Abstract
Objective
The use of an algorithm may facilitate measurement-based treatment and result in more rational therapy. We conducted a 1-year, open-label study to compare various outcomes of algorithm-based treatment (ALGO) for schizophrenia versus treatment-as-usual (TAU), for which evidence has been very scarce.
Methods
In ALGO, patients with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, fourth edition) were treated with an algorithm consisting of a series of antipsychotic monotherapies that was guided by the total scores in the positive and negative syndrome scale (PANSS). When posttreatment PANSS total scores were above 70% of those at baseline in the first and second stages, or above 80% in the 3rd stage, patients proceeded to the next treatment stage with different antipsychotics. In contrast, TAU represented the best clinical judgment by treating psychiatrists.
Results
Forty-two patients (21 females, 39.0 ± 10.9 years-old) participated in this study. The baseline PANSS total score indicated the presence of severe psychopathology and was significantly higher in the ALGO group (n = 25; 106.9 ± 20.0) than in the TAU group (n = 17; 92.2 ± 18.3) (P = 0.021). As a result of treatment, there were no significant differences in the PANSS reduction rates, premature attrition rates, as well as in a variety of other clinical measures between the groups. Despite an effort to make each group unique in pharmacologic treatment, it was found that pharmacotherapy in the TAU group eventually became similar in quality to that of the ALGO group.
Conclusion
While the results need to be carefully interpreted in light of a hard-to-distinguish treatment manner between the two groups and more studies are necessary, algorithm-based antipsychotic treatments for schizophrenia compared well to treatment-as-usual in this study.
Introduction
Measurement-based treatment is of high importance in every field of medicine including psychiatry,Citation1 and the process may be facilitated by the implementation of treatment recommendations and algorithms. However, there has been little evidence on treatments that are guided by an algorithm for psychotic disorders. In fact, a MEDLINE search (March 2013) could identify only two clinical trials that compared algorithm-based treatment (ALGO) with treatment-as-usual (TAU) in patients with schizophrenia.Citation2,Citation3 Furthermore, such a lack of clinical trials using ALGO appears to be the case for other psychiatric illnesses as well, including major depressive disorders.Citation4
Previously, the Texas Medication Algorithm Project found a significant decrease in the Brief Psychiatric Rating Scale (BPRS) scores during the first 3 months of ALGO when compared with the TAU group.Citation2 Over the subsequent 9 months, however, the TAU group demonstrated a significant decline in symptoms, eventually catching up with the ALGO group. Further, this study could not find significant differences in terms of quality of life, depression, and cognition, although ALGO patients had more frequent medication changes and a greater number of medication visits, especially in the early treatment stage.
Janssen et alCitation3 compared the effectiveness of a computer-based, guideline-oriented, decision-support system with TAU. The study could not find a significant effect in the Positive and Negative Syndrome Scale (PANSS) total scores at any time points during the 12-month treatment, although ALGO was favorable in terms of rehospitalization. As such, the authors concluded that an ALGO was feasible and effective.
However, these clinical trials generally targeted relatively stable outpatients. Indeed, the average BPRS score was 38.8 to 45.4 in the Texas Medication Algorithm Project.Citation2 The average score in the positive subscale of the PANSS was 13.8 to 14.5, and that for the negative subscale was 17.9 to 20.5 in the study by Janssen et alCitation3 (total PANSS score not provided), thereby leaving the usefulness of the ALGO unaddressed in more severe patient populations. Additionally, such very limited evidence on systematic measurement-based treatments in schizophrenia originated from the US and Germany, and further evidence from the rest of the globe is critical. In Japan, pharmacotherapy of schizophrenia has been notoriously characterized with high-dose antipsychotic polypharmacy.Citation5,Citation6 The successful implementation of a treatment algorithm is expected to counteract antipsychotic polypharmacy and facilitate monotherapy – the latter of which has been endorsed in the treatment of schizophrenia.Citation7
Based on such limited information, we report on the results from an open-label study in which a measurement-based treatment algorithm was adopted in the treatment of schizophrenia for the first time from Japan.
