Abstract
There have been significant advances in the treatment of psychiatric disease in the last half century, but it is still unclear which neural circuits are ultimately responsible for specific disease states. Fortunately, technical limitations that have constrained this research have recently been mitigated by advances in research tools that facilitate circuit-based analyses. The most prominent of these tools is optogenetics, which refers to the use of genetically encoded, light-sensitive proteins that can be used to manipulate discrete neural circuits with temporal precision. Optogenetics has recently been used to examine the neural underpinnings of both psychiatric disease and symptom relief, and this research has rapidly identified novel therapeutic targets for what could be a new generation of rational drug development. As these and related methodologies for controlling neurons ultimately make their way into the clinic, circuit-based strategies for alleviating psychiatric symptoms could become a remarkably refined approach to disease treatment.
Introduction
Within the last half century, there has been remarkable progress in the treatment of psychiatric disease. The first chemical antidepressants were discovered fortuitously in clinical trials designed to treat tuberculosis.Citation1 Since then, researchers in both academia and industry have developed an impressive array of related compounds, which work through similar mechanisms but treat a host of different psychiatric conditions.Citation2 These developments have led to an abundance of pharmacotherapies that have dramatically increased the number of available treatment options for psychiatrists. Unfortunately, currently available drugs still produce numerous deleterious side effects and, for a substantial number of patients, are largely ineffective in alleviating their disease symptoms. Consequently, there remains a strong need for more effective, targeted pharmacotherapies to treat psychiatric disease.
A better understanding of the neurobiological processes that underlie psychiatric disease states is expected to help drug development efforts. Accordingly, there has been a considerable effort to identify biological variables that consistently differ between healthy individuals and those with disease.Citation3 Studies in humans have implicated various circuits in specific psychiatric disease states using neuroimaging, genomic, and post mortem tissue analyses.Citation4 However, data produced from these studies are largely post hoc in nature and cannot be used to conclusively infer cause and effect. The attribution of causality to specific neuronal circuitry or reliable biomarkers has been hampered by technological limitations and overall etiological complexities.Citation5 In order to determine if a specific biological process is integral to a psychiatric condition, it must be experimentally manipulated in preclinical models of the disease. To this end, numerous animal models of psychiatric disease states have been developed.Citation6
One approach to modeling psychiatric disease in an animal is to use a behavioral task in which an animal’s performance is sensitive to drug treatments that have already proved to be effective in humans.Citation7 When new drugs produce behavioral effects that are similar to those elicited with proven pharmacotherapies, they are likely to have similar mechanisms of action to the old drugs and be similarly effective in treating the disease. This strategy provides strong predictive validity in modeling treatment of disease states and has been successfully used to screen novel compounds. However, its utility may be limited to compounds that act via the same mechanisms as those used to initially develop the behavioral model. An additional caveat to this line of research is that predictive validity in modeling treatment does not necessarily mean that the etiology or mechanisms underlying the disease in humans are relevant to the given model. Thus, while animal models of psychiatric disease offer a powerful approach to understand symptomatology, they may be less able to provide information about the neural processes that are ultimately responsible for the initiation of disease states.
The unclear neuronal substrates of psychiatric diseases and correspondingly slow progress of novel drug development have resulted in pharmaceutical companies recently abandoning development of psychiatric compounds.Citation8 To reinvigorate research, it may be necessary to take a more refined technological approach to the study of disease symptoms and treatment. The maladaptive behaviors associated with complex psychiatric disorders are likely the result of specific aberrant processes in discrete, albeit multiple, neural circuits. The development of optogenetics has provided researchers with a powerful set of tools to manipulate neural activity in genetically defined populations of cells in a pathway-specific manner.Citation9 In applying optogenetic strategies to established animal models of psychiatric disease, investigators have made rapid progress in defining the neural circuitry responsible for both psychiatric disease and symptom relief.
Optogenetic tools
Optogenetic approaches enable researchers to activate or inhibit groups of neurons, which can be defined by genetic identity and/or projection target. This is made possible through the targeted expression of light-sensitive proteins known as rhodopsins, which are responsive to specific wavelengths of light. Rhopdopsins are protein complexes that contain an opsin (channel) and a light-sensitive cofactor (retinal).Citation10 The opsins used in optogenetic strategies are generally derived from the type 1 class of prokaryotic rhodopsin proteins that support a covalent bond with an internalized retinal molecule.Citation11 The ionic environment around this covalent bond influences the spectral sensitivity of the protein, whereas the amino acid residues around it play a significant role in channel kinetics.Citation12 Photon absorption causes a conformational change in the rhodopsin protein, via retinal isomerization, which allows for ion translocation across the channel.
