Abstract
Purpose
We aimed to investigate the efficacy of a combined herbal formula and electroacupuncture (EA) for mild cognitive impairment (MCI), a neurodegenerative disease leading to dementia, and its underlying mechanisms of action.
Patients and Methods
This was a prospective open-label observational pilot study at Daejeon Korean Medicine Hospital of Daejeon University in South Korea from March 2022 to March 2023. We included six Korean patients (50% male) aged ≥ 45 years and < 85 years with MCI, a clinical dementia rating score of 0.5, and a Montreal Cognitive Assessment-Korea (MoCA-K) score ≤ 22. The exclusion criterion was impaired cognitive function. Patients received combined therapy, including a herbal formula and EA, for 12–24 weeks. We prescribed the herbal formulas Gamiguibi-tang, Yukmijihwang-tang, and Banhasasim-tang to the patients for at least 70% of the treatment period, in combination with EA. Moreover, we investigated changes in cognitive and cognition-related symptoms and cytokine expression in the blood following combined traditional medicine therapy. At baseline and after 12 and 24 weeks, we administered the MoCA-K and cognitive-related questionnaires. We analyzed network pharmacology to reflect the herbal formula intervention mechanism comprehensively.
Results
The median score [interquartile range] of MoCA-K at baseline was 19.5 [16.0, 22.0], which improved significantly (24.5 [24.0, 26.0], p < 0.01) over 24 weeks following combined therapy. We obtained no significant conclusion regarding cytokine changes due to the small sample size. In network pharmacology, we analyzed the brain, head, heart, peripheral nerves, peripheral nervous system, and pancreas as the enriched organs from the common targets of the three herbal formulas.
Conclusion
Combined herbal medicine and EA improved cognitive function in patients with MCI. We assume the underlying mechanism of herbal formulas to be antioxidative and anti-inflammatory changes in cytokine expression. Combined traditional medicine has potential therapeutic application in preventing MCI progression to dementia.
Plain Language Summary
This was a single-centered study focusing on the therapeutic effect of combined herbal medicine and electroacupuncture in patients with mild cognitive impairment, including a small number of participants, a relatively long treatment intervention of 12 weeks, and a follow-up assessment of 24 weeks. The intervention was a combination of a herbal formula and electroacupuncture treatment customized for each participant. The blood cytokine analyses of the participants were compared with the network analysis of the predicted target organs and pathways for the herbal formulas administered. Because each participant was not given the exact same intervention, we were unable to identify the specific treatment that produced the predicted effect. The observational study design of the study limited the ability to accurately assess causation between intervention and outcome. However, combined traditional medicine has potential therapeutic application in preventing mild cognitive impairment progression to dementia.
Introduction
Mild cognitive impairment (MCI) is a complex clinical condition characterized by neurodegenerative symptoms encompassing deficits in attention, learning, memory, and information processing speed. Its prevalence ranges between 18 and 22% globally and nationally,Citation1,Citation2 being a transitional phase between normal aging and Alzheimer’s disease (AD).Citation3,Citation4 Current clinical interventions for MCI are aimed at postponing this condition and remain notably limited.Citation5 Recent studies have highlighted the importance of eliminating amyloid-beta (Aβ) peptides—linked with AD—not only from the brain but also from peripheral organs, such as the liver, kidney, and gastrointestinal tract.Citation6,Citation7 Therefore, it is necessary to develop fundamental treatment strategies for cognitive impairment that are not limited to Aβ clearance in the brain, considering multiple peripheral organs.
Traditional complementary and alternative medicine (CAM) provides personalized treatment based on a holistic perspective.Citation8 Similar to CAM, traditional herbal formulas have been systematically shown to improve cognitive function and memory impairment and significantly decrease the number of patients who progress to dementia.Citation4,Citation9 Recently, an observational study was conducted to explore the role of Korean Medicine (KM) therapy in patients with MCI in a clinical setting.Citation10 Electroacupuncture (EA) involves inserting two or more acupuncture needles into the skin, through which a weak electric current is transmitted. When applied to specific head and neck acupoints, EA demonstrates superior cognitive improvement compared to anti-dementia medications. Furthermore, functional magnetic resonance imaging has revealed correlations between EA at these acupoints and dementia-associated brain surface areas.Citation11,Citation12
Herbal formula treatments, including Gamiguibi-tang (GGT; Gui Pi Tang [China]; Kamikihi-to [Japan]) and Yukmijihwang-tang (YMJ; Liu wei di huang tang [China]; Rokumijio-to [Japan]), have been used for MCI. GGT, a herbal formula widely used to treat psychological symptoms in East Asia, is a safe and effective treatment option for patients with amnestic MCI.Citation13 In addition, GGT combination therapy with acetylcholinesterase inhibitors, such as donepezil, maintains and prolongs the effects of donepezil in patients with AD.Citation14 To investigate the therapeutic mechanism of action of GGT, magnetic resonance imaging analysis on brain metabolites, neurotransmitters, and cerebral blood flow was performed in a previous study.Citation15 Assessment of cerebral blood flow after 24 weeks of GGT treatment revealed a significant treatment effect in the hippocampus of participants with MCI when compared with that in the placebo group. Additionally, the efficacy of YMJ in patients with MCI was reported in an observational study.Citation16 However, evidence on memory function improvement and the therapeutic mechanisms underlying herbal formula treatment in patients with amnestic MCI is lacking. Furthermore, the cognitive benefits of the chemical compounds contained in GGT and YMJ are known. Accumulating data support the effect of ginsenosides Rb1 and Rg1 and geniposide in GGT on improvements in cognitive function.Citation17–19 Moreover, loganin and 5-hydroxymethyl-2-furaldehyde in YMJ reportedly ameliorated cognitive deficits in AD-like animal models.Citation20,Citation21
While positive clinical findings for herbal formula treatment in MCI have been reported, its therapeutic mechanism of action, considering the complexity of traditional medicine, should be investigated based on network pharmacology. Network pharmacology explores the multidimensional mechanisms underlying drug action in diseases by analyzing the complex drug–gene–target–disease networkCitation22 and reveals the compound–protein/gene network of disease pathways describing complexities among biological systems, sharing a holistic philosophy similar to that of traditional medicine.Citation23 Recently, medication rules for herbal formulas for MCI were reported using network pharmacology.Citation24 MCI-related core targets were screened using multiple databases, and the corresponding core components were matched with important herbs (Danshen, Yanhusuo, Gancao, Gouteng, and Jiangxiang) using the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform to build a complex network of target-compounds. However, there are limitations in that the data sources and analyses mostly rely on specific databases derived from published literature. Moreover, experimental validation of prediction based on published results is lacking, necessitating additional biological confirmation.Citation24
In this observational study, we aimed to assess the effectiveness of combining herbal formulas with EA to enhance cognitive function in patients with MCI. Furthermore, we aimed to elucidate the therapeutic mechanisms underlying the effectiveness of the herbal formulas in patients with MCI using topological analysis and functional modules based on network pharmacology. We anticipate that this study will offer insights into selecting appropriate herbal formula prescriptions and EA acupoints for managing MCI within a conventional KM practice setting.