Methods
Inclusion criteria
Patients with schizophrenia, according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition,Citation8 were recruited. To be eligible, the patients were required to be 20 to 65 years old and show a score range of 11 to 90 in the Global Assessment of Functioning (GAF),Citation8 as well as a score of 3 (mild) to 6 (severe) in the Clinical Global Impression– Schizophrenia (CGI-SCH) overall severity subscale.Citation9 Also, all patients were required to have received antipsychotic treatment at chlorpromazine-equivalent doses of 150 mg to 2000 mg per day.Citation10
Exclusion criteria
Exclusion criteria included: a clear history of nonresponse or intolerance to any of the atypical antipsychotics (ie, olanzapine, risperidone, quetiapine. aripiprazole, perospirone, or blonanserin); active and uncontrolled somatic conditions; significant personality disorders or mental retardation severe enough to interfere with the ability to give consent; a potential of being pregnant; a significant risk of being suicidal; and active substance abuse.
Trial setting
This was a prospective, multisite, open-label study of ALGO in Japan. This clinical trial was conducted at eight hospitals (Keio University, Nippon Medical School, University of Yamanashi, Ohizumi Hospital, Inokashira Hosipital, Komagino Hospital, Asai Hospital, Kurumegaoka Hospital). The study was approved by the institutional review board at each participating site, and written informed consent was obtained from all patients after a full description of the study. This study received financial support from the Japanese Minister of Health, Labour, and Welfare. Data were collected from July 2009 through June 2012.
Outcome measurements
The primary outcome measure was a total score on the PANSS.Citation11 The secondary outcome measures aimed to address a variety of aspects in the illness and included the CGI-SCH,Citation9 GAF,Citation8 Drug-Induced Extra-Pyramidal Symptoms Scale (DIEPSS),Citation12 Targeted Inventory on Problems in Schizophrenia (TIP-Sz),Citation13 Functional Assessment for Comprehensive Treatment of Schizophrenia (FACT-Sz),Citation13 Medical Outcome Survey Short-form 36v2 Health Survey (SF-36v2),Citation14 Japanese version of Subjective Well-Being under Neuroleptics Scale (SWN-J),Citation15 and Drug Attitude Inventory (DAI-10).Citation16
The duration of the study was 52 weeks. All patients were evaluated every 4 weeks with the PANSS, CGI-SCH, GAF, DIEPSS, TIP-Sz, FACT-Sz, SF-36v2, SWN-J, and DAI-10. Rating of the PANSS was performed by independent, experienced psychologists who were unaware of the results of other outcome measures. The CGI-SCH, GAF, DIEPSS, TIP-Sz, and FACT-Sz were rated by each attending physician, and subjective measures were completed by the participants. Body weight and vital signs (ie, body temperature, heart rate, and blood pressure) were monitored throughout the study. Routine blood work was performed every 12 weeks.
For the sake of measurement-based treatment, we used the PANSS total score as a guide to decide subsequent treatments, as discussed below. Further, regular meetings were held to provide ongoing training and to ensure interrater reliability on the clinical scales. The ALGO group physicians were required to change psychopharmacological treatments based on the PANSS reduction rate. On the other hand, the psychopharmacological treatment of TAU physicians refected a best case-by-case clinical judgment, irrespective of the PANSS scores.
The study assignment to ALGO or TAU was decided by the physicians, and not by the patients or the facilities. In other words, the study doctors were allocated to treat patients, either using the algorithm or on a TAU basis. All TAU physicians belonged to Ohizumi Hospital, which was intended so as to not masquerade TAU as ALGO (ie, TAU physicians under study try to intentionally modify their usual practices and imitate ALGO to reflect perceived study outcome desirability). All of the study data were monitored by the central research coordinator (MK) in order to maintain research integrity.
Algorithm description
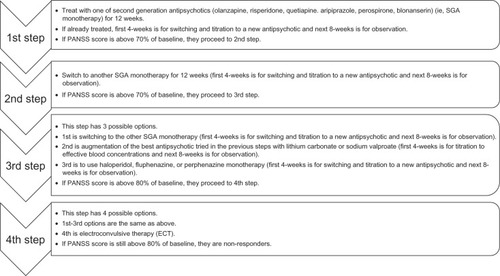
There are some differences among antipsychotics; however, it is currently recommended to base the choice of antipsychotics on the adverse events profile rather than efficacy. Our psychopharmacological treatment algorithm is composed of four stages and is shown in (the rationale for our algorithm can be found in ). This algorithm refects currently available evidence in an effort to optimize antipsychotic treatment while avoiding irrational polypharmacy:
Table 1 Algorithm description
Each treatment step lasted up to 12 weeks with antipsychotic monotherapy.
