Abstract
The prevalence of severe mental disorders has been rising annually. Electroconvulsive therapy (ECT) is considered a valuable treatment option in psychiatry for conditions such as schizophrenia and medication-resistant depression, especially when other treatments have proven insufficient. ECT rapidly improves patients’ mood, alleviates symptoms, and demonstrates significant therapeutic effects. Currently, the form of ECT used in clinical practice is modified electroconvulsive therapy (mECT), which is administered under general anesthesia. Accumulative evidence has confirmed that different anesthetic drugs, anesthetic-ECT time interval, anesthetic depth, and airway management can impact the outcomes of ECT. Therefore, this review aims to summarize the current impact of anesthesia factors on ECT, providing reference for clinical anesthesia during ECT procedures.
Keywords:
Introduction
The prevalence of mental disorders such as depression is steadily rising, indicating a worsening trend.Citation1,Citation2 These conditions not only cause subjective distress to patients, impacting their quality of life, but also disrupt family harmony, increase societal burdens, and pose a threat to the sustainable development of human society, thus becoming one of the foremost global public health issues.Citation3,Citation4
Currently, conventional pharmacological interventions for various mental disorders are limited in their effectiveness, requiring time to take effect, and exhibiting relatively low rates of relief and efficacy.Citation5,Citation6 Electroconvulsive therapy (ECT) refers to the method of treating diseases by stimulating the patient’s head with a specific amount of electric current, inducing seizure-like discharges in the cerebral cortex.Citation7 It is a valuable treatment option for depression, including severe and resistant forms.Citation8 ECT can rapidly control patients’ symptoms and improve their mood.Citation9,Citation10 When conventional drug therapies and psychological interventions fail to significantly improve the patient’s symptoms, ECT should be considered an important treatment option.Citation11
Initially, ECT was conducted without anesthesia, and the electrical stimulation and seizures during the procedure could potentially cause traumatic injuries to patients, such as fractures or dental damage.Citation12 With technological advancements, modern ECT has evolved into modified ECT (MECT), which is conducted under general anesthesia. Administering anesthesia and muscle relaxants can reduce patients’ fear of treatment and the associated risk of muscle spasms caused by treatment.Citation13 Compared to the original non-anesthetic method, this approach is safer.
Currently, accumulated evidence has focused on the relationship between anesthesia and ECT. A more appropriate choice of anesthesia helps improve the quality of seizures and thus enhances treatment effectiveness.Citation14 Different anesthetic drugs, anesthetic-ECT time interval (TI), depth of anesthesia, and airway management can influence the effectiveness of ECT. Therefore, we review the current study status, summarize the impact of anesthesia on ECT, and hope that anesthesiologists can provide better protection for patients during the ECT process.
Potential Mechanisms of ECT
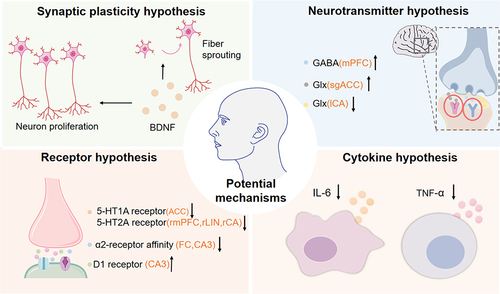
To date, multiple studies support the efficacy of ECT in treating various mental disorders, but its specific mechanisms remain not fully elucidated. Extensive experimental data suggests that ECT may be associated with changes in the central nervous system, including synaptic plasticity, neurotransmitter activity, receptor and cytokine hypothesis.