Opsins are broadly categorized based on their depolarizing or hyperpolarizing effects. Depolarizing opsins, or channelrhodopsins (ChRs), permit the translocation of cations into the intracellular space upon photon absorption and retinal isomerization. Channelrhodopsin-1 (ChR1) was the first of this type to be characterized, but it produces relatively small photocurrents and is not widely used.Citation13 Channelrhodopsin-2 (ChR2) has a higher photocurrent than its predecessor and has proved to be quite effective at altering neuronal firing patterns in mammalian tissue.Citation14,Citation15 It is maximally activated by 470 nm wavelength light and can sustain reliable firing up to 40 Hz. Multiple variants of ChR1 and ChR2 have recently been developed through point mutations and chimeric complexes in an attempt to improve or alter ion permeability (CatCH), channel kinetics (ChETA), and wavelength selectivity (C1V1)Citation12,Citation16 (see for a list of notable variants). The creation of red-shifted opsins, such as C1V1, has enabled independent excitation of two depolarizing opsins in the same tissue.Citation17,Citation18 In addition, longer wavelengths mitigate light scatter and absorption, thus promoting deeper tissue penetration.Citation19
Table 1 Summary of noteworthy opsin proteins commonly used in optogenetic research
In general, the photocurrent generated from opsin activation decays quickly following light cessation. This feature is an asset for most experimental designs, but certain behavioral paradigms benefit from prolonged manipulations. ChR2 variants known as stabilized-step function opsins significantly extend the open-state of the channel from a single pulse of blue light, which promotes a sustained depolarized state. This state increases the likelihood of firing in response to endogenous input, and can be inactivated with a single pulse of yellow light.Citation20 Recent step function opsin variants can induce depolarized states that last as long as 30 minutes following a single activating pulse of light.Citation21
Hyperpolarizing opsins, in contrast, are more limiting in both their overall characteristics and variety. Most hyperpolarizing opsins have an activation profile that is significantly red-shifted in comparison with the standard depolarizing ChRs. Potent inhibition can be achieved through the pumping of chloride ions into the neuron through halorhodopsin (eg, eNpHR3.0Citation22) or via expelling protons from the intracellular space using archaerhodopsin (eg, eArch3.0Citation16). However, despite their effectiveness, pumps are not energetically efficient, owing to their one proton to one ion photocycle,Citation23 and do not alter the cell’s input resistance.Citation24 Additionally, sustained halorhodopsin activation can disrupt chloride gradients, leading to a change in gamma-aminobutyric acid type A (GABAA) receptor reversal potential, thus eliciting a post-inhibitory excitatory state.Citation25 Recently, however, it has been shown that point mutations in a chimeric ChR protein can convert it into a chloride conducting channel.Citation26,Citation27 The slow mutant variant, known as slow ChloC, possess a 10-second long open-state during which neurons are effectively silenced.Citation26 Longer periods of neuronal inhibition can be achieved with a pharmacogenetic approach that utilizes designer receptors that are exclusively activated by designer drugs.Citation28
In addition to the advances in opsin-mediated excitatory and inhibitory neuronal activity, there has been success in engineering chimeric opsin proteins that directly alter intracellular signaling cascades through G-protein coupled receptors.Citation29 This class of proteins are known as OptoXRs, and they have been designed to incorporate bovine rhodopsin (type II opsin) into endogenously expressed adrenergicCitation30 and serotonergicCitation31 receptors. As a result of these developments, optogenetics provides a temporally refined and multifaceted toolkit of opsins allowing various controls on neuronal activity within genetically defined neuronal cell types.
To achieve selective opsin expression, DNA constructs encoding the opsins, often fused with a fluorescent protein marker (eg, yellow fluorescent protein), are introduced into neurons in an anatomically restricted manner, commonly by transgenic introduction, viral injection, or in vivo electroporation.Citation32–Citation35 Expression of opsins within neurons can be further restricted with the use of selective promoters or DNA recombination systems. For example, one popular expression protocol involves the use of Cre-recombinase, for its ability to invert gene orientations based on Cre-identifiable tags (lox sites) that flank opsin genes. Thus, cell-specific opsin expression can be achieved using transgenic animals that express Cre under a cell-specific gene promoter (eg, tyrosine hydroxylase), followed by viral infection carrying a payload with the opsin gene in an inverted orientation. As a result, only neurons expressing Cre will be able express the opsin gene since it will have been inverted to its correct orientation.
Once the proteins are expressed, they will diffuse within the membranes of cells and traffic to distal neural processes. This allows for the direct manipulation of spiking activity, either through stimulation of cell bodies or their distal processes. Thus, there are multiple dimensions by which photostimulation can be restricted, ie, anatomical location of DNA delivery, cell-type specific expression, and localized light delivery. When these approaches are used together, anatomically localized, genetically defined neural pathways can be repeatedly stimulated or inhibited, in vitro and in vivo.
Depression
Current treatments for clinical depression include pharmacotherapies that directly increase synaptic levels of serotonin and/or norepinephrine via reuptake inhibition. These two neurotransmitter systems have diffuse projections throughout the brain and are involved in a variety of functions, which may be the reason why individual patients respond to different antidepressant drugs in a highly variable manner. While antidepressant drugs act on these entire systems, it is presumed that only a subset of these projections are ultimately responsible for the therapeutic actions.Citation36,Citation37 This appears to be an area ripe for optogenetic analyses. However, antidepressant drugs typically require weeks of medication before symptoms alleviate, which complicates experimental designs and raises questions about the direct role of serotonin/norepinephrine in the therapeutic effects of antidepressant medications.
The initial optogenetic explorations of depression have instead attempted to dissect a nonpharmacological treatment for depression known as deep brain stimulation, a treatment that has been explored as an alternative therapeutic option for severely depressed individuals. Deep brain stimulation via implanted electrodes that target frontal cortical areas and the nucleus accumbens has been demonstrated to relieve symptoms of intractable depression in human subjects.Citation38–Citation42 It is unclear if there is a common neurobiological substrate mediating the therapeutic effects of electrical brain stimulation and antidepressant medication. Unlike drug-induced antidepressant effects, symptom relief following deep brain stimulation has a rapid onset. However, interpretation of the immediate effects of electrical stimulation is problematic, because it is not obvious how neural activity is altered by this manipulation. Electrical stimulation elicits nonspecific activation of all neuronal cell types and processes in a small area around the electrode. Since frontal cortical areas integrate information from throughout the brain and have projections that are similarly diffuse, it is difficult to attribute therapeutic effects to specific neural pathways. As such, electrical stimulation lacks the resolution needed to precisely define which local networks and cell types are responsible for the observed therapeutic changes.