In the first and second steps, when the posttreatment PANSS total score was above 70% of that at baseline (ie, the total PANSS score at the time of entry into the study), the patients were treated with the next antipsychotic.
The dose of each antipsychotic was decided flexibly on an individual basis, with the basic clinical rule of beginning treatment at a low dose and titrating upwards to search for the lowest effective dose in the first 4 weeks, while monitoring tolerability and adverse effects.
Antipsychotics included: olanzapine once daily, ~20 mg/day; risperidone once to three times daily, ~12 mg/day; quetiapine twice or three times daily, ~750 mg/day; perospirone (a Japanese serotonin-dopamine antagonist) once to three times daily, ~48 mg/day; blonanserin (another Japanese serotonin-dopamine antagonist) once to three times daily, ~24 mg/day; and aripiprazole once to two times daily, ~30 mg/day – all as recommended in their respective drug information sheets.
From the third step onward the PANSS threshold was reduced to 80% (as less resistant patients would have already responded before).
Antipsychotics also included haloperidol or fluphenazine, once to three times daily ~12 mg/day; and perphenazine once to two times daily ~48 mg/day.
In the event that augmentation therapy with lithium carbonate or sodium valproate was selected the dose was titrated upwards to effective blood concentrations (ie, lithium, 0.4~1.2 mEq/L; valproate, 50~125 ng/mL).
In all steps, the use of lorazepam ~6 mg/day for anxiety/insomnia/agitation and/or biperiden ~3 mg/day for extrapyramidal symptoms was permitted only when clinically necessary.
In the event that troublesome side effects argued against continuation of treatment, ALGO patients were allowed to go to the next step prematurely before the predetermined timelines at any treatment stages.
Statistical analyses
The differences between the ALGO and TAU groups in each rating scale were investigated. All analyses were conducted with last-observed-carried-forward principle, using the intention-to-treat population. Continuous variables were analyzed by Mann-Whitney’s U-test for nonnormal data, and categorical variables were evaluated by Student’s t-test. Study retention was compared using Kaplan-Meier survival analysis. A P-value of <0.05 was considered to indicate statistical significance (two-tailed).
Results
A total of 48 patients met the inclusion criteria for the study and provided informed consent. Six of these patients did not complete the baseline assessments, leaving 42 patients to be studied. The characteristics of the participants at baseline are shown in . There were 25 ALGO patients and 17 TAU patients. There were no significant differences in age, sex, duration of illness, previous number of admissions, or age at onset between the groups. Likewise, no significant differences were noted in the CGI-SCH, FACT-Sz, TIP-Sz, GAF, SWN-J, DIEPSS, and SF-36v2 scores. However, the baseline PANSS total score was significantly higher in the ALGO group than in TAU group (mean ± standard deviation [SD]: ALGO, 106.9 ± 20.0 and TAU, 92.2 ± 18.3; U-test P = 0.021). Therefore, changes in the PANSS total score were interpreted in terms of the percent reduction from baseline scores in order to take into account such imbalances in symptom severity between the groups.
Table 2 Baseline characteristics of ALGO and TAU groups
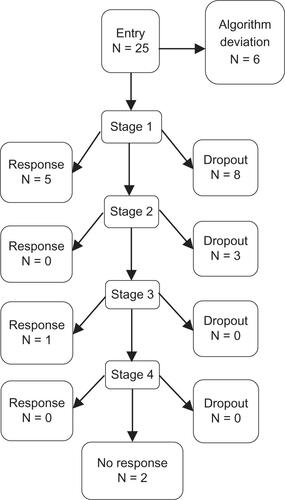
Our clinical trial resulted in a high dropout rate of 64% in the ALGO group and 71% in the TAU group (). The PANSS reduction rates in the two groups failed to show any significant differences at all times (at 24 weeks: ALGO mean ± SD reduction rate, 0.1738 ± 0.201; TAU, 0.152 ± 0.109; U-test P = 0.672; at 52 weeks: ALGO, 0.177 ± 0.194; TAU 0.187 ± 0.157; U-test P = 0.847) ( and ).
