Abstract
Purpose
To evaluate the effect of lurasidone on a new, patient Life Engagement scale in schizophrenia.
Patients and Methods
This post-hoc analysis included participants (ages 18 to 74) diagnosed with schizophrenia who were randomized to lurasidone (40 mg/day) or placebo in a 6-week double-blind efficacy study and those who continued in a subsequent 12-week open-label extension study during which patients received either 40 or 80/mg day lurasidone (flexibly dosed). Change in life engagement was measured using the Positive and Negative Syndrome Scale (PANSS) 11-item Life Engagement subscale score, and individual subscale items, at week 6 during the double-blind phase and extension phase week 12 during the open-label extension phase.
Results
Analysis focused on 478 subjects randomized to lurasidone or placebo during the 6-week trial, and 146 who received lurasidone during the extension phase. During the 6-week trial, there was a significantly greater change on the PANSS11 Life Engagement subscale score from baseline to week 6 in the lurasidone group compared to the placebo group (mean changes of −6.4 and −4.8, respectively, p = 0.006; effect size = 0.27). Further improvement was evident during the extension phase for patients who received lurasidone in both phases, with a mean change from double-blind baseline to week 12 of the open-label treatment phase of −10.1 on in PANSS11 Life Engagement subscale.
Conclusion
This post-hoc analysis suggests that lurasidone may improve life engagement in patients with schizophrenia, a meaningful outcome from patients’ perspective. Further studies are needed to confirm this effect.
Eudract Number
Trial registration: EudraCT Numbers: 2016-000060-42; 2016-000061-23.
Introduction
Schizophrenia is estimated to affect 21 million people worldwide.Citation1 Substantial burdens on both the individual with the disorder and society in general have been documented, with schizophrenia accounting for 12.66 million disability-adjusted life years.Citation2 Of concern is that this level of burden has increased significantly increased over the past 30 years.Citation2 Contributing to the burden of schizophrenia is the decreased quality of life and poor functioning, increased morbidity including cardiovascular disease, and lowered life expectancy associated with the disorder.Citation3–6 The worldwide economic burden of schizophrenia has been estimated to be 0.02–1.65% of the gross domestic product (GDP) of each country, with a large component (50–85%) of this economic burden attributable to indirect costs defined as productivity losses related to morbidity and premature mortality.Citation7
The problem of decreased quality of life in schizophrenia has recently been re-formulated and extended to encompass the broader, patient-centered outcome of “life engagement”.Citation8,Citation9 Life engagement as a concept is based on the patient’s perception of what is important and affects their quality of life including positive outcomes relating to cognition, vitality, motivation, and reward, and the ability to feel pleasure.Citation9 Life engagement has been found to be associated with increased well-being and a feeling of participation in meaningful family, social, and community activities.Citation9–11 It appears to be a patient-centered construct that is an important contributor to the ultimate treatment goal, not just symptom remission, but functional recovery. Furthermore, a recent report of an expert roundtable concluded that Initiating conversations about a patient’s engagement with life and monitoring it on an ongoing basis can strengthen the therapeutic alliance and provide insights about the patient’s progress toward recovery.Citation12
No specific instrument has yet been developed and characterized in terms of reliability or validity to evaluate the key facets of life engagement. To date there has been one effort to create a life engagement scale using a subset of items from the Positive and Negative Symptom Scale (PANSS) scale.Citation13 A modified Delphi process was used to select 11 items from the 30-item PANSS that captured patient engagement and well-being beyond improvement of the core symptoms of schizophrenia.Citation8 A principal components analysis found that the majority of these items clustered together, validating forming a life engagement subscale based on these items. In exploratory post-hoc analyses (to-date only available in abstract form) treatment with brexpiprazole was reported to have the potential to improve patient well-being and engagement with life.Citation8