According to the neuroplasticity hypothesis, a study shows that the levels of plasma brain-derived neurotrophic factor (BDNF) increase in patients with treatment-resistant schizophrenia after ECT.Citation15 ECT can treat mental disorders by inducing BDNF production to affect neuronal proliferation in the dentate gyrus and the sprouting of its efferent fibers.Citation16
ECT also improves depressive symptoms by affecting neurotransmitters. After ECT, for instance, there is an increase in glutamate (Glu) /glutamine (Glx) in the subgenual anterior cingulate cortex (sgACC), a decrease in Glx in the left hippocampus (ICA), and an increase in gamma-aminobutyric acid (GABA) concentration in the medial prefrontal cortex (mPFC). These changes may be associated with clinical improvements in mental disorders.Citation17–19
After ECT, the reduction of 5-hydroxytryptamine1A (5-HT1A) receptors in the anterior cingulate cortex (ACC), along with the decrease in 5-hydroxytryptamine2A (5-HT2A) receptors in the right hippocampal parahippocampal gyrus (rCA), right lingual gyrus (rLIN), and right medial prefrontal gyrus (rmPFC), may be associated with the improvement of depressive symptoms.Citation20,Citation21
There is an increased affinity of ɑ2 adrenergic receptors in the frontal cortex (FC) and hippocampus (CA) in patients with depression.Citation22,Citation23 The affinity of ɑ2 receptors at the aforementioned sites decreased after ECT, which might be one of the mechanisms by which ECT improves psychiatric symptoms.Citation23 Simultaneously, ECT can modulate the expression of dopamine receptors’ encoding genes, causing an upregulation of D1 receptors in the hippocampal CA3 region, thereby helping the treatment of severe mental disorders.Citation24
The cytokine hypothesis suggests that alterations in the levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) after ECT might be one of the therapeutic mechanisms. Activation of the inflammatory response system (IRS) in patients with depression may lead to an increased release of these cytokines, while the levels of these inflammatory factors significantly decrease after ECT.Citation25–27 This indicates that cytokine may be one of the reasons ECT contributes to improving mental disorders ().
Influence of Anesthetic Factors on ECT
Multiple studies indicate that anesthesia factors have a significant impact on ECT. During the process, the management of anesthesia can affect the treatment’s efficacy and the safety of patients. The quality of seizures during ECT is regarded as a pivotal factor and a potential indicator of treatment effectiveness.Citation28,Citation29 Seizure duration is an important indicator for the quality of seizure. Although the view that seizure duration is a primary determinant of treatment efficacy is changing, seizure durations of less than 25s or 15s are still believed to be less effective.Citation30 If the motor activity lasts at least 20s or the EEG activity lasts at least 30s, it is considered sufficient. For patients over 70 years of age, seizure duration is considered appropriate if motor activity exceeds 15 seconds or EEG activity exceeds 25 seconds.Citation31 Furthermore, central inhibition (concordance or the postictal suppression index), amplitude, interhemispheric coherence and autonomic activation are common seizure parameters that can reflect seizure quality.
Influence of Different Anesthetic Drugs on ECT
Different Intravenous Anesthetics
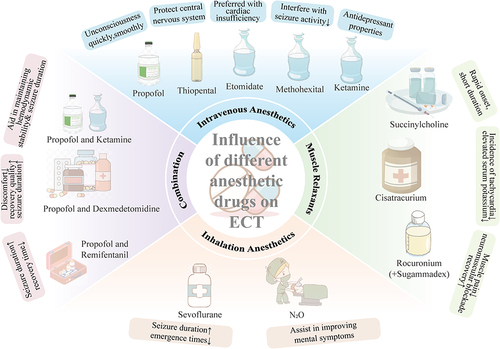
Propofol is a widely used intravenous anesthetic agent in general anesthesia.Citation32 Due to its excellent general anesthesia and sedative-hypnotic effects, and its ability to induce patients into unconsciousness quickly and smoothly, it has become one of the most commonly used intravenous anesthetics in ECT.Citation33 The anesthesia process induced by propofol is stable, with stable hemodynamic parameters and fewer complications during the recovery period, making it safer in patients with hypertension, cardiovascular disease, hyperthyroidism, and other diseases.Citation29,Citation34,Citation35 Compared to thiopental sodium, propofol can reduce acute cognitive impairments after ECT.Citation34,Citation36 These are significant advantages of propofol over other intravenous anesthetic drugs. However, the anticonvulsant effect of propofol may raises the seizure threshold, resulting in shorter seizure duration during ECT than thiopental sodium or etomidate.Citation29 Nonetheless, this study demonstrates that the seizure duration of ECT under propofol anesthesia still exceeds 25 seconds, thus maintaining the efficacy of the treatment.Citation29 Therefore, propofol remains the most commonly used anesthetic drug in ECT due to its demonstrated superiority in several aspects.