Materials and Methods
Study Design and Setting
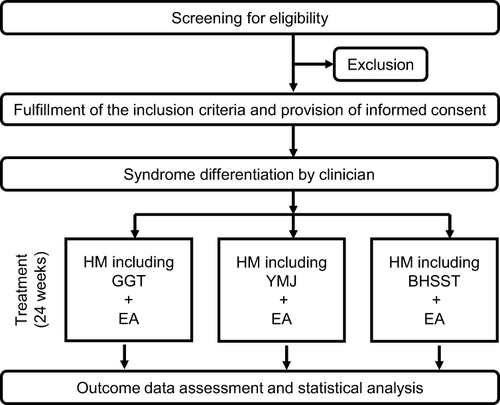
We designed a small, prospective, open-label, observational pilot study to investigate the efficacy of a combination of herbal formulas and EA in patients with MCI, as well as topological features and functional modules using network pharmacology. This clinical study was performed at Daejeon Korean Medicine Hospital of Daejeon University in South Korea from March 2022 to March 2023. Outcome assessments encompassed measurements of cognitive function, symptoms associated with cognitive dysfunction, and alterations in blood cytokine expression either 3 or 6 months following the participant’s initial visit. A flowchart of the study design is shown in and .
Table 1 Schedule for Enrollment, Clinical Outcomes, and Safety Assessment
Participants and Recruitment
Recruitment of participants began in August 2022. The inclusion criteria were as follows: age ≥ 45 years and < 85 years, mild neurocognitive disorder based on the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 diagnostic criteria,Citation25 a clinical dementia rating (CDR) score of 0.5, a Montreal Cognitive Assessment-Korea (MoCA-K) score ≤ 22,Citation26 understanding the contents of the questionnaire, and provision of informed consent.
The exclusion criteria were as follows: cranial lesions, brain damage, or mental retardation that could impair cognitive function; history of neurological diseases, including Huntington’s disease, hydrocephalus, and brain tumor; uncontrolled gastrointestinal, endocrine, and/or cardiovascular disease, despite dietary or medication management; uncontrolled diabetes not responsive to either hypoglycemic drugs or insulin; clinically significant liver disease (indicated by serum aspartate aminotransferase, alanine aminotransferase, and/or gamma-glutamyl transpeptidase values exceeding twice the upper limit of laboratory reference ranges) or kidney disease requiring dialysis; diagnosis of malignancies, such as aplastic anemia or cancer; unstable medical conditions (with determinations made by investigators following standard work procedures, considering vital signs and clinical laboratory examination); major psychiatric disorders, including schizophrenia, delusional disorder, bipolar disorder, and alcohol or substance abuse disorder, as per the DSM-5 diagnostic criteria; pregnancy, lactation, or inadequate use of contraception methods; inability to comprehend the consent form owing to mental retardation, emotional, or intellectual problems or practical difficulties conducting research; difficulty in assessment owing to blindness, hearing loss, and severe speech impairment in the participant; and ineligibility to participate in this clinical study per the coordinator’s discretion or demonstration of a non-cooperative attitude. Among the patients who visited Daejeon Korean Medicine Hospital for MCI treatment, six who met the inclusion criteria were recruited.
Intervention Procedures
Six patients with MCI received an average treatment duration of approximately 3 months, which combined an individualized herbal formula and EA at Daejeon Korean Medicine Hospital, affiliated with Daejeon University. The herbal formula and EA procedures were not restricted to a fixed protocol; instead, they were tailored based on the clinical judgment of a senior KM doctor with over 10 years of clinical experience, adapted to each patient’s specific condition in a real clinical setting. However, the EA procedures were routinely conducted, primarily targeting specific needling acupoints (GV20 and Ex-HN3), utilizing needle stimulation at 2 Hz, and maintaining a retention time of 20 min. All procedural methods, including the composition of prescribed herbal formulas, were recorded retrospectively.
Outcome Measurements
All participants were screened at baseline using cognitive scales, including the MoCA-K and CDR. Cognitive function was assessed using MoCA-K scores and the cognitive subscale of the AD Assessment Scale (ADAS-Cog). The Korean version was evaluated three times: before and 12 and 24 weeks after combination therapy. The MoCA-K consists of several subscales to evaluate an individual’s overall cognitive function, including visuospatial ability, executive function, attention-concentration-working memory, language, short-term memory recall, and orientation to time and place. The evaluation tool uses a 30-point scale, and the cut-off score for detecting MCI is 22/23.Citation26,Citation27 As an assessment tool associated with cognitive function, the Korean version of the Perceived Stress Scale (PSS),Citation28,Citation29 Beck Depression Inventory,Citation30 Insomnia Severity Index,Citation31,Citation32 Simplified Nutritional Appetite Questionnaire,Citation33,Citation34 and European Quality of Life-5 DimensionsCitation35 scores were evaluated at four distinct time points: before and 6, 12, and 24 weeks after combination therapy. To evaluate patient satisfaction following treatment, the Integrative Medicine Patient Satisfaction Scale (IMPSS)Citation36 scores were evaluated 6, 12, and 24 weeks after combination therapy.