Lurasidone is a second-generation antipsychotic approved for the treatment of people with schizophrenia in Japan, the United States, European Union, and many other countries worldwide. Lurasidone has high affinities for dopamine D2, serotonin 5-hydroxytryptamine (5-HT)2A, 5-HT7, and noradrenaline α2C receptors as antagonist, and 5-HT1A as partial agonist.Citation14,Citation15 In contrast to other atypical antipsychotics, lurasidone possesses similar binding affinities for the D2 and 5-HT2A receptors, but greater affinity for serotonin 5-HT1A receptors. In addition, lurasidone shows weak affinity for 5-HT2C, histamine H1 or muscarinic M1 receptors which are thought to be involved with metabolic syndrome effects, weight gain and sedation.Citation14
In the treatment of schizophrenia, a meta-analysis of 8 short-term (6 week) placebo-controlled studies conducted in the US, Europe, Asia, and South America reported that lurasidone was efficacious relative to placebo in terms of change in positive symptoms, negative symptoms, and general psychopathology.Citation16 The meta-analysis also found lurasidone to be well tolerated with only minimal effects on body weight, glucose, and lipid parameters.Citation16 Long-term continuation studies of 6 to 22 months in duration have found continued efficacy and continued minimal effects on body weight and metabolic parameters for lurasidone in the treatment of schizophrenia.Citation17–21
In a recent 6-week double-blind, placebo-controlled trial (JEWEL Study), lurasidone 40 mg/day again demonstrated efficacy and safety in a diverse patient population with acute schizophrenia.Citation22 A 12-week extension trial (JEWEL Extension Study) that enrolled patients completing the JEWEL 6-week study found continued improvement on efficacy measures.Citation23 No published studies that we are aware of (with the exception of the previously cited brexpiprazole abstractCitation8) have reported on the efficacy of marketed antipsychotic agents in improving life engagement. This is a significant gap in the treatment literature. The purpose of the current study is to evaluate the effect of lurasidone on life engagement in schizophrenia using data from the JEWEL StudyCitation22 and JEWEL Extension Study.Citation23
Methods
Overview
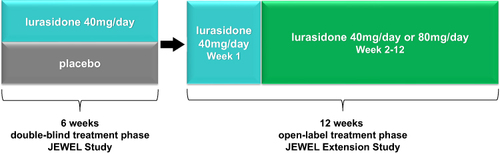
The study designs of the original JEWEL Study and JEWEL Extension Study are shown in . The JEWEL Study was a multicenter, randomized, double-blind, placebo-controlled, parallel-group study designed to evaluate the efficacy and safety of lurasidone 40 mg/day administered over a 6-week period in patients with acute schizophrenia (clinical trial registration, EudraCT number: 2016-000060-42; study initiated May, 2016 and completed November, 2018). A screening/washout phase (up to 21 days, including a 3- to 7-day single-blind washout phase) preceded the 6-week double-blind treatment phase. Patients were hospitalized during the single-blind washout and from baseline to Week 2. In the extension study (clinical trial registration: EudraCT Number: 2016-000061-23), eligible patients who completed the 6-week study, who were treated with either placebo or lurasidone 40 mg/day during the 6-week trial, were treated with flexibly-dosed 40 or 80 mg/day of open-label lurasidone for an additional 12 weeks. Efficacy assessments were conducted weekly throughout the 6-week double-blind study. During the extension study, visits occurred at open-label baseline and weeks 1, 2, 4, 8, and 12, with a follow-up visit at week 13.
The 6-week trial and extension phase were conducted, in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by the Ethics Committee at each participating center (Supplementary Tables 1 and 2). Following a detailed explanation of study procedures, written informed consent was obtained from each patient. At total of 73 clinical sites in 5 countries (Japan, Ukraine, Russia, Romania, Poland) participated.