Thiopental sodium is an ultra-short-acting barbiturate intravenous anesthetic. It reduces cerebral metabolic rate and contracts cerebral blood vessels, thereby lowering cerebral blood flow and intracranial pressure, which is beneficial for protecting the central nervous system during ECT.Citation37 In comparison to propofol, thiopental sodium has weaker anticonvulsant effects, leading to significantly prolonged and improved quality of seizures during ECT.Citation37 Meanwhile, patients who use thiopental sodium anesthesia exhibit better memory function.Citation38 However, other researchers have reached the opposite conclusion when studying the effects of thiopental sodium and propofol on the efficacy of ECT, thiopental sodium may pose greater risks of cognitive impairments and its recovery period is relatively long.Citation35,Citation39 Therefore, the effects of sodium thiopental on the central nervous system still need to be supported by further findings. In addition, thiopental sodium was once used for lethal injection and is no longer available for use in the United States due to this association.Citation40
Etomidate is an imidazole derivative, which can rapidly induce unconsciousness and exhibits brief anesthetic effects. It has been widely used in ECT.Citation41 Compared to ECT under propofol anesthesia, the use of etomidate as an anesthetic resulted in higher overall quality of seizures and longer duration of motor seizure activity.Citation35,Citation42,Citation43 Furthermore, etomidate can reduces cerebral blood flow, intracranial pressure, intraocular pressure, and cerebral oxygen consumption, making it the preferred drug for patients with cardiac insufficiency.Citation44 The study has shown that the use of etomidate may reduce the occurrence rate of cognitive impairments related to ECT.Citation45 Notably, etomidate can inhibit 11ß-hydroxylase and cholesterol metabolism, thus blocking the synthesis of corticosteroids.Citation46 Study showed that the effects of etomidate on adrenocortical function in ECT were transient. Based on the transient suppression of adrenocortical function by etomidate, it is recommended that etomidate should be administered at an interval of more than 48 hours during ECT.Citation47
Methohexital, a barbiturate salt with a specific methyl substitution at the C-2 position, is one of the commonly used intravenous anesthetic drugs in ECT.Citation48 In comparison to etomidate, methohexital achieves a shorter time to maximum sustained coherence (MSC) and lower systolic blood pressure after treatment.Citation49 Compared to propofol and thiopental sodium, methohexital is less likely to interfere with the seizure activity induced by ECT.Citation48,Citation50 Particularly, when using right unilateral electrode placement, patients receiving anesthesia with methohexital require a lower number of treatments than those under propofol anesthesia.Citation51 This suggests that methohexital may be a superior choice to propofol in the management of anesthesia in ECT.
Ketamine has long been used as an alternative anesthetic induction agent in ECT. In recent years, due to its inherent antidepressant properties, its role in ECT has gained increasing attention.Citation52 Some studies suggest that the clinical efficacy of ECT under ketamine anesthesia may be superior to that under thiopental sodium anesthesia.Citation53 Compared with propofol, ketamine anesthesia has a longer duration of ECT seizures and is most advantageous in central inhibition.Citation42 However, ketamine may lead to adverse reactions such as hallucinations, delirium, and deterioration in verbal memory.Citation42,Citation54 The duration of verbal memory deterioration remains unclear, and the impact of ketamine anesthesia on autobiographical memory also requires further study.Citation53 In future studies, we still need to focus on the effects of ketamine on patients’ cognitive function when applied to ECT to better guide clinical anesthesia protocols.