To evaluate changes in cytokine levels in serum samples collected from patients, we employed the Bio-Plex Pro Human Cytokine 27-plex Assay kit (Bio-Rad, Hercules, CA, USA). The kit enables the measurement of various cytokines, including fibroblast growth factor (FGF) basic, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17A, IL-1ra, macrophage inflammatory protein (MIP)-1α, MIP-1β, eotaxin, granulocyte-colony-stimulating factor (G-CSF), platelet-derived growth factor (PDGF)-BB, granulocyte-macrophage colony-stimulating factor (GM-CSF), regulated on activation normal T-cell expressed and presumably secreted (RANTES), interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IFN-γ inducible protein 10 (IP-10), vascular endothelial growth factor (VEGF), and monocyte chemoattractant protein-1 (MCP-1).
Serum samples were prepared from the blood specimens of the participants at the first (before treatment) and last visits (12 weeks after treatment). Cytokine antibody-coupled beads were first placed in 96-well microplates and incubated with the 4-fold diluted serum or standard serum for 30 min at room temperature with constant shaking. Subsequently, they underwent another 30-min incubation at room temperature with a biotin-labeled detection antibody, followed by a 10-min incubation with streptavidin-phycoerythrin, at room temperature. Incubation and shaking were performed at 850–900 rpm by covering the microplates with sealing tape. After further washing, assay buffer was added to each well to resuspend the beads, and the fluorescence intensity was measured using an automatic immunoassay analyzer (Bio-Rad Bio-Plex 200 array System). The cytokine concentration was assessed from the standard curve using the Bio-Plex Manager software (ver. 6.0, Bio-Rad), and the serum samples were analyzed in triplicate.
Pharmacological Network Analysis
The pinyin names (ren shen, bai zhu, fu ling, long yan rou, huang qi, dang gui, yuan zhi, chai hu, zhi zi, gan cao, mu xiang, da zao, gan jiang, suan zao ren, mu dan pi, ban xia, huang qin, huang lian, sheng jiang, shu di huang, shan yao, shan zhu yu, and ze xie) of the herbs consisting of the three herbal formulas (GGT, YMJ, and Banhasasim-tang) used in the observational study were inputted into the Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine database (http://bionet.ncpsb.org.cn/batman-tcm) to extract lists of targets per herb. The target lists were selected under the conditions of cutoff score > 30 and p-value < 0.05 and were merged for each formula; the target lists of the three formulas were derived after deduplication.
The target lists of the three formulas were entered into Venny (Ver. 2.1.0) (https://bioinfogp.cnb.csic.es/tools/venny) to obtain common and unique targets among the formulas, using a Venn diagram. Next, we inputted the target lists of formulas into the VarElect database (https://varelect.genecards.org/), using the phenotype keyword “mild cognitive impairment”, and retrieved only the target list with a score exceeding the median value of the score calculated using the VarElect database. The target lists of formulas extracted by matching to MCI were inputted to the GeneORGANizer database (http://geneorganizer.huji.ac.il/) and analyzed by setting the significance level α to 0.001, curation level to “confident + tentative”, frequency of phenotype to “typical”, the multiple comparison corrections to “false discovery rate”, and type of analysis to “Gene-based” in the advanced option mode. GeneORGANizer is a phenotype-based tool that directly shows the linkage between human genes and the body parts they affect.Citation37 We employed identical target lists of formulas associated with MCI to extract pathway lists referred to as SuperPath using the GeneAnalytics database (https://ga.genecards.org/). We focused exclusively on pathways that exceeded the median score determined by the GeneAnalytics database.
Statistical Analyses
Continuous or categorical variables are presented as the median, interquartile range (IQR), mean, and 95% confidence interval or as frequency and percentages, respectively. This study had a small sample size, and parametric statistical analyses were not suitable. Wilcoxon signed-rank tests were used to assess differences between the scores before and after treatment. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
Ethical Considerations and Study Registration
The study protocol was approved by the Research Ethics Committee of Daejeon Korean Medicine Hospital, Daejeon University (DJDSKH-21-BM-23) for the clinical study and the Institutional Review Board of the Korea Institute of Oriental Medicine (I-2206/006-001-01) for cytokine analysis of patient-derived serum samples. The Institutional Review Board was responsible for supervising all aspects of the study. Volunteer participants were recruited and registered, and written informed consent was obtained from all participants. The study protocol was registered in Clinical Research Information Service of the Republic of Korea (KCT0007838).
Patients were voluntarily involved in the study after seeing in-clinic and online posters distributed by the researchers. The patients were given detailed explanations of the intervention, including the strength of the combination treatment, possible side effects, and time of treatment. The patients were asked to sign a consent form if they agreed. We are planning to publish our findings and distribute them to patients with MCI visiting the hospital where we conducted the observational study, including study participants. This study was conducted in accordance with the Declaration of Helsinki.
Results
Demographic and Participant Characteristics
A total of six participants with MCI who met the eligibility criteria were recruited for medical treatment at Daejeon Korean Medicine Hospital and successfully completed the 6-month period of visits and assessments (). These participants had a mean (± standard deviation) age of 70.16 ± 3.43 years, with three (50%) of them being female. The demographic and other baseline characteristics and cognitive assessment results are presented in Supplementary Table 1.
Frequently Prescribed Herbal Formulas
The frequency of the total prescribed herbal formulas for the six patients with MCI in this observational clinical study was as follows: GGT > YMJ > Gami-Ondam-tang > Banhasasim-tang > Ukgan-san. Detailed descriptions of GGT, YMJ, and Banhasasim-tang, which were mainly prescribed to the participants in this study, are presented in Supplementary Table 2. For each participant, herbal formulas taken for a long period of time, for more than 70% of the total period of taking a herbal formula, were GGT in patients #4 and #6, YMJ in patients #1 and #2, and Banhasasim-tang in patient #3. Patient #5 did not receive long-term herbal formulas for over 70% of the total herbal formula intake period.