Participants
Patients (age 18–74) were eligible for the 6-week trial if a diagnosis of schizophrenia was confirmed by a clinical interview using the Mini-International Neuropsychiatric Interview (MINI)Citation24 6.0.0 diagnostic interview with Diagnostic and Statistical Manual of Mental Disorder, 4th Edition, Text Revision (DSM-IV-TR)Citation25 criteria. Further inclusion criteria were: a PANSS total score ≥ 80; a PANSS item score ≥ 4 (moderate) on 2 or more of the following PANSS items: delusions, conceptual disorganization, hallucinations, suspiciousness, or unusual thought content at both screening and baseline; a score of 4 (moderately ill) or higher on the Clinical Global Impressions-Severity of Illness (CGI-S)Citation26 at screening and baseline; an acute exacerbation of mainly positive symptoms (eg, exacerbation of delusion or hallucination; thought disorder) for no longer than 2 months prior to the screening visit and marked deterioration of function from baseline (by history); and able to be hospitalized from visit 2 (Washout) through visit 5 (Week 2) assessments. Key exclusion criteria were the following: continuous hospitalization for >3 months (90 days) immediately prior to screening, continuous hospitalization for >14 days for acute exacerbation of psychotic symptoms immediately prior to screening in patients who had been treated continuously with adequate doses of one or more antipsychotic agents for ≥ 4 weeks immediately prior to screening, a decrease of ≥ 20% in the PANSS total score between screening and baseline visits, PANSS total score below 80 at baseline, patient is considered to be at imminent risk of suicide or injury to self or others, history of treatment with clozapine for refractory psychosis or treatment-resistant schizophrenia, receiving a total dose of antipsychotic medication equivalent to ≥ 12.0 mg/day of haloperidol at the screening visit, received any depot antipsychotic drugs (sustained-release formulation) more recently than the minimum required washout prior to screening, received fluoxetine within 1 month of screening.
For the 12-week extension study, eligibility criteria included completion of the 6-week double-blind study and all assessments on the final visit of that phase. Patients were excluded from the extension study if they were judged to be an imminent risk of suicide or injury to self or others or answered “yes” to item 4 (active suicidal ideation with some intent to act, without specific plan) or item 5 (active suicidal ideation with specific plan and intent) on the Columbia-Suicide Severity Rating Scale (C-SSRS),Citation27 at the final visit of the preceding 6-week double-blind study, which also served as open-label baseline visit of the extension study. Also excluded from the extension study were patients who exhibited evidence of severe tardive dyskinesia, severe dystonia, or other severe movement disorder, as determined by the investigator, or who required treatment with any potent cytochrome P450 (CYP) 3A4 inhibitors or inducers during the preceding double-blind study.
Randomization and Masking
For the double-blind 6-week trial, randomization was implemented (1:1 ratio of drug to placebo) using an Interactive Voice/Web Response System (IXRS) performed at baseline. Patients, investigators, and all research staff remained unaware to treatment assignment until database lock and unblinding. Lurasidone and placebo were identical in packaging, labeling, schedule of administration, and appearance.
Drug Administration and Concomitant Medications
Study drug during the 6-week double-blind trial consisted of tablets containing either lurasidone 40 mg/day or placebo administered orally. During the 12-week extension study, study drug consisted of tablets containing lurasidone 40 mg and was administered orally, as one for 40 mg and two for 80 mg. During both the 6-week trial and 12-week extension study, study drug was administered once daily, in the evening, with food or within 30 minutes after eating.
During the 12-week extension study, all patients received open-label lurasidone 40 mg/day during week 1. Following week 1 of the open-label study, flexible dosing of lurasidone to 80 mg/day was permitted, if judged clinically necessary. An increase or decrease in dose could occur at each visit thereafter.
Prohibited medications included other antipsychotics, antidepressants, mood stabilizers, other psychotropics, and potent inducers or inhibitors of the CYP 3A4 enzyme system. Biperiden, trihexyphenidyl, diphenhydramine or promethazine were permitted if benztropine was not available for the management of treatment-emergent movement disorders, or if a subject had an inadequate response or intolerability to benztropine treatment. Treatment with propranolol (≤ 120 mg/day) was permitted as needed for akathisia. Concomitant use of lorazepam, zolpidem, temazepam, brotizolam, triazolam, lormetazepam, zopiclone, or eszopiclone was permitted within protocol-specified dose limits and timing constraints (not administered within 8 hours prior an assessment).
Life Engagement Assessment
The 11-item PANSS Life Engagement subscale was used as the primary measure of changes in life engagement.Citation8 This subscale consists of the sum of PANSS items N1, N2, N3, N4, N5, N6, G6, G7, G13, G15 and G16 (N=negative symptoms; G=general psychopathology). A principal components analysis had demonstrated that the majority of these items clustered together, providing evidence for creation of a subscale on life engagement.Citation8 The individual items on the PANSS11 Life Engagement subscale were also examined.