Figure 2 Improvements in the ALGO and TAU groups.
Abbreviations: ALGO, algorithm-based treatment; TAU, treatment-as-usual; PANSS, Positive and Negative Syndrome Scale.
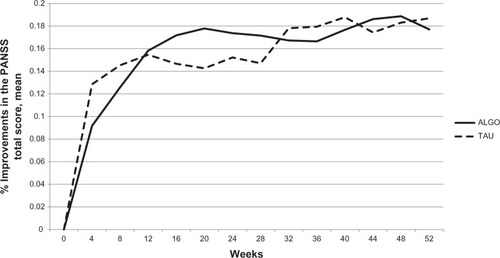
Table 3 Changes in clinical scales for ALGO and TAU patients
Likewise, we could not find any evidence for significant differences in the ALGO group compared to the TAU group regarding secondary outcomes. For instance, the results of the CGI-SCH as well as the GAF and TIP-Sz corroborated the PANSS results and were nonsignificant (). Further, there were no significant differences in extrapyramidal symptoms as assessed by the DIEPSS, and only two cases (one in each group) needed an anti-Parkinson drug. One patient in the ALGO group responded to risperidone, but later showed dystonia, which was resolved by switching to quetiapine.
Chlorpromazine-equivalent antipsychotic dosage (mean ± SD) was 386.6 ± 258.5 mg for the ALGO group and 540.4 ± 362.5 mg for the TAU group at 24 weeks, which failed to reach statistical significance likely due to the limited number of patients, a wider variability in dosage requirements, and more attritions at later treatment weeks (). There were no safety concerns for unexpected adverse reactions in both treatment groups throughout the study.
Looking at the treatment details (), of the 25 ALGO cases entered, six responded, eleven prematurely dropped out, six deviated from the algorithm, and two completed the study without satisfying our response criteria. Alternatively, the response rate was 26.3% (5/19) in the first step, 33.0% (1/3) in the third step, but 0% for the rest of the steps. On the other hand, the response rate in the TAU group was 23.5% (4/17) according to the ≤70% threshold, and 41.2% (7/17) for the 80% cutoff in the PANSS. Nine patients dropped out in the midst of treatment, and two completed the algorithm but did not respond according to our PANSS criteria.
As for the antipsychotics selected in the ALGO group (), risperidone, olanzapine, and aripiprazole were popular. It was found that treatments selected in the TAU group were very similar in quality to those in the ALGO group. In fact, psychopharmacological therapy was “completely” identical to our algorithm in six instances, and in four other cases, it conformed to our algorithm in that lorazepam was only replaced with other benzodiazepines (). The average dose of antipsychotics was not significantly different between the groups (). A small number of patients treated with their respective antipsychotics did not allow meaningful statistics to shed light on differential effects among antipsychotics.
Discussion
This study represents one of very few studies to evaluate the usefulness and feasibility of measurement and algorithm-based, sequential pharmacotherapy with antipsychotics for patients with schizophrenia in real-world clinical settings.Citation20 The ALGO group compared well with the TAU group, although patients in this study presented with relatively severe psychopathology in that the PANSS total score at baseline was high at 106.9 and 92.2, respectively, for each group. Furthermore, a majority of patients were inpatients in this study, which is in contrast to previous studies that solely included outpatients.Citation17 TAU treatment that was intended to represent a best case-by-case clinical judgment was akin in quality to the ALGO in this study (ie, no differences emerged between the treatment groups), which significantly complicated the interpretation of our results.
We failed to find an advantage in terms of the primary outcome – the PANSS total score – as well as in a variety of relevant secondary outcome measures that encompassed global functioning (GAF and FACT-Sz), extrapyramidal symptoms (DIEPSS), problems and overall severity (TIP-Sz, CGI-SCH), subjective well-being (SWN-J), quality of life (SF-36v2), and drug preferences (DAI-10).Citation27 We could not find any significant superiority of ALGO compared to TAU, but our data suggest that ALGO might manage more severe illness with a smaller amount of antipsychotics. Our work indicates that testing ALGO for schizophrenia is a feasible clinical goal.