Outcome Measure for Cognitive-Related Function and Changes in Cytokine Levels
At baseline, the median score [IQR] of the MoCA-K for cognitive assessment among all participants with MCI was 19.5 [16.0, 22.0]. After 24 weeks, significant differences in the MoCA-K scores were observed (24.5 [24.0, 26.0]; p = 0.0313). Similarly, the median [IQR] ADAS-Cog score for cognitive assessment was 16.5 [11.0, 18.0] at baseline, with significant differences in ADAS-Cog scores noted over 24 weeks (7.5 [3.0, 11.0]; p = 0.0313). The median [IQR] PSS score for stress measurement was 19.5 [16.0, 20.0] at baseline, with significant differences in PSS scores noted over 24 weeks (17.0 [14.0, 19.0]; p = 0.0313). However, in other instruments, no statistically significant score changes before and after combination treatment were observed. The changes in the outcome scores after combination therapy are presented in . Regarding the IMPSS, the median score [IQR] was 2.5 [2.0, 4.0] after 6 weeks of intervention and 2.0 [1.0, 2.0] over 24 weeks.
Table 2 Outcomes Changes Associated with Herbal Medicine
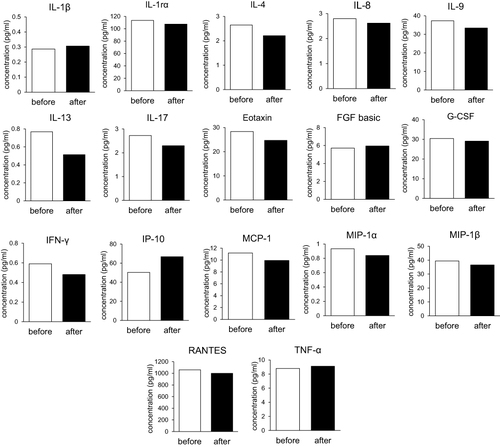
In terms of molecular markers in the blood, 17 of the 27 tested cytokines in the multiplex analysis exhibited over 1.5-fold changes 24 weeks after combination treatment with the herbal formula and EA compared to baseline. These altered cytokines included IL-1β, IL-1ra, IL-4, IL-8, IL-9, IL-13, IL-17, eotaxin, FGF basic, G-CSF, INF-γ, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α. However, a common pattern of altered cytokine levels was not observed in any of the six patients ().
Figure 2 Cytokine levels before and after herbal formula treatment. Graphs represent an average concentration of cytokines in six patients.
Enrichment Analysis of Function and Pathways Related to Cytokines
Network pharmacology analysis was performed on the three herbal formulas, GGT, YMJ, and Banhasasim-tang, that were frequently prescribed to the participants. The target lists of the three formulas (GGT, 891 targets; YMJ, 454 targets; and Banhasasim-tang, 974 targets) resulted in 80 common targets. Specifically, there were 138 common targets between GGT and Banhasasim-tang, with six unique targets for GGT, 11 unique targets for Banhasasim-tang, and no unique targets for YMJ. The numbers of targets of the three formulas extracted from the VarElect database by matching MCI were 79, 80, and 47, respectively, with 42 common targets derived from these three formulas (Supplementary Figure 1; Supplementary Data 1).
We analyzed the SuperPath pathway specific to cytokines in the three formulas and their common targets. The pathways analyzed from common targets of the three formulas were the IL-4 and IL-13 signaling, development of VEGF signaling via VEGFR2-generic cascades, and IL-9 signaling pathways. Common pathways among the three formulas were the IL-4 and IL-13 signaling pathway, immune response IL-23 signaling pathway, development of VEGF signaling via VEGFR2-generic cascades, IL-18 signaling pathway, and IL-9 signaling pathway. The unique pathways were the IL-10 signaling pathway in GGT, IL-1 family signaling pathways, IL-10 signaling, TNF superfamily-human ligand-receptor interactions and their associated functions, IL-17 family signaling pathways, and the TNFR1 pathway in Banhasasim-tang. A full list of these pathways is provided in Supplementary Data 2.
Subanalysis by Herbal Formulas
Changes in cytokine levels were organized according to each formula (Supplementary Figure 2). There were no common changes in cytokine levels across the three herbal formulations. The common cytokines changed by YMJ and GGT were IL-4, IL-17, eotaxin, INF-γ, and MCP-1. Banhasasim-tang altered IL-13 and IP-10 levels, similar to YMJ and GGT. GGT primarily induced alterations in T-cell cytokines, including IL-1β, IL-4, IL-17, and INF-γ. Notably, a consistent reduction in IFN-γ levels was observed in patients #1 and #2 over a 12-week post-treatment period with GGT. Conversely, Banhasasim-tang influenced IL-13 and IP-10 levels. YMJ primarily stimulated the changes in CC chemokines, such as eotaxin, MCP-1, MIP-1α, MIP-1β, and RANTES.
The results of the organ enrichment analysis of the three formulas and common targets analyzed using the GeneORGANizer database are shown in Supplementary Figure 3. The enriched organs analyzed from the common targets of the three formulas were the brain, head, heart, peripheral nerves, peripheral nervous system, and pancreas. The organs commonly enriched in GGT and Banhasasim-tang were the spinal cord, liver, lung, kidney, bone marrow, skin, small intestine, esophagus, intestine, blood vessels, large intestine, upper limb, rectum, stomach, eye, mouth, lower limb, spleen, hand, foot, toes, forearm, arm, digits, wrist, breast, prostate, coagulation system, neck, elbow, shoulder, anus, knee, fingers, ankle, and hip. The unique enriched organs were the chest wall, red blood cells, shin, scapula, and thigh in GGT; only the gallbladder in Banhasasim-tang; and none in YMJ. A full list of enriched organs is presented in Supplementary Data 3.
Discussion
This observational clinical study showed the effectiveness of combination treatment with a herbal formula and EA in patients with MCI, and network pharmacology analysis revealed the therapeutic mechanism. Our results showed that cognitive function, including the MoCA-K and PSS scores, improved after herbal formula therapy. The herbal formulas that each participant took for the longest period of time were GGT, YMJ, and Banhasasim-tang. These herbal formulas have been used to treat patients with cognitive impairment.Citation14,Citation16 To identify the therapeutic mechanisms of the herbal formulas, changes in cytokine levels in patient-derived serum and cytokine-related pathways were investigated using network pharmacology. In six participants with MCI, cytokines that were altered included IL-1β, IL-1ra, IL-4, IL-8, IL-9, IL-13, IL-17, eotaxin, FGF basic, G-CSF, INF-γ, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α.