Statistical Analyses
The analysis set for the comparison of lurasidone (40 mg/day) and placebo during the 6-week double-blind phase consisted of all Participants who received any study drug during this phase. For analyses of changes during the extension phase, we focused on the subgroup who received lurasidone (40 mg/day) during both the 6-week study and participated in the extension phase receiving flexibly dosed (40 or 80 mg/day) lurasidone.
For the comparison of lurasidone and placebo during the 6-week trial, the primary endpoint for the current analysis was least squares (LS) mean change from double-blind baseline to week 6 in the PANSS11 Life Engagement subscale. This measure was analyzed using a mixed model for repeated measures (MMRM), with fixed factors of pooled study center, visit, treatment and treatment-by visit interaction, and baseline PANSS11 Life Engagement subscale score as a covariate. Missing data were handled using the assumption that the data are missing at random (MAR). This approach allows the inclusion of all available data points without the need for imputation and is appropriate under the MAR. All weekly assessments during the 6-week trial were included. A specific contrast was included to estimate the lurasidone-placebo difference at week 6. An unstructured covariance matrix was used for the within-subject correlation. The Kenward-Roger’s approximation was used to calculate the denominator degree of freedom. The individual PANSS11 Life Engagement items were analyzed in the same way. Effect sizes were calculated as the least squares mean difference divided by the model estimate of standard deviation.
Descriptive statistics were used to summarize changes in life engagement during the 12-week extension phase. Mean change from double-blind baseline to open-label baseline and to endpoint during the extension phase were calculated for the PANSS11 Life Engagement subscale score and for the individual PANSS11 Life Engagement subscale items. For participants with missing assessment at week 12 of the extension phase, a last observation carried forward (LOCF) endpoint was derived.
All statistical inference was performed with two-sided tests at the significance level of 0.05. All data analyses were conducted using SAS Version 9.4.
Results
Patient Disposition and Baseline Characteristics
There were 593 patients screened for 6-week double-blind study, and a total of 483 were entered in the study, consisting of 247 randomized to lurasidone and 246 randomized to placebo (one of these did not placebo and was excluded from the analysis). Furthermore, a postbaseline PANSS assessment was not obtained for 2 lurasidone patients and 3 placebo patients resulting in a final lurasidone and placebo analysis sample size of 245 and 233, respectively. No patients were considered noncompliant during the 6-week trial. Of this total of 478 patients, 375 patients (78.5%; lurasidone, 199; placebo, 176) completed the 6-week study, and 289/375 (77.1%) enrolled in the 12-week extension study (from lurasidone 40–80 mg/d, 148; 40 mg/day; placebo, 141). Two patients from the lurasidone 40 mg/day 6-week study group did not have assessments after the open-label baseline and therefore were excluded, leaving 146 patients for analyses of change in life engagement during the extension phase. A total of 54 patients (18.7%) discontinued the extension study. The primary reasons for discontinuation of the extension study were withdrawal by patient (9.7%) and adverse event (5.9%).
Patients were, on average, about 40 years old and there was a similar number of men and women (). Demographic and clinical characteristics of the analysis sample for the 6-week double-blind trial were similar between the lurasidone and placebo groups. Sample sizes during the double-blind study by country were Japan 107, Ukraine 193, Russia 146, Romania 23, and Poland 9.
Table 1 Demographic and Clinical Characteristics of Sample: Double-Blind Baseline (JEWEL Study) and Open-Label Baseline (JEWEL Extension Study)
During the double-blind trial, concomitant medications in safety population were used by 42.1% (n=104) of those in the lurasidone group and 45.1% (n = 106) of those in the placebo group. These were primarily anxiolytics (n=66, 26.7%, for lurasidone; n=74, 31.5% for placebo), most commonly lorazepam, and hypnotics/sedatives (n=50, 20.2% for lurasidone; n=53, 22.6% for placebo), most commonly brotizolam. Antiparkinsonian medications were used by 4.9% (n=12) of those in the lurasidone group and 1.3% (n=3) of those in the placebo group.