Different Inhalation Anesthetics
Sevoflurane is an inhalation anesthetic with low airway irritation. Due to its ability to shorten anesthesia induction and emergence times, and its milder suppression effects on the respiratory and cardiovascular systems, it’s widely used for anesthetic induction in patients undergoing ECT.Citation55,Citation56 For patients with severe needle phobia or intolerance to intravenous anesthetics, sevoflurane may be more suitable for inducing general anesthesia.Citation56 Compared to propofol, sevoflurane can prolong the duration of ECT-induced seizures.Citation57,Citation58 Nevertheless, it may increase blood pressure, heart rate, elevate the risk of arrhythmias, which can be mitigated by reducing or discontinuing administration post-induction.Citation56,Citation58 In conclusion, sevoflurane is a relatively suitable drug for anesthesia induction in ECT. But when using sevoflurane, it is necessary to pay close attention to the patient’s status, detect complications in time and take appropriate measures.
Nitrous oxide (N₂O) is a slightly sweet-smelling inhalation anesthetic that is relatively safe and easy to manage. N₂O can induce central sympathetic nervous system stimulation similar to ECT and trigger the release of endogenous opioids, potentially benefiting improvement in psychiatric symptoms for treatment-resistant depression patient.Citation59 Considering the antidepressant and sedative-analgesic effects of N₂O and its mild inhibitory effect on the respiratory system, it can be used as an adjunctive drug during ECT for patients with severe anxiety, psychological disorders, difficulty with intravenous injection, or needle phobia.Citation60 Although N₂O is beneficial for improving mental symptoms, more clinical studies are needed to evaluate its impact on seizure time, efficacy, and adverse reactions in ECT.
Different Muscle Relaxants
Succinylcholine, due to its rapid onset and short duration of action, is considered the preferred muscle relaxant for ECT. It can achieve the desired muscle blocking in most patients.Citation61 Compared with the cisatracurium group, the succinylcholine group had a significantly shorter duration of induced seizure, time to return of spontaneous respiration from seizure ending, and duration of apnea.Citation62 This is due to the different pharmacokinetics between the two muscle relaxants.
For patients with contraindications to succinylcholine (such as pseudocholinesterase deficiency, hyperkalemia, family history of malignant hyperthermia, glaucoma, spinal cord injury, paralysis, or mobility problems due to other conditions), non-depolarizing muscle relaxants should be considered.Citation63 Comparing the hemodynamic changes and serum potassium levels during ECT, it has revealed that the incidence of tachycardia is lower in the cisatracurium group, and the levels of elevated serum potassium are significantly lower.Citation62 Therefore, the non-depolarizing muscle relaxant cisatracurium is a safer choice for patients who are unable to use succinylcholine.Citation62 In addition, Sugammadex is a drug with a cyclodextrin structure that selectively binds to rocuronium, antagonizing the neuromuscular blocking induced by rocuronium. Compared to succinylcholine, the combination of rocuronium and Sugammadex can alleviate muscle pain and headache after ECT, and meanwhile, the recovery of neuromuscular blockade in patients is faster.Citation64 For patients who cannot tolerate succinylcholine, the combination of rocuronium and Sugammadex is a safer and more reasonable option.
Combination of Propofol and Ketamine
In recent years, some studies have recommended the combined anesthesia of propofol and ketamine during ECT. Studies have revealed that their hemodynamic effects balance each other when used in combination, thereby aiding in maintaining hemodynamic stability. And meanwhile, the concurrent use of propofol and ketamine reduces ketamine-associated adverse effects such as hallucinations and delirium. Ketamine counteracts the anticonvulsant effect of propofol, thereby prolonging the duration of ECT induced seizures and achieving better therapeutic effects.Citation42,Citation54,Citation65 This suggests that propofol combined with ketamine anesthesia may be a better choice for ECT compared to single administration.
