolesc. 2011;34(2):337–347. doi:10.1016/j.adolescence.2010.04.004
- Mori T, Ito S, Kita T, et al. Oxidative stress in methamphetamine-induced self-injurious behavior in mice. Behav Pharmacol. 2007;18(3):239–249. doi:10.1097/FBP.0b013e328153dae1
- Kita T, Miyazaki I, Asanuma M, Takeshima M, Wagner GC. Dopamine-induced behavioral changes and oxidative stress in methamphetamine-induced neurotoxicity. Int Rev Neurobiol. 2009;88:43–64.
- Mori T, Ito S, Kita T, Sawaguchi T. Effects of dopamine- and serotonin-related compounds on methamphetamine-induced self-injurious behavior in mice. J Pharmacol Sci. 2004;96(4):459–464. doi:10.1254/jphs.FPJ04040X
- Denef JF, Many MC, van den Hove MF. Iodine-induced thyroid inhibition and cell necrosis: two consequences of the same free-radical mediated mechanism? Molec Cell Endocrinol. 1996;121(1):101–103. doi:10.1016/0303-7207(96)03848-8
- Song Y, Driessens N, Costa M, et al. Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab. 2007;92(10):3764–3773. doi:10.1210/jc.2007-0660
- Chao G, Zhu Y, Fang L. Retrospective analysis of the correlation between uric acid and thyroid hormone in people with normal thyroid function. J Diabetes Res. 2019;2019:5904264. doi:10.1155/2019/5904264
- Kong Y, Liu C, Zhang C, et al. Association between serum uric acid levels and suicide attempts in adolescents and young adults with major depressive disorder: a retrospective study. Neuropsychiatr Dis Treat. 2022;18:1469–1477. doi:10.2147/NDT.S368471
- Li T, Walsh JR, Ghishan FK, Bai L. Molecular cloning and characterization of a human urate transporter (hURAT1) gene promoter. Biochim Biophys Acta. 2004;1681(1):53–58. doi:10.1016/j.bbaexp.2004.10.001
- Llull L, Amaro S, Chamorro Á. Response to letter regarding article, “uric acid therapy improves clinical outcome in women with acute ischemic stroke. Stroke. 2015;46(11):e242.
- Hatterer JA, Herbert J, Hidaka C, Roose SP, Gorman JM. CSF transthyretin in patients with depression. Am J Psychiatry. 1993;150(5):813–815.
- Kritz-Silverstein D, Schultz ST, Palinska LA, Wingard DL, Barrett-Connor E. The association of thyroid stimulating hormone levels with cognitive function and depressed mood: the Rancho Bernardo study. J Nutr Health Aging. 2009;13(4):317–321. doi:10.1007/s12603-009-0029-6
- Andrewes HE, Hulbert C, Cotton SM, Betts J, Chanen AM. Ecological momentary assessment of nonsuicidal self-injury in youth with borderline personality disorder. Personal Disord. 2017;8(4):357–365. doi:10.1037/per0000205