During the 12-week extension study, concomitant medications used included antiparkinsonian (n=21, 7.3%), anxiolytics (n=66, 22.8%), hypnotics/sedatives (n=49, 17.0%), and other antipsychotics (n=36, 12.5%). Concomitant use of an antipsychotic was a protocol violation. The most commonly used anxiolytic was lorazepam (n=55, 19%), while brotizolam (n=24, 8.3%), eszopiclone (n=12, 4.2%), and triazolam (n=10, 3.5%) were the more commonly used hypnotics/sedatives.
Comparison of Lurasidone and Placebo on Change in Life Engagement During 6-Week Trial
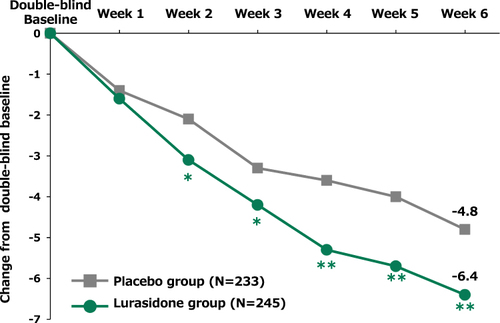
Over the course of the 6-week double-blind trial, there was significantly greater improvement on the Changes PANSS11 Life Engagement subscale score from double-blind baseline to week 6 of the double-blind treatment phase for lurasidone compared to placebo (p = 0.006; effect size = 0.27). LS mean changes in PANSS11 Life Engagement subscale score were −6.4 in the lurasidone group and −4.8 in the placebo group (). The lurasidone-placebo difference was particularly evident in three of the PANSS11 Life Engagement items that showed significant differences in LS mean change from double-blind baseline to double-blind week 6. These were “N5 Difficulty in abstract thinking” (p = 0.020; effect size = 0.23), “G15 Preoccupation” (p = 0.003; effect size = 0.30), and “G16 Active social avoidance” (p < 0.001; effect size = 0.36) ().
Table 2 Mean Change from Baseline in PANSS11 Life Engagement Item Scores
Changes in Life Engagement During Open-Label Extension Study
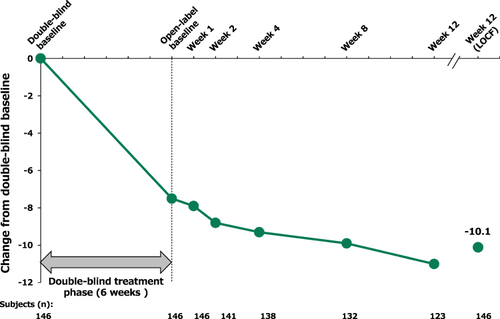
For patients who received lurasidone (40 mg/day) during the 6-week trial and continued on lurasidone (40 or 80 mg/day, flexibly dosed) during the 12-week extension study, there was evidence of continued improvement on the PANSS11 Life Engagement subscale score. Mean change from double-blind baseline to week 12 (LOCF) of the open-label treatment phase in PANSS11 Life Engagement subscale was −10.1 for this group (). Descriptively, all PANSS11 Life Engagement item scores showed further improved during the subsequent open-label treatment phase for patients that received lurasidone during both the 6-week trial and 12-week extension study ().
Discussion
The Results of this exploratory post-hoc analysis suggest that lurasidone may improve life engagement in patients with schizophrenia. Compared to placebo, the decrease in the PANSS11 Life Engagement subscale score during the double-blind treatment phase in the lurasidone group was mainly attributable to the decrease in the baseline scores of three items “N5 Difficulty in abstract thinking”, “G15 Preoccupation” and “G16 Active social avoidance”, with effect sizes of 0.23, 0.30, and 0.36, respectively. The reason why these three items had the largest treatment effect is unclear, though it may be partly attributable to the fact that these items had the highest baseline severity scores. Preoccupation and Difficulty in abstract thinking both measure autistic-like absorption with internally generated thoughts or feelings, or idiosyncratic thinking that impairs normative abstract cognitions. These two items, together with Active social avoidance, all have high face validity with the construct of Life Engagement, and we suspect that additional scale validation research may identify these items as part of a revised ‘core’ Life Engagement scale.
In addition to the improvement observed during the first 6 weeks of double-blind treatment, continued improvement was observed in the PANSS11 Life Engagement subscale and its constituent items over the course of the subsequent 12-week extension phase. The lack of a double-blind placebo comparator makes it difficult to draw confident inferences regarding the long-term effect of lurasidone on the lifetime engagement.