In this clinical trial, patients in the ALGO and TAU groups were not randomly assigned or matched. Treatment allocation to the ALGO or the TAU group was decided according to the physician, and not according to the patients or the facilities. We have made an effort to avoid “treatment contamination” by recruiting all TAU patients from a single institution, but this study suffered from a high dropout rate. This may be related to the fact that the patients appeared to have rather symptomatic illness, especially considering the baseline PANSS score, although no formal assessments were made for treatment resistance.Citation28 Nevertheless, 64.3% of patients were inpatients, with involuntary admission accounting for 74.0% of patients. In addition, this was a 1-year study and many of these patients with suboptimal insight and substantial psychopathology were not successful for continued treatment. Other patients were simply inaccessible due to location. This is relevant in Japan, since there are patients who cannot choose which hospitals to be admitted to in case of compulsory hospitalization where a risk of harming oneself or others is noted. In such cases, patients frequently go back to their hometown, making an establishment of catchment area extremely complicated in a crowded city, while this is highly desirable for longer-term studies. Location issues accounted for six dropouts in each group.
Miller et alCitation2 argued that algorithm-based intervention encouraged clinicians to be more aggressive in treating residual symptoms, which is compatible with our clinical trial. In the TAU group, only seven cases switched their antipsychotics in the middle of treatment. On the other hand, patients in the ALGO group experienced more antipsychotic trials, which is in part due to the fact that patients failing to meet the predetermined cutoff points should uniformly have gone to the next step in the study protocol. One important consideration is to take into account the stage of the illness, since first-episode patients are known to be responsive to antipsychotic medications, although they are sensitive as well;Citation29 in addition, the greater number of antipsychotics that patients failed to respond to, the less likely they were to be responsive to another.Citation30 How aggressive goal setting could be or how realistic it should be among patients with schizophrenia in general, or with patients exhibiting some specific characteristics (eg, treatment-resistant schizophrenia [TRS]) would be a matter for further investigation.
While this study represents one of a few endeavors of measurement-based treatment and ALGO in schizophrenia,Citation2,Citation3 and while it tries to capture important domains of the illness with the use of independent raters for the main outcome of interest, there are a number of limitations to be noted. First, the number of participants was rather small, and there was a high premature dropout rate, which limits the representativeness of the sample and leaves a possibility of a type-2 error. We stratified patients by physicians and cannot know for sure how the study design may have potentially biased the results; however, a lack of patient-level randomization, as is typical for clinical trials, is an obvious limitation. Potential individual differences in the effectiveness and safety of antipsychotics could not be inferred from this small study.Citation31 Second, the availability of newer antipsychotics was limited. While perispirone and blonanserin were included as atypical agents, amisulpiride, ziprasidone, paliperidone, asenapine, lurasidone, iloperidone, and clozapine were not evaluated. This is especially true for clozapine, which shows superiority for TRS,Citation32 but this drug was unavailable in Japan at the time of the study. While clozapine has been regarded as the third medication, its position needs to be further examined in the treatment algorithm of schizophrenia.Citation33 Third, treatment allocation was guided by the physicians and not by the patients or the facilities. As such, physicians’ prescribing tendencies could not be controlled for. This issue has affected the results in a significant manner, as discussed. Fourth, our patients exhibited relatively severe illness, and the baseline PANSS score was higher in the ALGO group than in the TAU group. Although a higher score may indicate more room for improvement, it may contrarily be an indication of more difficult-to-treat conditions. Fifth, a concept of ALGO treatment may sound the opposite to individually-tailored treatment.Citation34 We believe both perspectives are important. Finally, there has been no unequivocal consensus on what constitutes reasonable assessment measures, but the PANSS has been regarded as a “standard.”Citation35 In this context, we used the PANSS as the primary outcome measure. Although a formal sample size calculation would have been ideal, it was not feasible as a lack of good data on this topic prevented us to perform sample size estimation. In fact, the past few studies have failed to find an effect size that was large enough to allow for a realistic number of patients for inclusion.
A fair interpretation of the results of this study appears to be that ALGO compares well to TAU, rendering ALGO a rational approach and a topic of further scrutiny. In TAU, ten patients received treatment, which was – in essence – the same with our algorithm (see Results). Altogether, the treatment results of these 35 patients (25 from the ALGO group and ten from the TAU group), which are shown in and are equivalent to a 27% reduction of the total PANSS score over the treatment period, could serve as an indicator of the overall usefulness of our algorithm in these symptomatic patients (see SuzukiCitation36 for past evidence on the PANSS/BPRS scores in TRS patients).