Combination of Propofol and Dexmedetomidine
The adjuvant medication for general anesthesia, dexmedetomidine, is an adrenergic receptor agonist that contributes to maintaining hemodynamic stability in patients. Compared to sole administration of propofol, combining propofol with dexmedetomidine prolongs the average seizure duration during ECT, reduces the incidence of patient agitation, and enhances treatment satisfaction.Citation66 Additionally, the analgesic properties of dexmedetomidine alleviate patient discomfort during ECT, improving overall recovery quality.Citation33
Combination of Propofol and Remifentanil
Most of the anesthetic drugs used in ECT have anticonvulsant effects, which can shorten seizure duration.Citation67,Citation68 Adding remifentanil to propofol extends seizure duration, shortens recovery time after ECT, and concurrently reduces the occurrence of adverse reactions.Citation42,Citation69 This combination might serve as a viable alternative for ECT in patients requiring higher stimulus intensity to prolong seizure duration ().Citation70
Influence of anesthetic-ECT time interval on ECT
TI refers to the duration between administering anesthesia drugs and applying electrical currents. As most intravenous anesthetics used in ECT have anticonvulsant properties, the plasma-brain concentration of anesthetics during seizure induction affects seizure occurrence.Citation28 Variations in the TI indirectly reflect the concentration of anesthetics during stimulation, potentially impacting the intensity and quality of induced seizures, thus influencing the efficacy of ECT.Citation29,Citation71
Anesthetic drugs require sufficient time to take effect, ensuring patients enter an unconscious state before receiving ECT. If the TI is too short, the effects of anesthetic drugs may be inadequate, leading to patient discomfort or consciousness during electrical stimulation, thereby increasing treatment risks. The longer TI, the levels of anesthesia in the blood and the brain are decreased at the time of seizure induction because of redistribution to other tissues, which may produce a reduction in anticonvulsant effects, an increase in seizure quality.
Within a safe range, the longer the time interval between the injection of anesthetic drugs and the application of electric current, the longer the duration of induced seizures and the higher the quality of seizures.Citation71–73 Anesthesia given is thiopentone (2–4 mg/kg), with succinylcholine administered (0. 5–1 mg/kg) for muscle relaxation. Long TI (>2 min) can induce better seizure quality compared to short TI (<2 min), and all measures (amplitude, postictal suppression, regularity and general seizure quality) have a better score.Citation71 Besides, anesthesia given was propofol (1–2 mg/kg) and succinylcholine (0. 5–1 mg/kg). Amplitude, regularity, stereotypy, post-ictal suppression and duration show a similar pattern.Citation72 In general, greater than 2 minutes is a recommended tipping point.
The optimal TI may vary individually and can be influenced by the type and dosage of anesthetic agents used.Citation73 Routine monitoring of the TI in clinical practice is recommended, optimizing it whenever possible to maximize the quality of seizures induced during ECT.
Influence of Anesthetic Depth on ECT
The depth of anesthesia plays a crucial role in ECT. Sufficient depth of anesthesia can elevate treatment risks and side effects, while insufficient depth may lead to patient pain during electrical stimulation, resulting in muscle reactions and potential physical harm. To assess the depth of anesthesia, the bispectral index (BIS), a physiological measure that identifies a patient’s anesthetic state and level of consciousness by monitoring electroencephalogram (EEG) signals, is recommended. In clinical practice, BIS can be used to assess anesthesia depth during ECT, determining the optimal timing for seizure induction to enhance the quality of seizures.Citation31,Citation68,Citation74
The depth of anesthesia not only determines the safety and comfort of patients under electrical stimulation, but also influences the quality of seizures.Citation13,Citation75 Studies have indicated a significant correlation between the mean BIS value before ECT and the duration of seizures induced after administering anesthetics like propofol, methohexital, and others.Citation30,Citation76,Citation77 Excessive doses of propofol can shorten the duration of seizures in patients.Citation78 For instance, At doses greater than 1mg /kg, propofol can reduce the duration of seizures by about 45%.Citation79 In a comparison of two groups, one receiving anesthesia with 1 mg/kg propofol and 1 mg/kg succinylcholine, 20 patients exhibited a longer seizure duration with a mean BIS of 65 before ECT stimulation (46±10 seconds), compared to a BIS of 48. 5 before ECT stimulation (31±5 seconds).Citation30 Furthermore, In patients anesthetized with sodium thiopental and succinylcholine, higher BIS values (65. 7) before stimulation induced better seizures and required only a lower stimulation dose.Citation31 And meanwhile, BIS score is also correlated positively with central inhibition, coherence and maximal heart rate, but not with midictal amplitude.Citation31 These data suggest that the BIS score 65 is a good choice for stimulus. However, further assessment is needed to evaluate the relationship between BIS and seizure quality under a wider variety of anesthetic drugs.