According to a review paper by Brosseron et alCitation38 five group classifications of blood markers, including cytokines, other immune signaling-related regulators, and their receptors, are based on the disease progression of MCI and AD. In our study, the cytokines that exhibited alteration predominantly belonged to groups 2 and 3. Specifically, group 2 encompassed cytokines such as IL-1β and TNF-α, which demonstrated a gradual but steady increase over time throughout the course of AD. Group 3 included cytokines such as MCP-1 and IP-10, which reached their peak levels in mild AD or during the transition from MCI to AD. Based on network pharmacology, the common target pathways related to cytokines matched with the three formulas (GGT, YMJ, and Banhasasim-tang) and MCI were IL-4, −9, −13, and VEGF. Cytokines, including IL-4, IL-9, and IL-13, are commonly identified in real-world patient serum and network pharmacology prediction analyses. Our results suggest that changes in cytokines, including IL-4, IL-9, and IL-13, following herbal formula therapy in patients with MCI may be related to improved cognitive impairment and therapeutic mechanisms, such as antioxidant and anti-inflammatory effects.
In addition, the cytokines commonly identified based on real-world and network pharmacology analyses in our study are reported to be associated with cognitive dysfunction. IL-9 levels are significantly increased in patients with AD. In a study by Saresella et alCitation39 which involved immunophenotypic and functional analysis of Aβ stimulated T lymphocytes in patients with AD, MCI, and healthy individuals, cytokines, including IL-21, IL-6, IL-23, and IL-9, were significantly upregulated in patients with AD. This upregulation coincided with a shift in post-thymic differentiation pathways, leading to the accumulation of differentiated effector T lymphocytes. Regarding IL-13, a cross-sectional study by Serre-Miranda et alCitation40 found that plasma from senior individuals with poor cognitive performance exhibited increased concentrations of IL-1β, IL-8, and IL-13 compared to that in individuals with better cognitive performance. These cytokines were suggested as potential contributors to predicting an individual’s cognitive performance. Additionally, in a previous animal study, IL-4 and IL-13 were reported to play critical roles in neuronal death by regulating oxidative stress in neurodegenerative diseases such as AD.Citation41
IL-4 and IL-13 are endogenously expressed in reactive microglia and are upregulated in activated microglia/macrophages, mediating prothrombin kringle-2-induced neurotoxicity in the rat cerebral cortex. A previous study suggested that the deleterious actions of IL-4 and/or IL-13 may be involved in oxidative stress-mediated neurodegenerative diseases. Consistent with this, our clinical study indicated a tendency for decreased expression of IL-4 and IL-13 following treatment with herbal formulations in patients with MCI, which can be attributed to a decrease in oxidative stress. However, in another previous study, IL-4 and IL-13 were shown to be anti-inflammatory factors that induce polarization toward M2 phenotype microglia, which secrete anti-inflammatory cytokines.Citation42 Therefore, IL-4 and IL-13 inhibit Aβ-induced microglial polarization and pro-inflammatory activity.Citation43 Overall, conflicting results on the role of peripheral immune molecules in AD are being reported; therefore, further large-scale clinical research studies to explore immune-related cytokines in participant groups composed of healthy seniors, MCI, and AD are needed.
We conducted a subanalysis focusing on the primary herbal formulas administered to each participant. Six patients with MCI received several herbal formulas over a 24-week period in a real-world clinical setting. Herbal formulas taken for over 70% of the treatment period included GGT in patients #4 and #6, YMJ in patients #1 and #2, and Banhasasim-tang in patient #3. To predict how the targets of the three herbal formulas were enriched or depleted in the organs, organ enrichment analysis was performed using GeneORGANizer. The enriched organs analyzed from the common targets of the three formulas were the brain, head, heart, peripheral nerves, peripheral nervous system, and pancreas. Most of these organs were the same as those enriched in the YMJ formula. YMJ ameliorates brain injury in animal models.Citation39,Citation40 In a study by Eom et alCitation44 using a chronic restraint stress mouse model, YMJ ameliorated the impairment of brain hippocampal memory ability. Additionally, YMJ improved cognitive function in a d-galactose-induced aging mouse model by regulating lipid metabolism and oxidative stress and ameliorated hippocampal synaptic ultrastructure damage.Citation45 In this study, the common target organ of the herbal formulas that improved cognitive function was the brain. However, Aβ peptide has been reported to be removed from the peripheral organ systemCitation6,Citation7 and our analysis identified peripheral organs as target sites for the herbal formulas used in this study. Therefore, further studies are needed to confirm cognitive impairment, considering multiple peripheral organs based on the holistic perspective of traditional medicine.
We also addressed the changes in the cytokine profiles using each formula. GGT administered to patients #4 and #6 mainly changed T-cell cytokines, such as IL-1β, IL-4, IL-17, and INF-γ, that play a role in neuroinflammation. Among the altered cytokines, IL-1β is known to be associated with the progression of MCI and/or AD. IL-1β levels exhibited a significant increase in both plasma and cerebrospinal fluid (CSF) among individuals with amnestic MCI and AD; however, it was notably higher in patients with AD than in those with MCI.Citation46,Citation47 Conversely, a meta-analysis by Su et alCitation48 revealed that the concentration of IL-1β constantly increased in patients with AD, with no consistent results in patients with MCI. These results imply that IL-1β is not specific and sufficient to only patients with MCI. IFN-γ, an essential cytokine in the immune system, plays a critical role in microglia-induced neural network and neurodegeneration.Citation49 In our study, IFN-γ levels consistently declined 12 weeks after treatment in both GGT-administered patients #1 and #2. Belkhelfa et alCitation50 reported higher IFN-γ levels in patients with mild or severe AD compared to those with moderate AD and MCI using plasma samples from Algerian patients. Nevertheless, there is limited evidence to firmly establish the clinical relevance of IFN-γ to MCI or AD, despite several studies suggesting it as a potential therapeutic target for AD.Citation50,Citation51
In clinical studies, the association between IL-4 or IL-17 and the progression or pathophysiology of MCI remains relatively unexplored. In the case of patient #3, who received Banhasasim-tang, changes in IL-13 and IP-10 levels were observed in serum samples. Recently, Mayagoitia et alCitation52 reported that the expression of IL-13 and IP-10 increased in the brains of amyloid precursor protein knock out mice, using multiplexed immunoassay and histological analysis. This study indicated numerous IL-13-positive cells in the medial prefrontal cortex and ventral hippocampus, while IP-10 was expressed in the medial prefrontal cortex and hippocampus in a mouse model. However, clinical evidence addressing the association of IL-13 and IP-10 with MCI or AD remains limited.