Conclusion
To conclude, the results showed that algorithm-based antipsychotic treatment compared well to TAU, but the results need to be interpreted in a context of limitations in the study, especially nearly indistinguishable psychopharmacotherapy between ALGO and TAU. Further investigations are clearly warranted on this highly pertinent topic.
Acknowledgments
The authors are grateful to Drs Motoyasu Ishii, Yasushi Moriyama, Norifusa Sawada, Kaoru Shimizu, Masayuki Tomita, Shinya Kojima, and Leina Tatsumi at Ohizumi Hospital for participating in this clinical trial as Treatment-As-Usual doctors. The authors are also grateful to Ms Fumie Saito at Keio University Hospital.
Supplementary materials
Figure S1 Study retention of ALGO and TAU groups.
Abbreviations: ALGO, algorithm-based treatment; TAU, treatment-as-usual.
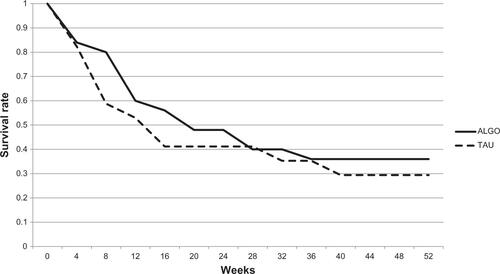
Figure S2 Mean chlorpromazine-equivalent dosage.
Abbreviations: ALGO, algorithm-based treatment; TAU, treatment-as-usual.
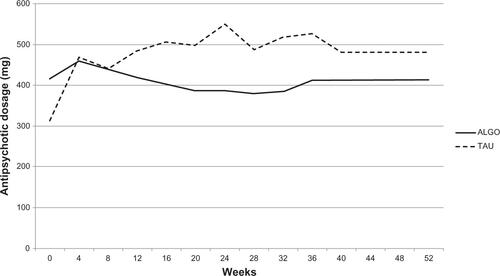
Figure S4 Outcome and antipsychotic choice in the ALGO group.
Abbreviations: ALGO, algorithm-based treatment; RIS, risperidone; OLZ, olanzapine; ARP, aripiprazole; PER, perospirone; BLO, blonanserin; QTP, quetiapine; VPA, valproate acid; Li, lithium carbonate.
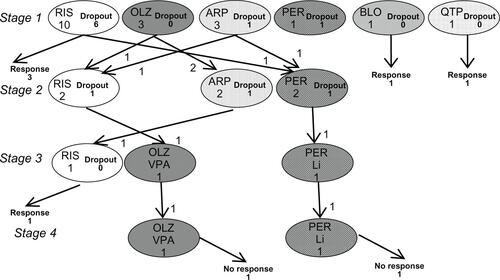
Figure S5 Outcome and antipsychotic choice in TAU patients, which “completely” and “nearly completely” conformed to our algorithm.
Notes: Those receiving treatment that “nearly completely” conformed to our algorithm used another single benzodiazepine than lorazepam. One patient showed severe akathisia with aripiprazole, which was resolved by a switch to risperidone.
Abbreviations: TAU, treatment-as-usual; RIS, risperidone; OLZ, olanzapine; ARP, aripiprazole.
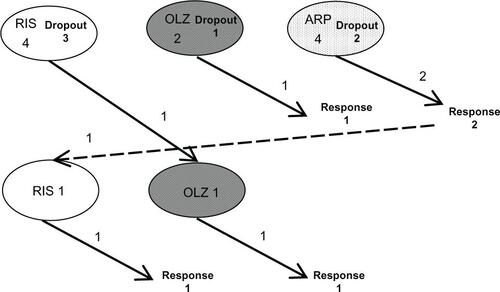
Table S1 The average dosage of SGAs in ALGO and TAU patients
Table S2 Treatment results of 35 patients (25 from ALGO and ten from TAU who received “nearly completely” or “completely” equal treatment with our algorithm)
Disclosure
Role of funding source: This study has received funding from a Health and Labour Sciences Research Grant for Research on Psychiatric and Neurological Diseases and Mental Health (H20-KOKORO-003) from the Japanese Ministry of Health, Labour and Welfare to the last author MK. The funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Dr Hirano has received manuscript or speaker’s fees from Dainippon Sumitomo Pharma, Eli Lilly, Meiji Pharma, and GlaxoSmith Kline Janssen Pharma.