In addition, patients’ BIS scores in the process of awakening from anesthesia after a seizure vary widely, so the BIS may not accurately predict awakening after an electric shock.Citation77,Citation80 Hence, determining anesthesia depth also requires a combination of clinical observation and the patient’s overall condition to ensure safe and effective treatment administration.
Influence of Airway Management on ECT
In ECT, preoxygenation and hyperventilation can improve seizure quality. Hyperventilation has been associated with longer seizure duration, reduced need to increase charge across a course of ECT to keep seizure duration.Citation81 Higher transcutaneous partial pressure of oxygen (tcpO2) to transcutaneous partial pressure of carbon dioxide (tcpCO2) ratio is associated with better seizure quality.Citation82 Using laryngeal mask can provide controlled hyperventilation (CHV) through monitoring the levels of CO2 during hyperventilation and then to improve seizure quality.Citation83 Besides, protocolized hyperventilation (PHV) reduces the patient’s tcpCO2 values throughout ECT, and prolongs seizure duration.Citation84 This suggests that employing PHV and CHV in ECT may be a straightforward and effective strategy to improve efficacy without altering anesthetic dosages, making it more applicable in routine clinical practice.Citation84
However, some studies have suggested that this result may be related to TI rather than hyperventilation. The study used linear mixed effects models to analyze the effect of TI and hyperventilation on seizure quality and found that hyperventilation is not associated with better seizure quality.Citation71 Therefore, the relationship between hyperventilation and ECT efficacy needs to be further evaluated. Currently, there are few studies on airway management in ECT, and more findings are needed to obtain optimal management protocols for better anesthesia management while improving the efficacy of ECT.
Standard airway management in clinical settings is achieved through mask ventilation, but difficulties arise during the induction of generalized seizures in ECT. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) is a method that enhances apnea tolerance and permits apnea oxygenation.Citation85,Citation86 Some studies have revealed no significant difference in seizure duration between THRIVE and mask ventilation, indicating that THRIVE may not affect the efficacy of ECT.Citation85 Additionally, THRIVE usage has shown no airway complications such as nasal injury, hoarseness or pneumothorax in patients, indicating it can be an effective alternative for difficult airway or patients with mask phobia.Citation85,Citation86 Simultaneously, it has been observed that THRIVE leads to a sustained rise in carbon dioxide levels after seizure termination.Citation85 Given the unclear clinical consequences of short-term elevated carbon dioxide levels, further study is needed to assess their impact on ECT efficacy.Citation85
Adverse Reactions of ECT
Although ECT has demonstrated significant therapeutic efficacy, it also accompanies certain adverse reactions, which can be attributed to anesthesia, anticholinergic drugs, muscle relaxants, electrical stimulation, or seizures.Citation9
General Adverse Reactions
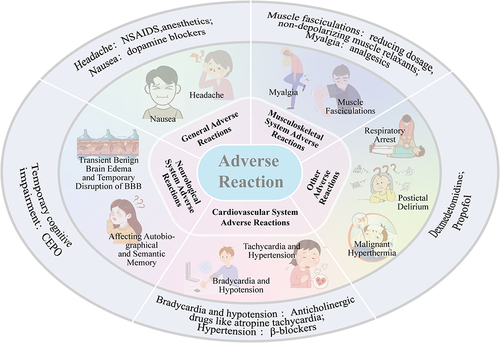
ECT may induce nausea and headache. There are several theories concerning the etiology of headache after ECT, such as vascular changes, increased cerebral blood flow, stimulation of 5-HT2 receptors, succinylcholine inducing contractions, and changes in blood pressure.Citation87 Headache can be relieved through non-steroidal anti-inflammatory drugs (NSAIDS) or anesthetics.Citation87,Citation88 Nausea may result from the effects of anesthesia and can be treated with antiemetic drugs such as dopamine blockers.Citation89 Nausea and headache induced by ECT are usually mild and typically diminish within 6 hours after ECT.Citation90