Researchers’ attempts to dissect stimulation-induced antidepressant effects with optogenetic tools have focused on alleviating depression in animal models of the disease. One of these animal models is known as the forced swim test, where laboratory rodents are placed in tanks of water and scored for time spent actively struggling versus passively floating.Citation43,Citation44 Antidepressant treatments increase the amount of time that animals spend struggling. Other models of interest expose animals to stressful events, which creates a behavioral phenotype that is sensitive to chemical antidepressant treatments.Citation45 Optogenetic studies employing these animal models (summarized in ) demonstrate that photostimulation of neurons in the medial prefrontal cortex can elicit antidepressant-like behavior in both forced swim and chronic social defeat models of depression.Citation46–Citation49 Additional research has demonstrated that optogenetic stimulation directed to the specific medial prefrontal cortex projections that innervate the dorsal raphe nucleus is sufficient to produce antidepressant-like effects.Citation48 The dorsal raphe nucleus is the principal source of ascending serotonin projections and the neurotransmitter system that most antidepressant pharmacological treatments act upon.Citation50 Thus, convergent lines of evidence implicate the dorsal raphe nucleus and its regulation by the medial prefrontal cortex in the treatment of depression.
Table 2 Contribution of various brain regions and circuits to the expression of depression-like symptoms
Despite the strong evidence implicating the medial prefrontal cortex-dorsal raphe nucleus circuit in treating symptoms of depression, many ambiguities remain regarding its precise role in this process. This pathway appears to preferentially excite GABAergic interneurons in the dorsal raphe nucleus,Citation46,Citation51–Citation53 suggesting that excitatory medial prefrontal cortex input to the dorsal raphe nucleus may act to depress serotonin levels via feed-forward inhibition. Consistent with this idea, pharmacological inhibition of the medial prefrontal cortex has been shown to enhance both stress-induced serotonin activity and “learned helplessness” behavior.Citation54 Collectively, these optogenetic and pharmacological studies support the provocative conclusion that acute decreases in serotonin function are capable of producing antidepressant-like effects. Similar behavioral effects have been demonstrated by reducing serotonin function via dorsal raphe nucleus negative-feedback autoreceptor activationCitation55 or overexpression.Citation56 These findings go against studies in humans in which acute depletion of serotonin synthesis decreases mood in people with a history of major depressive disorder. Thus, “antidepressant” responses in some behavioral models might be produced by any perturbation of serotonin signaling, regardless of directionality of effect.
In contrast with the forced swim test, optogenetic research exploiting the chronic social defeat model of depression has supported the more conventional conclusion that enhancing serotonin neuron activity in the dorsal raphe nucleus reduces depression-like behavior. Specifically, social defeat experiences have been demonstrated to sensitize GABAergic interneuron function in the dorsal raphe nucleus, and photoinhibition of this population of neurons prevents the acquisition of defeat-induced social avoidance.Citation57 Further, bidirectional manipulations of medial prefrontal cortex-dorsal raphe nucleus circuitry can modulate defeat behavior in positive or negative ways, accordingly.Citation46 A greater understanding of these behavioral models and the microcircuitry of the dorsal raphe nucleus will help explain how different neural inputs are integrated in this region of the brain.
Other medial prefrontal cortex projection targets, in addition to the dorsal raphe nucleus, have recently been explored as well. Optogenetic stimulation to the medial prefrontal cortex projections to the nucleus accumbens have been shown to produce antidepressant-like effects in mice that exhibited depressive symptomatology following cholecystokinin infusions into the medial prefrontal cortex.Citation58 This work highlights a potential role for the nucleus accumbens in depression, which is a region that has been somewhat overlooked in relation to this disease state. While the nucleus accumbens does receive serotonin and norepinephrine input,Citation59,Citation60 it is most prominently innervated by dopaminergic fibers. Photoactivation of midbrain dopamine neurons that innervate the nucleus accumbens has been shown to elicit antidepressant-like effects in both the tail suspension and forced swim tests.Citation61 On the other hand, activation of accumbens-projecting dopamine cell bodies exacerbates the effects of social defeat stress,Citation62 so this system may not have a simple role in depressive symptomatology. Indeed, midbrain dopamine neuron stimulation has been reported to produce opposite effects on sucrose preference in different studies, which suggests that specific conclusions may be more attributable to experimental parameters than depression in general.Citation61–Citation63 More research is needed to clarify how dopamine and its downstream effectors regulate mood in relation to chronic depression.
Anxiety
Anxiety disorders are generally characterized by excessive feelings of apprehension in the absence of any immediate threat. This category of disorders is broad, as it includes generalized anxiety, obsessive-compulsive, and post-traumatic stress disorders.Citation64 Modern medications used to treat anxiety disorders largely target serotonin and GABAergic neurotransmitter systems; however, drugs that block norepinephrine function appear to have particular utility in post-traumatic stress disorder.Citation65,Citation66
Research into the neurobiology of anxiety has largely focused on the amygdala and its extended compartment in the bed nucleus of the stria terminalis (BNST), as these regions are critical for the manifestation of fear. Early studies in laboratory animals found that electrical stimulation of the amygdala produced behavioral and autonomic responses that resemble a state of fear, whereas lesion or inactivation of this region inhibited the expression of experimentally induced anxiety and conditioned fear.Citation67 The basolateral amygdala, where multiple pathways converge, appears to be the locus of associative fear learning. Neural activity here is acutely sensitive to the presentation of previously learned, fear-evoking stimuli.Citation68 Neurons in the basolateral complex innervate the central nucleus of the amygdala, which is the primary output structure of the amygdala nuclei. This region regulates the behavioral and physiological manifestations of fear.Citation69,Citation70 The BNST, which primarily receives input from within the amygdala, serves as an additional output relay station.Citation71
Recent optogenetic studies have extended this model of amygdala circuitry and highlighted a role for this structure in behavioral measures of anxiety (). Photostimulation of the basolateral amygdala is sufficient to induce fear, and any stimuli paired with this stimulation can become fear-inducing.Citation72 In addition, activity in this region is critical for the consolidation of fear memories.Citation73 These studies have supported the classical view of the amygdala as a critical site of fear learning. Other optogenetic studies have broadened this view by showing that neighboring projections out of the basolateral amygdala can mediate opposing effects on fear and anxiety. Photostimulation of basolateral complex projections to the central lateral amygdala reduces anxiety in the elevated plus maze,Citation74 whereas projections to the CA1 region of the hippocampus increase these and other measures of anxiety.Citation75,Citation76 This work underscores the importance of characterizing the role of adjacent structures and discrete projections. Indeed, the hippocampus is similarly nonuniform, as photostimulation of the ventral, but not dorsal, dentate gyrus area elicits a robust anxiolytic effect.Citation77,Citation78
Figure 1 Schematic depiction of brain circuits controlling anxiety.