Dr Watanabe has received manuscript or speaker’s fees from Astellas Pharma, Dainippon Sumitomo Pharma, Eli Lilly Japan, GlaxoSmithKline, Janssen Pharmaceutical, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Otsuka Pharmaceutical, Pfizer Japan, Shionogi, Yoshitomiyakuhin, and grants from Dainippon Sumitomo Pharma, GlaxoSmithKline, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Otsuka Pharmaceutical, and Pfizer Japan.
Dr Suzuki has received manuscript or speaker’s fees from Dainippon Sumitomo Pharma, Eli Lilly, Astellas Pharma, Novartis Pharma, and Meiji Seika Pharma.
Dr Uchida has received grants from Pfizer and Dainippon-Sumitomo Pharma, and speaker’s honoraria from Otsuka Pharmaceutical, Eli Lilly, Novartis Pharma, Shionogi, GlaxoSmithKline, Yoshitomi Yakuhin, Dainippon-Sumitomo Pharma, and Janssen Pharmaceutical.
Dr Nagasawa has received speaker’s fees from Dainippon Sumitomo Pharma and Otsuka Pharmaceutical.
Dr Hara has received speaker’s fees from Otsuka Pharmaceutical, Pfizer Japan, GlaxoSmithKline, Yoshitomi Yakuhin, Takeda Pharmaceutical.
Dr Inagaki has received grants from the Japan Pharmaceutical Manufacturer Association and Janssen Pharma, and manuscript or speaker’s fees from Janssen Pharma, Astellas Pharma, Novartis Pharma, Eli Lilly Japan, Otsuka Pharmaceutical, Meiji Seika Pharma, and MSD Japan.
Dr Motohashi has received grants from Astellas Pharma, GlaxoSmithKline, Otsuka Pharmaceutical, Pfizer Japan, Sanofi-Aventis, and Yoshitomi Yakuhin.
Dr Mimura has received grants, consultant fees, and/or speaker’s honoraria from Asahi Kasei, Astellas, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Meiji, Mochida, Novartis, Otsuka, Pfizer, Shionogi, and Yoshitomi.
The other authors do not have any conflicts of interests in relation to this manuscript. The authors report no other conflicts of interest in this work.
References
- CorrellCUKishimotoTNielsenJKaneJMQuantifying clinical relevance in the treatment of schizophreniaClin Ther20113312B16B3922177377
- MillerALCrismonMLRushAJThe Texas medication algorithm project: clinical results for schizophreniaSchizophr Bull200430362764715631256
- JanssenBLudwigSEustermannHImproving outpatient treatment in schizophrenia: effects of computerized guideline implementation – results of a multicenter-study within the German research network on schizophreniaEur Arch Psychiatry Clin Neurosci20102601515719876665
- TrivediMHRushAJCrismonMLClinical results for patients with major depressive disorder in the Texas Medication Algorithm ProjectArch Gen Psychiatry200461766968015237079
- SuzukiTUchidaHWatanabeKYagiGKashimaHA clinical case series of switching from antipsychotic polypharmacy to monotherapy with a second-generation agent on patients with chronic schizophreniaProg Neuropsychopharmacol Biol Psychiatry200428236136914751434
- SuzukiTUchidaHTanakaKFReducing the dose of antipsychotic medications for those who had been treated with high-dose antipsychotic polypharmacy: an open study of dose reduction for chronic schizophreniaInt Clin Psychopharmacol200318632332914571152
- HasanAFalkaiPWobrockTWorld Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for SchizophreniaWorld Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistanceWorld J Biol Psychiatry201213531837822834451
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th edArlington, VAAmerican Psychiatric Association1994
- HaroJMKamathSAOchoaSSOHO Study GroupThe Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophreniaActa Psychiatr Scand Suppl2003416162312755850
- InagakiAInadaT[Dose equivalence of psychotropic drugs. Part XXII: dose equivalence of depot antipsychotics III: risperidone long-acting injection]Japanese Journal of Clinical Psychopharmacology20101313491353 Japanese
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- InadaTYagiGMiuraSExtrapyramidal symptom profiles in Japanese patients with schizophrenia treated with olanzapine or haloperidolSchizophr Res2002572–322723812223254