Musculoskeletal System Adverse Reactions
Depolarizing muscle relaxants can cause muscle fasciculations and myalgia, which can be alleviated by reducing the dosage or using non-depolarizing muscle relaxants or analgesics.Citation91 Seizures may result in forceful contractions of the trunk and limbs, potentially causing damage to muscles, joints, teeth, and bones. Pre use of anesthesia and muscle relaxants to ensure full effectiveness before treatment can help avoid these risks.Citation92 During ECT induced seizures, the anesthesiologist should also pay close attention to the patient’s general condition, promptly detect and manage complications of the musculoskeletal system, and strive to minimize damage.
Cardiovascular System Adverse Reactions
Hemodynamic changes induced by seizures may increase the risk of cardiovascular events, particularly in patients with pre-existing cardiovascular diseases.Citation93 Bradycardia and hypotension caused by ECT are associated with the excitation of the parasympathetic nervous system, which may lead to transient cardiac arrest, premature ventricular contractions (PVCs), and bradycardia.Citation37,Citation91,Citation94 Anticholinergic drugs like atropine aid in preventing such complications.Citation37,Citation94 Another report suggests that the occurrence of PVCs may be associated with electrolyte disturbances and increased catecholamine release due to ECT, suggesting that PVCs may be a multifactorial outcome.Citation95 Additionally, ECT may induce sympathetic nervous system excitation, manifested as tachycardia and hypertension.Citation91,Citation94 Administering β-blockers to high-risk patients during ECT can reduce these events.Citation91,Citation94
Neurological System Adverse Reactions
During ECT, the body increases cerebral blood flow to meet the increased oxygen demand caused by epileptic seizures. The resulting increase in intracranial pressure can cause transient benign brain edema and temporary disruption of blood-brain barrier (BBB) function.Citation96 In addition, ECT may cause structural and functional changes in the brain.Citation97
ECT may affect autobiographical and semantic memory.Citation91 Autobiographical memory refers to the mixed memories of personal complex life events, while semantic memory pertains to general knowledge and rules. However, although the short-term cognitive impairment of ECT is well established, the relationship between ECT and persistent memory impairment, especially autobiographical memory, remains controversial.Citation98 Patients’ memory status prior to treatment and the time of assessment after ECT treatment have an impact on the results of the assessment of autobiographical memory.Citation99 Therefore, the evaluation methods need to be refined to further determine the effect of ECT on autobiographical memory.
Other Adverse Reactions
Apart from the aforementioned adverse reactions, using depolarizing muscle relaxants like succinylcholine may lead to life-threatening complications such as respiratory arrest and malignant hyperthermia.Citation91 Postictal delirium (PID) is also a relatively common side effect following ECT.Citation100,Citation101 Pretreatment with dexmedetomidine before ECT significantly reduced the incidence of PID.Citation100 Also, propofol is a safe and effective drug for the treatment of PID ().Citation101
Material and Methods
For this review we systematically searched and screened PubMed for articles related to the influence of anesthesia factors on electroconvulsive therapy (ECT) from 2000 to 2023. Key words including “mental disorders”,“ECT”,“anesthesia factors”.