Abbreviations: BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CEA, central nucleus of the amygdala; CRF R2, corticotropin-releasing factor receptor 2; D1R, dopamine receptor D1; GABA, gamma-aminobutyric acid; Glu, glutamate; HIPP, hippocampus; hypothal, hypothalamus; LS, lateral septum; mPFC, medial prefrontal cortex; oxy, oxytocin; VTA, ventral tegmental area.
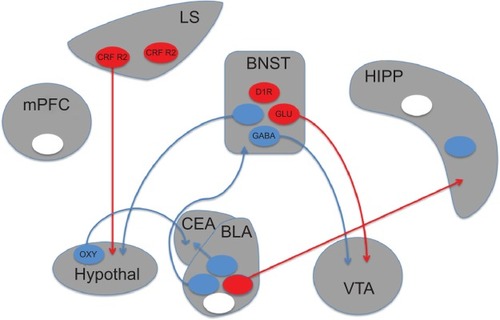
Anxiety regulation within the extended amygdala is also complex. The BNST contains two parallel output pathways to the ventral tegmental area (VTA), ie, a GABAergic pathway that is anxiolytic and a glutamatergic pathway that is anxiogenic.Citation79 The behavioral and physiological components of anxiety can be dissociated via other BNST projections as well. Stimulation of BNST to lateral hypothalamus projections produces avoidance of open arms in an elevated plus maze, but has no effect on respiration rate. Conversely, stimulation of BNST to parabrachial nucleus projections increases respiratory rate without altering plus maze behavior.Citation80 Stimulation of BNST cell bodies directly can produce various responses that depend on the exact location and genetic cell type targeted by the stimulation, factors that likely influence which output pathways are preferentially recruited.
Beyond the extended amygdala, a role for the lateral septum in anxiety has also been demonstrated with optogenetics. Although this structure has been classically associated with anxiolytic responses,Citation81 optogenetic stimulation of cells expressing corticotropin-releasing factor receptor type 2 produces anxiogenic effects.Citation82 This effect was demonstrated to occur via GABAergic projections to the anterior hypothalamus. The hypothalamus contains oxytocin-producing neurons that project to the amygdala and participate in the regulation of anxiety. Selective stimulation of this pathway reduced expression of conditioned fear by directly activating GABAergic neurons in the central lateral amygdala.Citation83
Obsessive-compulsive (OCD) disorder is a specific subset of anxiety disorders that is characterized by repetitive behaviors aimed at relieving anxiety related to intrusive, irrational thoughts. As with general anxiety disorders, obsessive-compulsive disorder can be treated with antidepressant pharmacotherapies. Research into OCD has revealed a role of striatal circuitry in the expression of stereotyped, repetitive actions. A genetic mouse model of OCD has been developed in which mice lack the postsynaptic scaffolding protein Sapap3.Citation84 This gene is expressed strongly in the striatum, and its deletion results in defective corticostriatal circuitry. Mice lacking the gene exhibit high levels of anxiety and excessive grooming behavior, both of which are reversed with chronic antidepressant treatment. Dorsal striatal medium spiny neurons in these mutant mice exhibit exaggerated responses to the presentation of cues associated with grooming.Citation85 Photostimulation of lateral orbitofrontal cortical projections to the dorsal striatum attenuated this striatal neuron hyperactivity by activating fast-spiking interneurons in the striatum. Other recent studies that have examined the orbitofrontal projections to the ventral striatum in wild-type mice found that photostimulation of this pathway can increase grooming.Citation86 While this phenomenon was only produced following chronic stimulation, it persisted for 2 weeks and could be reversed by chronic antidepressant treatment. These studies highlight the importance of corticostriatal circuitry in OCD, but future studies need to clarify where stimulation could be targeted to ultimately alleviate symptoms of the disorder. Indeed, a recent study implicates the more lateral orbitofrontal cortex in controlling the shift from habitual to goal-directed actions.Citation87
Addiction
Drug abuse disorders are characterized by compulsive drug use in the face of adverse consequences. Highly addictive substances seem to disrupt the processes of self-control associated with normal reward-seeking behavior. Initial research mapping out regions of the brain involved in reward processing and motivation began over a half century ago with electrical intracranial self-stimulation experiments.Citation88 Recent studies that have used optogenetic techniques to extend this research have already confirmed that stimulation of midbrain dopamine neurons in the VTA and substantia nigra pars compacta is highly reinforcing.Citation89–Citation92 Although the predominant effect of VTA dopamine neuron stimulation is to reinforce and motivate operant behavior, an aversive subcircuit has also been identified, that consists of VTA dopaminergic neurons receiving input from the lateral habenula and projecting to the medial prefrontal cortex.Citation93 In addition, photoactivation of GABAergic interneurons in the VTA has proved to be aversive.Citation94,Citation95 This research has largely confirmed ideas that have been developed over the preceding years, but a new level of detail is starting to emerge.
The reinforcing effects of midbrain dopamine neuron activity are believed to be mediated primarily via projections to the striatum,Citation96–Citation98 although it has been recently shown that VTA-habenula projections also contribute to this effect.Citation99 Within the striatum, multiple glutamatergic inputs, in addition to the dopaminergic pathway, can reinforce instrumental behavior.Citation100–Citation102 Projections out of the striatum are segregated into two populations based on the expression of certain receptor proteins in the efferent neurons as well as projection targets.Citation103–Citation105 Optogenetic activation of these neuronal populations produces opposing behavioral effects, as photostimulation of dopamine D1 receptor-expressing neurons (direct pathway neurons) produces reward-related behavior and photostimulation of dopamine D2 receptor-expressing neurons (indirect pathway neurons) produces aversion.Citation106 More refined behavioral studies are necessary to expand upon this simple reward/aversion dichotomy.