Due to the relatively recent focus on life engagement in the schizophrenia treatment literature, there is little basis for comparison of the current results to findings in other studies. The one study (available only in Abstract form) that combined three short-term (6 week) trials of brexpiprazole found a drug-placebo difference effect size of 0.31 on change on the PANSS11 Life Engagement subscale, similar to the effect size (0.27) found here.Citation8 The mean baseline PANSS total score across the three trials, however, was about 96 (reported hereCitation28), somewhat lower than the mean baseline PANSS total score of about 102 in the current study. The higher baseline PANSS total score in the current study (equal to approximately a half SD) suggests greater severity of illness relative to the brexpiprazole study. However, it is unclear whether baseline severity, or other between-study differences in patient populations or study design, might have influenced the effect of each study drug (or time-to-effect) on the lifetime engagement outcome. It should be noted that in a randomized, double-blind clinical trial designed to demonstrate the efficacy of active drug, an effect size in the range of 0.30 is generally considered to be “small”Citation29 when compared to placebo (between-group effect size). However, a significant drug versus placebo difference may not represent a clinically meaningful improvement in a patient’s outcome. The magnitude of within-patient (pre-post) improvement is often considered to be a more sensitive indicator of clinically meaningful change, and the within-patient, baseline-to-endpoint improvement in the current study demonstrates a standard mean difference of >1.0 for the three Life Engagement item (“N5 Difficulty in abstract thinking”, “G15 Preoccupation”, “G16 Active social avoidance”) with mean item severity shifting from a moderate level at Baseline to a mini-to-mild level of severity at endpoint (3.8–3.9 to 2.4–3.1).Citation30
More research is needed to determine the importance of life engagement as a construct, and as a pre-condition for a patient’s full return to functioning in the community, both socially and occupationally. More research is also needed to clarify the exact mechanism through which lurasidone improves life engagement. A recent study, however, indicates that impairments in cognitive function in schizophrenia correlate strongly with assessments of “life activities – household”, “participation in society”, and “getting along with people”, all dimensions that overlap with the concept of “life engagement”.Citation31 Lurasidone has been found to improve cognitive function,Citation32,Citation33 social cognition,Citation34 and social function,Citation35 in patients with schizophrenia. Thus, it is possible that lurasidone improves life engagement through the improvement of cognitive function, social cognition and/or social function.
As discussed in detail elsewhere, there is an unmet need to improve clinical attention to life engagement in the treatment of schizophrenia.Citation9 In formulating treatment plans and goals, however, physicians must be aware that their self-set treatment goals may not be consistent with the patient’s treatment goals. A discordance between physician and patient treatment goals, if unaddressed, has been found to be associated with reduced adherence and relatively poorer treatment outcomes.Citation36 Greater attention to life engagement in both clinical and research settings is warranted. The current study provides preliminary support for incorporating these patients’ needs into treatment plans and the evaluation of treatment outcomes when lurasidone is prescribed.
Several limitations of the current study need to be mentioned. The most important limitation is that the current results were based on a post-hoc analysis of data from the JEWEL Study and JEWEL Extension Study, and thus must be considered to be exploratory, and need to be confirmed by a randomized, double-blind, prospective study. Further research needs to confirm current findings prospectively in a hypothesis testing trial. Another limitation is that patients included in this study met specific inclusion and exclusion criteria. Therefore, the extent to which the findings generalize beyond the type of sample recruited in the JEWEL Study is uncertain. A further limitation is that the PANSS11 Life Engagement subscale has not undergone standard validity and reliability testing, including assessing whether it has sensitivity to change, and the extent to which the scale, as a Baseline and a change measure is correlated with improvements in other psychiatric symptoms, such as positive or negative symptoms. Scale development work in this area would help advance research on life engagement in schizophrenia. The PANSS11 Life Engagement subscale, however, has the advantage of being contained within the widely used PANSS scale. This provides the opportunity for life engagement to be examined post-hoc in other schizophrenia treatment studies to determine the extent to which different agents improve life engagement.