- SuzukiTUchidaHNomuraKNovel rating scales for schizophrenia – Targeted Inventory on Problems in Schizophrenia (TIP-Sz) and Functional Assessment for Comprehensive Treatment of Schizophrenia (FACT-Sz)Schizophr Res20081062–332833618804960
- WareJEKosinskiMDeweyJEHow to Score Version Two of the SF-36 Health SurveyLincoln, RIQualityMetric, Incorporated2000
- NaberDMoritzSLambertMImprovement of schizophrenic patients’ subjective well-being under atypical antipsychotic drugsSchizophr Res2001501–2798811378316
- HoganTPAwadAGEastwoodRA self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validityPsychol Med19831311771836133297
- MooreTABuchananRWBuckleyPFThe Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 updateJ Clin Psychiatry200768111751176218052569
- LehmanAFKreyenbuhlJBuchananRWThe Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003Schizophrenia Bull2004302193217
- WeidenPJDiscontinuing and switching antipsychotic medications: understanding the CATIE schizophrenia trialJ Clin Psychiatry200768Suppl 1121917286523
- SuzukiTUchidaHWatanabeKHow effective is it to sequentially switch among Olanzapine, Quetiapine and Risperidone? – A randomized, open-label study of algorithm-based antipsychotic treatment to patients with symptomatic schizophrenia in the real-world clinical settingPsychopharmacology2007195228529517701027
- TakahashiHKamataMYoshidaKIshigookaJHiguchiHSwitching to olanzapine after unsuccessful treatment with risperidone during the first episode of schizophrenia: an open-label trialJ Clin Psychiatry200667101577158217107250
- LiebermanJAStroupTSMcEvoyJPClinical Antipsychotic Trials of Intervention Effectiveness (CATIE) InvestigatorsEffectiveness of antipsychotic drugs in patients with chronic schizophreniaNew Engl J Med2005353121209122316172203
- JonesPBBarnesTRDaviesLRandomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS)Arch Gen Psychiatry200663101079108717015810
- National Institute for Health and Care ExcellenceNice clinical guidelines Schizophrenia (update) (CG82). Core interventions in the treatment and management of schizophrenia in primary and secondary care (update). NICE 2009 Available from: http://publications.nice.org.uk/schizophrenia-cg82Accessed 01 Jan 2013
- LeuchtSMcGrathJKisslingWLithium for schizophreniaCochrane Database Syst Rev20033CD00383412917990
- SuzukiTUchidaHTakeuchiHAugmentation of atypical antipsychotics with valproic acid. An open-label study for most difficult patients with schizophreniaHum Psychopharmacol200924862863819946935
- RemingtonGFoussiasGAgidOProgress in defining optimal treatment outcome in schizophreniaCNS Drugs201024192020030416
- SuzukiTRemingtonGMulsantBHDefining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendationPsychiatry Res20121971–21622429484
- FleischhackerWWKeetIPKahnRSEUFEST Steering CommitteeThe European First Episode Schizophrenia Trial (EUFEST): rationale and design of the trialSchizophr Res2005782–314715616055308
- AgidOArenovichTSajeevGAn algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysisJ Clin Psychiatry201172111439144421457676
- LeuchtSCorvesCArbterDEngelRRLiCDavisJMSecond-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysisLancet20093739657314119058842
- KaneJHonigfeldGSingerJMeltzerHClozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazineArch Gen Psychiatry19884597897963046553
- AgidOFoussiasGSinghSRemingtonGWhere to position clozapine: re-examining the evidenceCan J Psychiatry2010551067768420964947
- UchidaHMamoDCPollockBGPredicting plasma concentration of risperidone associated with dosage change: a population pharmacokinetic studyTher Drug Monit201234218218722377743
- SuzukiTWhich rating scales are regarded as ‘the standard’ in clinical trials for schizophrenia? A critical reviewPsychopharmacol Bull2011441183122506437
- SuzukiTRemingtonGArenovichTTime course of improvement with antipsychotic medication in treatment-resistant schizophreniaBr J Psychiatry2011199427528022187729