As individuals repeatedly abuse addictive substances, addictive behaviors develop that are marked by a loss of self-control. Research into the mechanisms of this process has focused on the neural plasticity that addictive drugs produce within reward circuitry.Citation107–Citation110 Within the striatum, drug exposure appears to preferentially potentiate glutamatergic inputs onto direct pathway striatal neurons.Citation109,Citation111 Individual animals with greater potentiation onto the other projection neurons in the striatum, ie, the indirect pathway neurons, show resistance to compulsive drug use.Citation111 Following prolonged withdrawal, potentiation of formerly silent basolateral amygdala-striatum synapses is prominent.Citation112 Pharmacological reversal of this potentiation reverses behavioral manifestation of incubation of drug craving. Numerous pathways, however, appear to be sensitive to drug exposure. Within the dorsomedial nucleus accumbens, drug-induced synaptic potentiation has also been shown to occur within the glutamatergic hippocampus-striatum pathway.Citation101 In addition, direct pathway striatal neurons (dopamine D1 receptor-expressing), which project back to the VTA and target GABAergic interneurons, are also potentiated by repeated drug use.Citation113
Several studies have focused on plasticity occurring within the medial prefrontal cortex. Individual rats that exhibit compulsive drug-seeking show intrinsic hypoactivity of medial prefrontal cortex neurons,Citation114 while presynaptic enhancement occurs in the prefrontal cortex-accumbens projection.Citation115 The causal role of cortical areas in addiction appears to be highly complex. Whereas 1 Hz stimulation of prefrontal cortex cell bodies inhibits compulsive drug-seeking,Citation114 inhibition of the prefrontal cortex-accumbens pathway reduces both reinstatement of cocaine-seekingCitation116 and compulsive alcohol self-administration.Citation117 Furthermore, depotentiation of the prefrontal cortex-accumbens pathway reverses cocaine-induced psychomotor sensitization.Citation109 Thus, while optogenetics have allowed for the ability to study pathway specificity in drug-induced plasticity, future studies are crucial for determining the role of parameters such as drug type and schedule in mediating this plasticity.
Schizophrenia, autism, and beyond
Schizophrenia and autism involve a constellation of symptoms that are not easy to model in rodents. One avenue of research into schizophrenia has focused on neurobiological markers seen within human populations, rather than attempting to recapitulate behavioral models of specific symptoms.Citation118 For example, cortical gamma oscillations (30–80 Hz), which are altered in schizophrenic patients, can be driven by optogenetic stimulation of fast-spiking cortical interneurons.Citation119,Citation120 This manipulation alters the gating of sensory information, which is a common symptom of schizophrenia. A related line of research examines how disruptions in the cellular balance of excitation and inhibition within neural networks underlie deficits in social behavior.Citation121 Recent research has found that increasing excitatory, but not inhibitory, input to pyramidal neurons of the prefrontal cortex causes social and cognitive disturbances in rodents.Citation21 This experiment utilized a step function opsin in conjunction with red-shifted opsins to simultaneously alter excitatory and inhibitory components in cortical microcircuits.
Autism spectrum disorders can manifest as repetitive actions and reduced social interaction, which are symptoms that animal studies have tried to address in relation to OCDCitation85–Citation87 and depression.Citation46–Citation49 As the symptomatology of many psychiatric diseases overlap, some rodent behavioral studies have the potential to address common underlying circuit disturbances. However, there are numerous and obvious differences between people suffering from autism spectrum disorders and those with OCD. For example, people with autism are generally not bothered by their repetitive behaviors, whereas compulsions in OCD are a significant source of anxiety. Additional studies are needed to delineate the neural circuit disruptions that may be common across disorders versus those that are unique to specific psychiatric diseases.
Significant progress has also been made with optogenetic tools in counteracting neural circuit disruptions that underlie certain neurological disorders, such epilepsy and Parkinson’s disease, which are comparatively easy to model in animals. Epileptiform activity can be completely shut down with photoinhibitions targeted to excitatory neurons as well as with photostimulations targeted to inhibitory neurons.Citation122–Citation124 This work is comparable with the advances seen in Parkinson’s disease, in which movement deficits have been minimized in animal models through photostimulations and inhibitions targeted to discrete pathways and groups of neurons.Citation125–Citation127 The research on neurological disorders benefits from having straightforward behavioral assays that are directly relevant to the treatment of human disease.
Conclusion
Optogenetics offers valuable tools for studying the neurobiology of psychiatric disease in animal models. The use of genetically-encoded, light-sensitive proteins, which can activate and inhibit discrete neural circuits, is revolutionizing behavioral neuroscience. When integrated with established techniques, ie, brain imaging, genetic manipulations, behavioral assays, and electrophysiology, optogenetics can help uncover the circuitry responsible for complex psychiatric disease states. To date, these tools have been used preclinically to advance our understanding of these phenomena in both rodent models and in nonhuman primates. There has been early success with this research in delineating previously overlooked neural pathways that should be a target in future drug discovery efforts. However, a great deal of clarification is still needed in this work, particularly in the cases where different publications have supported disparate conclusions. As our understanding of the neurobiology of various psychiatric disease states advances, optogenetic tools may eventually be used as therapeutic agents in human patients. This research promises to advance therapeutic drug development and to help refine the groupings of diverse symptomatology present within many psychiatric diseases.
Acknowledgments
This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2014-05069) as well as with funding from the National Institute on Drug Abuse Intramural Research Program at the National Institutes of Health.
Disclosure
The authors report no conflicts of interest in this work.