YMJ principally changed levels of the CC chemokines, such as eotaxin, MCP-1, MIP-1α, MIP-1β, and RANTES. Notably, MIP-1α exhibited a marked change in both patients #4 and #6. Chemokines, a subfamily of cytokines, play a pivotal role in inflammatory diseases by controlling the recruitment of effector leukocytes.Citation53 Several groups have reported the potential of eotaxin and MCP-1 as selective markers of MCI and AD. Bettcher et alCitation54 reported that MCP-1 and eotaxin-1 play potentially selective roles in memory dysfunction in MCI and AD. Morgan et alCitation55 demonstrated that MCP-1, eotaxin, and soluble complement receptor type 1 can serve as moderately predictive markers for differentiating between MCI and AD in an inflammatory biomarker study of AD plasma within the AddNeuroMed, a significant cross-European cohort. MIP-1α, MIP-1β, and RANTES commonly bind and activate the CC-chemokine receptor 5, a crucial factor in various inflammatory diseases including AD, to display their effects.Citation56 Quantitative analysis of signaling proteins revealed increased epidermal growth factor, glial cell-derived neurotrophic factor, and MIP1 levels in AD; MIP4 in MCI; and RANTES in MCI and AD in human plasma samples from healthy controls and patients with MCI or AD.Citation57 In our study, YMJ also altered the concentration of T-cell cytokines IL-4, IL-9, IL-17, INF-γ, and TNF-α in the serum of patients with MCI. The potential effects of YMJ on cognitive improvement in patients with MCI may be attributed to the modulation of CC chemokines by T-cell cytokines.
It is important to explore biomarkers for preventing and treating diseases in modern medicine, whether in Western or traditional medicine approaches. Currently, the most broadly used biomarkers for AD are Aβ and tau proteins in CSF. However, CSF markers are limited by variations in diagnostic accuracy and invasive lumbar puncture in predicting the progression from MCI to AD.Citation58 Therefore, blood-based molecular markers, including cytokines, may be beneficial tools for diagnosing MCI and AD to overcome the limitations of CSF markers. Several groups have studied the development of blood-based markers in MCI or AD; however, their conclusions remain controversial. Future studies should consider the standardization of assays, clinical study protocols, and patient charts.
Despite its strengths, our study had some limitations. First, our clinical study was a small observational study without a placebo control, which cannot exclude the possibility of the placebo effect and the influence of the learning effect. Consequently, further randomized controlled clinical trials exploring the interaction between EA and herbal medicine combination therapy on MCI are warranted. Second, several formulas were prescribed to or within patients. Therefore, it is difficult to derive representative herbal formulas for MCI and specify their therapeutic mechanisms. In addition, external efficacy was compromised due to multi-ingredient herbal formulas in a small number of clinical events. Third, even though we could analyze cytokine-related pathways using network pharmacology, we were unable to determine whether the three herbal formulas activated or inhibited cytokine activity. Further large-scale randomized controlled trials with follow-up observations are needed to confirm the effects of cognitive improvement and the mechanisms underlying network pharmacology in patients with MCI. More information on the collective characterization of patients and herbal formulas is required to develop a biomarker set for MCI in the blood. Therefore, planning personalized medicine that reflects the characteristics of individual patients would be helpful.
Conclusion
The combination therapy of EA and herbal formulas appears promising for enhancing cognitive function in patients with MCI. The network pharmacological analysis for herbal formulas in our study provided insight into possible therapeutic mechanisms, such as anti-oxidation and anti-inflammation, via peripheral immune molecule alterations based on real-world clinical studies. In addition, cytokine changes and target-enriched organs were analyzed using subgroup analysis for each frequently used herbal formula, but no significant. Conclusion was obtained due to the small sample size. However, the candidate mechanisms of herbal formulas related to cognitive improvement are presented and should be proven through follow-up studies.
Abbreviations
Aβ, amyloid-beta; AD, Alzheimer’s disease; ADAS-Cog, AD Assessment Scale; BHSST, Banhasasim-tang; CAM, complementary and alternative medicine; CDR, clinical dementia rating; CSF, cerebrospinal fluid; DSM, Diagnostic and Statistical Manual of Mental Disorders; EA, electroacupuncture; FGF, fibroblast growth factor; G-CSF, granulocyte-colony-stimulating factor; GGT, Gamiguibi-tang; IFN, interferon; IL, interleukin; IMPSS, Integrative Medicine Patient Satisfaction Scale; IP-10, inducible protein 10; IQR, interquartile range; MCI, mild cognitive impairment; MCP-1, monocyte chemoattractant protein-1; MIP, macrophage inflammatory protein; MoCA-K, Montreal Cognitive Assessment-Korea; PSS, Perceived Stress Scale; RANTES, regulated on activation normal T-cell expressed and presumably secreted; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; YMJ, Yukmijihwang-tang.
Ethics Approval
This study was approved by the Institutional Review Board (approval number: DJDSKH-21-BM-23) for clinical studies and by the Institutional Review Board of the Korea Institute of Oriental Medicine (approval number: I-2206/006-001-01) for cytokine analysis of patient-derived serum. They were responsible for supervising all aspects of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
Data Sharing Statement
Original data (crude data) from this study are available by contacting the corresponding author via email.