Conclusion
These exploratory results of a post-hoc analysis suggest that lurasidone (40 or 80 mg/day) may improve life engagement in patients with schizophrenia. Further studies are required to confirm the validity and reliability of the PANSS11 Life Engagement subscale. Once a validated Life Engagement scale is confirmed, then a more ambitious research program can be undertaken to determine: (1) whether existing (or future) antipsychotic agents have differential treatment effects on this outcome; (2) the dose-response relationships of effective agents; and (3) the extent to which improvement in Life Engagement contributes to the ultimate treatment goal of recovery, indicating symptomatic remission with a return to pre-illness levels of functioning.
Disclosure
I Miura reports personal fees from Sumitomo Pharma Co., Ltd., Eisai, Janssen, Meiji Seika Pharma, MSD, Otsuka, Takeda, Tanabe-Mitsubishi, Towa, Yoshitomi, Viatris, outside the submitted work. H Maruyama, F Sano, R Sakaguchi, and K Okamoto are full-time employees of Sumitomo Pharma Co., Ltd. The authors report no other conflicts of interest in this work.
Acknowledgments
We thank the patients and providers who participated in the study. We also thank Kentaro Takai who is a member of Sumitomo Pharma Co., Ltd. for providing support in performing the post-hoc analyses. Medical writing assistance was provided by Edward Schweizer, MD, of Paladin Consulting Group, Inc. (Concord, MA).
Data Sharing Statement
The data supporting the findings of this study have not been made publicly available due to ethical restrictions.
Additional information
Funding
References
- World Health Organization. Schizophrenia fact sheet. 2016. Available from: https://www.who.int/news-room/fact-sheets/detail/schizophrenia. Accessed March 20, 2023.
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi:10.1016/S0140-6736(18)32279-7
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi:10.1001/archpsyc.64.10.1123
- Tandberg M, Sundet K, Andreassen O, Melle I, Ueland T. Occupational functioning, symptoms and neurocognition in patients with psychotic disorders: investigating subgroups based on social security status. Soc Psychiatry Psychiatr Epidemiol. 2013;48:863–874. doi:10.1007/s00127-012-0598-2
- Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–180. doi:10.1002/wps.20420
- He H, Liu Q, Li N, et al. Trends in the incidence and DALYs of schizophrenia at the global, regional and national levels: results from the Global Burden of Disease Study 2017. Epidemiol Psychiatr Sci. 2020;29:e91. doi:10.1017/S2045796019000891
- Chong HY, Teoh SL, Wu DB, et al. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357–373. doi:10.2147/NDT.S96649
- Ismail Z, Pedersen AM, Thase ME, et al. Effect of brexpiprazole on engagement in patients with schizophrenia: post-hoc analysis of three studies. Schizophr Bull. 2020;46(Suppl 1):S208–S209. doi:10.1093/schbul/sbaa030.503
- Correll CU, Ismail Z, McIntyre RS, Rafeyan R, Thase ME. Patient functioning and life engagement: unmet needs in major depressive disorder and schizophrenia. J Clin Psychiatry. 2022;83(4):LU21112AH1. doi:10.4088/JCP.LU21112AH1
- Ho MY, Cheung FM, Cheung SF. The role of meaning in life and optimism in promoting well-being. Pers Individ Dif. 2010;48(5):658–663. doi:10.1016/j.paid.2010.01.008
- Bartrés-Faz D, Cattaneo G, Solana J, et al. Meaning in life: resilience beyond reserve. Alzheimers Res Ther. 2018;10(1):47. doi:10.1186/s13195-018-0381-z
- Correll CU, Ismail Z, McIntyre RS, Rafeyan R, Thase ME. Patient Functioning, Life Engagement, and Treatment Goals in Schizophrenia. J. Clin. Psychiatry. 2022;83(5 LU21112AH2). doi:10.4088/JCP.LU21112AH2
- Kay S, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi:10.1093/schbul/13.2.261
- Ishibashi T, Horisawa T, Tokuda K, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther. 2010;334:171–181. doi:10.1124/jpet.110.167346