References
- LoomerHPSaundersJCKlineNSA clinical and pharmacodynamic evaluation of iproniazid as a psychic energizerPsychiatr Res Rep Am Psychiatr Assoc1957812914113542681
- KapurSMamoDHalf century of antipsychotics and still a central role for dopamine D2 receptorsProc Neuropsychopharmacol Biol Psychiatry20032710811090
- WiedemannKBiomarkers in development of psychotropic drugsDialogues Clin Neurosci20111222523421842620
- HymanSERevitalizing psychiatric therapeuticsNeuropsychopharmacology20143922022924317307
- KrystalJHStateMWPsychiatric disorders: diagnosis to therapyCell201415720121424679536
- RazafshaMBehforuziHHaratiHAn updated overview of animal models in neuropsychiatryNeuroscience201324020421823473749
- ChadmanKKYangMCrawleyJNCriteria for validating mouse models of psychiatric diseasesAm J Med Genet B Neuropsychiatr Genet2009150B11118484083
- MillerGIs pharma running out of brainy ideas?Science201032950250420671165
- TyeKMDeisserothKOptogenetic investigation of neural circuits underlying brain disease in animal modelsNat Rev Neurosci20121325126622430017
- ZhangFWangLPBoydenESDeisserothKChannelrhodopsin-2 and optical control of excitable cellsNat Methods2006378579216990810
- RothschildKJArgadePVEarnestTNThe site of attachment of retinal in bacteriorhodopsin. A resonance raman studyJ Biol Chem198210859285956807975
- GunaydinLAYizharOBerndtASohalVSDeisserothKHegemannPUltrafast optogenetic controlNat Neurosci20101338739220081849
- NagelGOlligDFuhrmannMChannelrhodopsin-1: a light-gated proton channel in green algaeScience2002282395239812089443
- NagelGSzellasTKateriyaSChannelrhodopsin-2, a directly light-gated cation-selective membrane channelProc Natl Acad Sci U S A200325139401494514615590
- BoydenESZhangFBambergENagelGDeisserothKMillisecond-time scale, genetically targeted optical control of neural activityNat Neurosci200581263126816116447
- MattisJTyeKMFerencziEAPrinciples for applying optogenetic tools derived from direct comparative analysis of microbial opsinsNat Methods2012915917222179551
- PriggeMSchneiderFTsunodaSPColor-tuned channelrhodopsins for multiwavelength optogeneticsJ Biol Chem2012287318043181222843694
- ErbguthKPriggeMSchneiderFHegemannPGottschalkABimodal activation of different neuron classes with the spectrally red-shifted channelrhodopsin chimera C1V1 in Caenorhabditis elegansPLoS One20127e4682723056472
- YaroslavskyANSchulzePCYaroslavskyIVSchoberRUlrichFSchwarzmaierHJOptical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral rangePhys Med Biol2002472059207312118601
- BerndtAYizharOGunaydinLAHegemannPDeisserothKBistable neural state switchesNat Neurosci20081222923419079251
- YizharOFennoLEPriggeMNeocortical excitation/inhibition balance in information processing and social dysfunctionNature201147717117821796121
- GradinaruVZhangFRamakrishnanCMolecular and cellular approaches for diversifying and extending optogeneticsCell201014115416520303157
- BambergETittorJOsterheltDLight-driven proton or chloride pumping by halorhodopsinProc Natl Acad Sci U S A1993906396438380643
- ZhangFWangLPBraunerMMultimodal fast optical interrogation of neural circuitryNature200744663363917410168
- RaimondoJVKayLEllenderTJAkermanCJOptogenetic silencing strategies differ in their effects on inhibitory synaptic transmissionNat Neurosci2012151102110422729174
- WietekJWiegertSAdeishviliNConversion of channelrhodopsin into a light-gated chloride channelScience201434440941224674867
- BerndtALeeSYRamakrishnanCDeisserothKStructure-guided transformation of channelrhodopsin into a light-activated chloride channelScience201434442042424763591
- RoganSCRothBLRemote control of neuronal signallingPharmacol Rev20116329151321415127
- MorenoJLHollowatTGonzález-MaesoJG protein-coupled receptor heterocomplexes in neuropsychiatric disordersProg Mol Biol Transl Sci201311718720523663970
- AiranRDThompsonKRFennoLBernsteinHDeisserothKTemporally precise in vivo control of intracellular signallingNature20094581025102919295515
- OhEMaejimaTLiuCDenerisEHerlitzeSSubstitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptorJ Biol Chem2010285308253083620643652
- BrittJPMcDevittRABonciAUse of channelrhodopsin for activation of CNS neuronsCurr Protoc Neurosci20122216
- SpartaDRStamatakisAMPhillipsJLHovelsoNvan ZessenRStuberGDConstruction of implantable optical fibers for long-term optogenetic manipulation of neural circuitsNat Protoc20127122322157972
- MattisJTyeKMFerencziEAPrinciples for applying optogenetic tools derived from direct comparative analysis of microbial opsinsNat Methods2012915917222179551
- TingJTFengGRecombineering strategies for developing next generation BAC transgenic tools for optogenetics and beyondFront Behav Neurosci2014811124772073
- InvernizziRWPariniSSacchettiGChronic treatment with reboxetine by osmotic pumps facilitates its effect on extracellular noradrenaline and may desensitize a2-adrenoreceptors in the prefrontal cortexBr J Pharmacol200113218318811156576
- Bach-MizrachiHUnderwoodMDTinAEllisSPMannJJArangoVElevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicidesMol Psychiatry20081350751318180753
- NahasZAndersonBSBorckardtJBilateral epidural prefrontal cortical stimulation for treatment-resistant depressionBiol Psychiatry20106710110919819427
- BewernickBHHurlemannRMatuschANucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depressionBiol Psychiatry20106711011619914605
- SchlaepferTECohenMXFrickCDeep brain stimulation to reward circuitry alleviates anhedonia in refractory major depressionNeuropsychopharmacology20083336837717429407
- MaybergHSLozanoAMVoonVDeep brain stimulation for treatment-resistant depressionNeuron20054565166015748841
- GrubertCHurlemannRBewernickBHNeuropsychological safety of nucleus accumbens deep brain stimulation for major depression: effects of 12-month stimulationWorld J Biol Psychiatry20111251652721736514
- CryanJFValentinoRJLuckiIAssessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming testNeurosci Biobehav Rev20052954756915893822
- PorsoltRDLe PichonMJalfreMDepression: a new animal model sensitive to antidepressant treatmentsNature1977266730732559941
- WillnerPChronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMSNeuropsychobiology2005529011016037678
- ChallisCBeckSGBertonOOptogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeatFront Behav Neurosci201484324596546
- KumarSBlackSJHultmanRCortical control of affective networksJ Neurosci2013331116112923325249
- WardenMRSelimbeyogluAMirzabekovJJA prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge2012492428432
- CovingtonHE3rdLoboMKMazeIAntidepressant effect of optogenetic stimulation of the medial prefrontal cortexJ Neurosci201030160821609021123555
- JacobsBLAzmitiaECStructure and function of the brain serotonin systemPhysiol Rev1992721652291731370
- HajosMRichardsCDSzekelyADSharpTAn electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the ratNeuroscience199887951089722144
- JankowskiMPSesackSRPrefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neuronsJ Comp Neurol200446851852914689484
- CeladaPPuigMVCasanovasJMGuillazoGArtigasFControl of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptorsJ Neurosci2001219917992911739599
- AmatJBarattaMVPaulEBlandSTWatkinsLRMaierSFMedial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleusNat Neurosci2005836537115696163
- SchreiberRDe VryJNeuroanatomical basis for the antidepressant-like effects of the 5-HT(1A) receptor agonists 8-OH-DPAT and ipsapirone in the rat forced swimming testBehav Pharmacol1993462563611224231
- McDevittRAHiroiRMackenzieSMSerotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behaviorBiol Psychiatry20116978078721353664
- ChallisCBouldenJVeerakumarARaphe GABAergic neurons mediate the acquisition of avoidance after social defeatJ Neurosci201333139781398823986235
- VialouVBagotRCCahillMEPrefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosBJ Neurosci2014343878388724623766
- BerridgeCWStratfordTLFooteSLKelleyAEDistribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbensSynapse1997272302419329158
- BockstaeleEJBiswasAPickelVMTopography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbensBrain Res19936241881988252391
- TyeKMMirzabekovJJWardenMRDopamine neurons modulate neural encoding and expression of depression-related behaviourNature201349353754123235822
- ChaudhuryDWalshJJFriedmanAKRapid regulation of depression-related behaviours by control of midbrain dopamine neuronsNature201349353253623235832
- LammelSTyeKMWardenMRProgress in understanding mood disorders: optogenetic dissection of neural circuitsGenes Brain Behav201413385123682971
- KesslerRCBerglundPDemlerOJinRMerikangasKRWaltersEELifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replicationArch Gen Psychiatry20056259360215939837
- SkolnickPAxioselective anxiolytics: on a quest for the holy grailTrends Pharmacol Sci20123361162022981367
- RaskindMAPetersonKWilliamsTA trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and AfghanistanAm J Psychiatry20131701003101023846759
- DavisMThe role of the amygdala in fear and anxietyAnnu Rev Neurosci1992153533751575447
- FanselowMSLeDouxJEWhy we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdalaNeuron19992322923210399930
- EhrlichIHumeauYGrenierFCiocchiSHerryCLuthiAAmygdala inhibitory circuits and the control of fear memoryNeuron20096275777119555645
- JohansenJPCainCKOstroffLELeDouxJEMolecular mechanisms of fear learning and memoryCell201114750952422036561
- WellerKLSmithDAAfferent connections to the bed nucleus of the stria terminalisBrain Res19822322552707188024
- JohansenJPHamanakaHMonfilsMHOptical activation of lateral amygdala pyramidal cells instructs associative fear learningProc Natl Acad Sci U S A2010107126921269720615999
- HuffMLMillerRLDeisserothKMoormanDELaLumiereRTPosttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in ratsProc Natl Acad Sci U S A20131103597360223401523
- TyeKMPrakashRKimSYAmygdala circuitry mediating reversible and bidirectional control of anxietyNature201147135836221389985
- Felix-OrtizACBeyelerASeoCLepplaCAWildesCPTyeKMBLA to vHPC inputs modulate anxiety-related behaviorsNeuron20137965866423972595
- Felix-OrtizACTyeKMAmygdala inputs to the ventral hippocampus bidirectionally modulate social behaviorJ Neurosci20143458659524403157
- KheirbekMAHenRDorsal vs ventral hippocampal neurogenesis: implications for cognition and moodNeuropsychopharmacology20113637337421116266
- KheirbekMADrewLJBurghardtNSDifferential control of learning and anxiety along the dorsoventral axis of the dentate gyrusNeuron20137795596823473324
- JenningsJHSpartaDRStamatakisAMDistinct extended amygdala circuits for divergent motivational statesNature201349622422823515155
- KimSYAdhikariALeeSYDiverging neural pathways assemble a behavioural state from separable features in anxietyNature201349621922323515158
- SheehanTPChambersRARussellDSRegulation of affect by the lateral septum: implications for neuropsychiatryBrain Res Brain Res Rev2004467111715297155
- AnthonyTEDeeNBernardALerchnerWHeintzNAndersonDJControl of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuitCell201415652253624485458
- KnoblochHSCharletAHoffmannLCEvoked axonal oxytocin release in the central amygdala attenuates fear responseNeuron20127355356622325206
- WelchJMLuJRodriguizRMCortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant miceNature200744889490017713528
- BurguiereEMonteiroPFengGGraybielAMOptogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviorsScience20133401243124623744950
- AhmariSESpellmanTDouglassNLRepeated cortico-striatal stimulation generates persistent OCD-like behaviorScience20133401234123923744948
- GremelCMCostaRMOrbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actionsNat Commun20134226423921250
- OldsJMilnerPPositive reinforcement produced by electrical stimulation of septal area and other regions of rat brainJ Comp Physiol Psychol19544741942713233369
- WittenIBSteinbergEELeeSYRecombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcementNeuron20117272173322153370
- IlangoAKesnerAJKellerKLStuberGDBonciAIkemotoSSimilar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversionJ Neurosci20143481782224431440
- KimKMBarattaMVYangALeeDBoydenESFiorilloCDOptogenetic mimicry of the transient activation of dopamine neurons by natural reward is sufficient for operant reinforcementPLoS One20127e3361222506004
- RossiMASukharnikovaTHayrapetyanVYYangLYinHHOperant self-stimulation of dopamine neurons in the substantia nigraPLoS One20138e6579923755282
- LammelSLimBKRanCInput-specific control of reward and aversion in the ventral tegmental areaNature201249121221723064228
- TanKRYvonCTuriaultMGABA neurons of the VTA drive conditioned place aversionNeuron2012731173118322445344
- van ZessenRPhillipsJLBudyginEAStuberGDActivation of VTA GABA neurons disrupts reward consumptionNeuron2012731184119422445345
- StuberGDBrittJPBonciAOptogenetic modulation of neural circuits that underlie reward seekingBiol Psychiatry2012711061106722196983
- BrittJPMcGeheeDSPresynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbensJ Neurosci2008281672168118272687
- CagniardBBeelerJABrittJPMcGeheeDSMarinelliMZhuangXDopamine scales performance in the absence of new learningNeuron20065154154716950153
- StamatakisAMJenningsJHUngRLA unique population of ventral tegmental area neurons inhibits the lateral habenula to promote rewardNeuron2013801039105324267654
- RussoSJNestlerEJThe brain reward circuitry in mood disordersNat Rev Neurosci20131460962523942470
- BrittJPBenaliouadFMcDevittRAStuberGDWiseRABonciASynaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbensNeuron20127679080323177963
- StuberGDHnaskoTSBrittJPEdwardsRHBonciADopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamateJ Neurosci2010308229823320554874
- KheirbekMABrittJPBeelerJAIshikawaYMcGeheeDSZhuangXAdenylyl cyclase type 5 contributes to corticostriatal plasticity and striatum-dependent learningJ Neurosci200929121151212419793969
- GerfenCRThe neostriatal mosaic: multiple levels of compartmental organization in the basal gangliaAnnu Rev Neurosci1992152853201575444
- Bertran-GonzalezJHerveDGiraultJAValjentEWhat is the degree of segregation between striatonigral and striatopallidal projections?Front Neuroanat2010413620953289
- KravitzAVTyeLDKreitzerACDistinct roles for direct and indirect pathway striatal neurons in reinforcementNat Neurosci20121581681822544310
- BrittJPBonciAOptogenetic interrogations of the neural circuits underlying addictionCurr Opin Neurobiol20132353954523375167
- JayanthiSMcCoyMTChenBMethamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanismsBiol Psychiatry201476475624239129
- PascoliVTuriaultMLuscherCReversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviourNature2012481717522158102
- JacobsBCreswellJBrittJFordKBogenJZaidelEQuantitative analysis of cortical pyramidal neurons after corpus callosotomyAnn Neurol20035412613012838530
- BockRShinJHKaplanARStrengthening the accumbal indirect pathway promotes resilience to compulsive cocaine useNat Neurosci20131663263823542690
- LeeBRMaYYHuangYHMaturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine cravingNat Neurosci2013161644165124077564
- BocklischCPascoliVWongJCCocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental areaScience20133411521152524072923
- ChenBTYauHJHatchCRescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seekingNature201349635936223552889
- SuskaALeeBRHuangYHDongYSchluterOMSelective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaineProc Natl Acad Sci U S A201311071371823267100
- StefanikMTMoussawiKKupchikYMOptogenetic inhibition of cocaine seeking in ratsAddict Biol201318505322823160
- SeifTChangSJSimmsJACortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intakeNat Neurosci2013161094110023817545
- WangXCarlenMOptogenetic dissection of cortical information processing-shining light on schizophreniaBrain Res20121476313722578471
- CardinJACarlenMMeletisKDriving fast-spiking cells induces gamma rhythm and controls sensory responsesNature200945966366719396156
- SohalVSZhangFYizharODeisserothKParvalbumin neurons and gamma rhythms enhance cortical circuit performanceNature200945969870219396159
- RubensteinJLThree hypotheses for developmental defects that may underlie some forms of autism spectrum disorderCurr Opin Neurol20102311812320087182
- LedriMMadsenMGNikitidouLKirikDKokaiaMGlobal optogenetic activation of inhibitory interneurons during epileptiform activityJ Neurosci2014343364337724573293
- PazJTDavidsonTJFrechetteESClosed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injuryNat Neurosci201316647023143518
- Krook-MagnusonEArmstrongCOijalaMSolteszIOn-demand optogenetic control of spontaneous seizures in temporal lobe epilepsyNat Commun20134137623340416
- KravitzAVFreezeBSParkerPRRegulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitryNature201046662262620613723
- YoonHHParkJHKimYHOptogenetic inactivation of the subthalamic nucleus improves forelimb akinesia in a rat model of Parkinson diseaseNeurosurgery20147453354124463495
- GradinaruVMogriMThompsonKRHendersonJMDeisserothKOptical deconstruction of parkinsonian neural circuitryScience200932435435919299587
- KleinlogelSFeldbauerKDempskiRUltra light-sensitive and fast neuronal activation with the Ca2+ permeable channelrhodopsin CatChNat Neurosci20111451352021399632
- ChowBYHanXDobryASHigh-performance genetically targetable optical neural silencing by light-driven proton pumpsNature20104639810220054397