Additional information
Funding
References
- Chen P, Cai H, Bai W, et al. Global prevalence of mild cognitive impairment among older adults living in nursing homes: a meta-analysis and systematic review of epidemiological surveys. Transl Psychiatry. 2023;13(1):88. doi:10.1038/s41398-023-02361-1
- Lee JS, Kang MJ, Lee O, Lee H, Kwak M, Yu W. Korean Dementia Observatory; 2022:1–175.
- Chehrehnegar N, Nejati V, Shati M, et al. Early detection of cognitive disturbances in mild cognitive impairment: a systematic review of observational studies. Psychogeriatrics. 2020;20:212–228.
- Wang W, Diwu Y, Liu Q, et al. Chinese herbal medicine for mild cognitive impairment using Mini-Mental State Examination: a systematic review and meta-analysis. Medicine. 2021;100(38):e27034. doi:10.1097/MD.0000000000027034
- Jongsiriyanyong S, Limpawattana P. Mild cognitive impairment in clinical practice: a review article. Am J Alzheimers Dis Other Demen. 2018;33(8):500–507. doi:10.1177/1533317518791401
- Xiang Y, Bu XL, Liu YH, et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol. 2015;130(4):487–499. doi:10.1007/s00401-015-1477-1
- Wang J, Gu BJ, Masters CL, Wang Y-J. A systemic view of Alzheimer disease – insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol. 2017;13(10):612–623. doi:10.1038/nrneurol.2017.111
- Deng T. Syndrome differentiation and treatment: an essence of traditional Chinese medicine. Tradit Chin Med J. 2005;1:1–4.
- Liang N, Chen Y, Yang S, et al. Chinese herbal medicine for mild cognitive impairment: a systematic review of randomized controlled trials. Front Neurol. 2022;13:903224. doi:10.3389/fneur.2022.903224
- Choi Y, Kim YE, Jerng UM, et al. Korean traditional medicine in treating patients with mild cognitive impairment: a multicenter prospective observational case series. Evid Based Complement Alternat Med. 2020;2020:4323989. doi:10.1155/2020/4323989
- Cao J, Huang Y, Meshberg N, Hodges SA, Kong J. Neuroimaging-based scalp acupuncture locations for dementia. J Clin Med. 2020;9(8):2477. doi:10.3390/jcm9082477
- Kim H, Kim HK, Kim SY, Kim YI, Yoo HR, Jung IC. Cognitive improvement effects of electro-acupuncture for the treatment of MCI compared with Western medications: a systematic review and meta-analysis. BMC Complement Altern Med. 2019;19(1):13. doi:10.1186/s12906-018-2407-2
- Shin HY, Kim HR, Jahng GH, et al. Efficacy and safety of Kami-guibi-tang for mild cognitive impairment: a pilot, randomized, double-blind, placebo-controlled trial. BMC Complement Med Ther. 2021;21(1):251. doi:10.1186/s12906-021-03428-6
- Ishida K. Effect of donepezil and kamikihito combination therapy on cognitive function in Alzheimer’s disease: retrospective study. Trad Kampo Med. 2016;3(2):94–99. doi:10.1002/tkm2.1045
- Cho SY, Kwon S, Shin HY, et al. Treatment evaluation of Kami Guibi-tang on participants with amnestic mild cognitive impairment using magnetic resonance imaging on brain metabolites, gamma-aminobutyric acid, and cerebral blood flow. J Appl Clin Med Phys. 2021;22(11):151–164. doi:10.1002/acm2.13443
- Choi Y, Kim AR, Lee JY, et al. Herbal medicine for patients with cognitive impairment: an observational study. Neuropsychiatr Dis Treat. 2021;17:3183–3194. doi:10.2147/NDT.S333569
- Yang Y, Li S, Huang H, et al. Comparison of the protective effects of ginsenosides Rb1 and Rg1 on improving cognitive deficits in SAMP8 mice based on anti-neuroinflammation mechanism. Front Pharmacol. 2020;11:834. doi:10.3389/fphar.2020.00834
- Mook-Jung I, Hong HS, Boo JH, et al. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res. 2001;63(6):509–515. doi:10.1002/jnr.1045
- Zhao C, Zhang H, Li H, et al. Geniposide ameliorates cognitive deficits by attenuating the cholinergic defect and amyloidosis in middle-aged Alzheimer model mice. Neuropharmacology. 2017;116:18–29. doi:10.1016/j.neuropharm.2016.12.002
- Zhou Y, Luo D, Shi J, et al. Loganin alleviated cognitive impairment in 3×Tg-AD mice through promoting mitophagy mediated by optineurin. J Ethnopharmacol. 2023;312:116455. doi:10.1016/j.jep.2023.116455
- Lee Y, Gao Q, Kim E, et al. Pretreatment with 5-hydroxymethyl-2-furaldehyde blocks scopolamine-induced learning deficit in contextual and spatial memory in male mice. Pharmacol Biochem Behav. 2015;134:57–64. doi:10.1016/j.pbb.2015.04.007
- Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110–1111. doi:10.1038/nbt1007-1110
- Zhang R, Zhu X, Bai H, Ning K. Network pharmacology databases for Traditional Chinese Medicine: review and assessment. Front Pharmacol. 2019;10:123. doi:10.3389/fphar.2019.00123
- Chang Z, Wang YC, Tian D, et al. Medication rules in herbal medicine for mild cognitive impairment: a network pharmacology and data mining study. Evid Based Complement Alternat Med. 2022;2022:2478940. doi:10.1155/2022/2478940
- Sachs-Ericsson N, Blazer DG. The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment. Aging Mental Health. 2015;19(1):2–12. doi:10.1080/13607863.2014.920303
- Lee JY, Dong Woo L, Cho SJ, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008;21(2):104–110. doi:10.1177/0891988708316855
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi:10.1038/nrn2639
- Nielsen MG, Ørnbøl E, Vestergaard M, et al. The construct validity of the Perceived Stress Scale. J Psychosom Res. 2016;84:22–30.