- Horisawa T, Ishiyama T, Ono M, Ishibashi T, Taiji M. Binding of lurasidone, a novel antipsychotic, to rat 5-HT7 receptor: analysis by [3H]SB-269970 autoradiography. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:132–137. doi:10.1016/j.pnpbp.2012.08.005
- Zheng W, Cai DB, Yang XH, et al. Short-term efficacy and tolerability of lurasidone in the treatment of acute schizophrenia: a meta-analysis of randomized controlled trials. J Psychiatr Res. 2018;103:244–251. doi:10.1016/j.jpsychires.2018.06.005
- Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27:165–176. doi:10.1097/YIC.0b013e32835281ef
- Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74:507–515. doi:10.4088/JCP.12m08084
- Loebel A, Cucchiaro J, Xu J, Sarma K, Pilalov A, Kane JM. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res. 2013;147:95–102. doi:10.1016/j.schres.2013.03.013
- Correll CU, Cucchiaro J, Silva R, Hsu J. Long-term safety and effectiveness of lurasidone in schizophrenia: a 22-month, open-label extension study. CNS Spectrums. 2016;21:393–402. doi:10.1017/S1092852915000917
- Higuchi T, Ishigooka J, Iyo M, Hagi K. Safety and effectiveness of lurasidone for the treatment of schizophrenia in Asian patients: results of a 26-week open-label extension study. Asia Pac Psychiatry. 2020;12:e12377. doi:10.1111/appy.12377
- Iyo M, Ishigooka J, Nakamura M, et al. Efficacy and safety of lurasidone in acutely psychotic patients with schizophrenia: a 6-week, randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2021;75(7):227–235. doi:10.1111/pcn.13221
- Iyo M, Ishigooka J, Nakamura M, et al. Safety and effectiveness of lurasidone in patients with schizophrenia: a 12-week, open-label extension study. Neuropsychiatr Dis Treat. 2021;17:2683–2695. doi:10.2147/NDT.S320021
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th Ed. Text Rev). Washington, DC: American Psychiatric Press; 2000.
- Guy W. ECDEU Assessment Manual for Psychopharmacology (Revised), (ADM) 76–338 Edn. Rockville, MD: National Institute of Mental Health; 1976.
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. doi:10.1176/appi.ajp.2011.10111704
- Marder SR, Meehan SR, Weiss C, Chen D, Hobart M, Hefting N. Effects of brexpiprazole across symptom domains in patients with schizophrenia: post hoc analysis of short- and long-term studies. Schizophr Bull Open. 2021;2(1):sgab014. doi:10.1093/schizbullopen/sgab014
- Cohen J. Statistical Power Analysis for the Behavioral Sciences (Second Edition). Erlbaum; 1988.
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. doi:10.1037//0022-006x.59.1.12
- Veleva I, Stoychev K, Stoimenova-Popova M, Mineva-Dimitrova E. Impact of cognitive disturbances and clinical symptoms on disability in patients with paranoid schizophrenia: a study of a Bulgarian clinical sample. Int J Environ Res Public Health. 2023;20(3):2459. doi:10.3390/ijerph20032459
- Harvey PD, Ogasa M, Cucchiaro J, Loebel A, Keefe RS. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. Ziprasidone Schizophr Res. 2011;127(1–3):188–194. doi:10.1016/j.schres.2011.01.004
- Harvey PD, Siu CO, Hsu J, Cucchiaro J, Maruff P, Loebel A. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: a short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur Neuropsychopharmacol. 2013;23:1373–1382. doi:10.1016/j.euroneuro.2013.08.003
- Harvey PD, Siu CO, Ogasa M, Loebel A. Effect of lurasidone dose on cognition in patients with schizophrenia: post-hoc analysis of a long-term, double-blind continuation study. Schizophr Res. 2015;166(1–3):334–338. doi:10.1016/j.schres.2015.06.008
- Miura I, Sano F, Sakaguichi R, et al. Effect of lurasidone on social functioning in schizophrenia: post-hoc analysis of the JEWEL Study. J Clin Psychiatry. 2024;85(1):23m14881. doi:10.4088/JCP.23m14881
- Bridges JFP, Slawik L, Schmeding A, et al. A test of concordance between patient and psychiatrist valuations of multiple treatment goals for schizophrenia. Health Expect. 2013;16(2):164–176. doi:10.1111/j.1369-7625.2011.00704.x