- Mirza SS, Ikram MA, Bos D, Mihaescu R, Hofman A, Henning Tiemeier H. Mild cognitive impairment and risk of depression and anxiety: a population-based study. Alzheimers Dement. 2017;13(2):130–139. doi:10.1016/j.jalz.2016.06.2361
- Cerri LQ, Justo MC, Clemente V, Gomes AA, Pereira AS, Marques DR. Insomnia Severity Index: a reliability generalisation meta-analysis. J Sleep Res. 2023;32:e13835.
- Brownlow JA, Miller KE, Gehrman PR. Insomnia and cognitive performance. Sleep Med Clin. 2020;15(1):71–76. doi:10.1016/j.jsmc.2019.10.002
- Wilson MM, Thomas DR, Rubenstein LZ, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005;82(5):1074–1081. doi:10.1093/ajcn/82.5.1074
- Oh SY, Koh SJ, Baek JY, et al. Validity and reliability of Korean version of Simplified Nutritional Appetite Questionnaire in patients with advanced cancer: a multicenter, longitudinal study. Cancer Res Treat. 2019;51:1612–1619.
- Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30(3):647–673. doi:10.1007/s11136-020-02688-y
- Lee SW, Lyu YR, Kim SY, et al. Efficacy and safety of GHX02 in the treatment of acute bronchitis and acute exacerbation of chronic bronchitis: a Phase II, randomized, double-blind, placebo-controlled, multicenter trial. Front Pharmacol. 2021;12:761575.
- Gokhman D, Kelman G, Amartely A, Gershon G, Tsur S, Carmel L. Gene ORGANizer: linking genes to the organs they affect. Nucleic Acids Res. 2017;45:W138–W145.
- Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol. 2014;50(2):534–544. doi:10.1007/s12035-014-8657-1
- Saresella M, Calabrese E, Marventano I, et al. Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav Immun. 2011;25:539–547.
- Serre-Miranda C, Roque S, Santos NC, et al. Cognition is associated with peripheral immune molecules in healthy older adults: a cross-sectional study. Front Immunol. 2020;11:2045. doi:10.3389/fimmu.2020.02045
- Jeong JY, Chung YC, Jin BK. Interleukin-4 and interleukin-13 exacerbate neurotoxicity of prothrombin kringle-2 in cortex in vivo via oxidative stress. Int J Mol Sci. 2019;20(8):1927. doi:10.3390/ijms20081927
- Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, López-Vales R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. 2016;64(12):2079–2092. doi:10.1002/glia.23041
- Xie L, Zhang N, Zhang Q, et al. Inflammatory factors and amyloid β-induced microglial polarization promote inflammatory crosstalk with astrocytes. Aging. 2020;12(22):22538–22549. doi:10.18632/aging.103663
- Eom TM, Kwon HH, Shin N, et al. Traditional Korean herbal formulae, Yuk-Mi-Ji-Hwang-Tang, ameliorates impairment of hippocampal memory ability by chronic restraint stress of mouse model. J Ethnopharmacol. 2020;260:113102. doi:10.1016/j.jep.2020.113102
- Liu B, Chen B, Yi J, et al. Liuwei dihuang decoction alleviates cognitive dysfunction in mice with D-galactose-induced aging by regulating lipid metabolism and oxidative stress via the microbiota-gut-brain axis. Front Neurosci. 2022;16:949298. doi:10.3389/fnins.2022.949298
- Scarabino D, Peconi M, Broggio E, et al. Relationship between proinflammatory cytokines (IL-1beta, IL-18) and leukocyte telomere length in mild cognitive impairment and Alzheimer’s disease. Exp Gerontol. 2020;136:110945. doi:10.1016/j.exger.2020.110945
- Rui W, Xiao H, Fan Y, et al. Systemic inflammasome activation and pyroptosis associate with the progression of amnestic mild cognitive impairment and Alzheimer’s disease. J Neuroinflammation. 2021;18(1):280. doi:10.1186/s12974-021-02329-2
- Su C, Zhao K, Xia H, Xu Y. Peripheral inflammatory biomarkers in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Psychogeriatrics. 2019;19(4):300–309. doi:10.1111/psyg.12403
- Kann O, Almouhanna F, Chausse B. Interferon γ: a master cytokine in microglia-mediated neural network dysfunction and neurodegeneration. Trends Neurosci. 2022;45(12):913–927. doi:10.1016/j.tins.2022.10.007
- Belkhelfa M, Rafa H, Medjeber O, et al. IFN-γ and TNF-α are involved during Alzheimer disease progression and correlate with nitric oxide production: a study in Algerian patients. J Interferon Cytokine Res. 2014;34(11):839–847. doi:10.1089/jir.2013.0085
- Yin Z, Herron S, Silveira S, et al. Identification of a protective microglial state mediated by miR-155 and interferon-γ signaling in a mouse model of Alzheimer’s disease. Nat Neurosci. 2023;26(7):1196–1207. doi:10.1038/s41593-023-01355-y
- Mayagoitia K, Tolan AJ, Shammi S, et al. Loss of APP in mice increases thigmotaxis and is associated with elevated brain expression of IL-13 and IP-10/CXCL10. Physiol Behav. 2021;240:113533. doi:10.1016/j.physbeh.2021.113533
- Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944–2971. doi:10.1111/febs.14466
- Bettcher BM, Fitch R, Wynn MJ, et al. MCP-1 and eotaxin-1 selectively and negatively associate with memory in MCI and Alzheimer’s disease dementia phenotypes. Alzheimers Dement. 2016;3:91–97.
- Morgan AR, Touchard S, Leckey C, et al. Inflammatory biomarkers in Alzheimer’s disease plasma. Alzheimers Dement. 2019;15(6):776–787. doi:10.1016/j.jalz.2019.03.007
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–621. doi:10.1056/NEJMra052723
- Marksteiner J, Kemmler G, Weiss EM, et al. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2011;32(3):539–540. doi:10.1016/j.neurobiolaging.2009.03.011
- Bayer AJ. The role of biomarkers and imaging in the clinical diagnosis of dementia. Age Ageing. 2018;47(5):641–643. doi:10.1093/ageing/afy004