Conclusion
ECT is a crucial therapeutic approach for patients with severe mental disorders. The impact of anesthesia on ECT cannot be overlooked. It’s imperative to conduct a comprehensive evaluation of the patient before treatment and tailor medication selection accordingly. Providing adequate respiratory support is essential while closely monitoring the onset of each medication and the time intervals between anesthesia and the electrical shock stimulus to ensure that the patient reaches a sufficient depth of anesthesia. The treatment should aim to optimize the balance between patient efficacy and safety, minimizing the occurrence of adverse reactions.
To sum up, future research on anesthetics in ECT should aim to optimizing treatment protocols through comparative studies, understanding long-term effects. We can improve the patient experience through personalized anesthetic selection. Interdisciplinary collaboration between anesthesiologists and psychiatrists will be crucial in advancing this field.
Abbreviations
ECT, Electroconvulsive therapy; mECT, Modified electroconvulsive therapy; BDNF, Brain-derived neurotrophic factor; Glu, Glutamate; GABA, Gamma-aminobutyric acid; Glx, Glutamine; sgACC, Subgenual anterior cingulate cortex; ICA, Left hippocampus; mPFC, Medial prefrontal cortex; 5-HT1A, 5-hydroxytryptamine 1A; ACC, Anterior cingulate cortex; rCA, Right hippocampal parahippocampal gyrus; rLIN, Right lingual gyrus; FC, Frontal cortex; CA, Hippocampus; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor-α; IRS, Inflammatory response system; MSC, Maximum sustained coherence; N2O, Nitrous oxide; BIS, Bispectral index; EEG, Electroencephalogram; tcpO2, Transcutaneous partial pressure of oxygen; tcpCO2, Transcutaneous partial pressure of carbon dioxide; CHV, Controlled hyperventilation; PHV, Protocolized hyperventilation; THRVE, Transnasal humidified rapid-insufflation ventilatory exchange; NSAIDS, Non-steroidal anti-inflammatory drugs; CEPO, Carbamylated erythropoietin; PID, Postictal delirium.
Disclosure
The authors report no conflicts of interest in this work.
References
- Smith K. Mental health: a world of depression. Nature. 2014;515(7526):181.
- Bodnar A, Krzywotulski M, Lewandowska A, et al. Electroconvulsive therapy and cognitive functions in treatment-resistant depression. World J Biol Psychiatry. 2015;17(2):159–164. doi:10.3109/15622975.2015.1091501
- Birnbaum HG, Kessler RC, Kelley D, Ben-Hamadi R, Joish VN, Greenberg PE. Employer burden of mild, moderate, and severe major depressive disorder: mental health services utilization and costs, and work performance. Depression Anxiety. 2010;27(1):78–89. doi:10.1002/da.20580
- Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatric Serv. 2014;65(8):977–987. doi:10.1176/appi.ps.201300059
- Turner EH. Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry. 2019;6(12):977–979. doi:10.1016/S2215-0366(19)30394-3
- Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in depression: a meta-analytic review. J ect. 2004;20(1):13–20. doi:10.1097/00124509-200403000-00004
- Espinoza RT, Kellner CH. Electroconvulsive Therapy. New Engl J Med. 2022;386(7):667–672. doi:10.1056/NEJMra2034954
- Ninke T, Bayerl S, Groene P. Anesthesia for electroconvulsive therapy. Der Anaes. 2021;70(4):271–279. doi:10.1007/s00101-020-00831-5
- Koitabashi T, Oyaizu T, Ouchi T. Low bispectral index values following electroconvulsive therapy associated with memory impairment. J Anesth. 2009;23(2):182–187. doi:10.1007/s00540-008-0722-3
- Lin CY, Chen IM, Tsai HJ, Wu CS, Liao SC. Effectiveness of electroconvulsive therapy on treatment-resistant depressive disorder: a population-based mirror-image study. J Psychiatr Res. 2020;121:101–107.
- Kellner CH, Obbels J, Sienaert P. When to consider electroconvulsive therapy (ECT). Acta Psych Scand. 2020;141(4):304–315. doi:10.1111/acps.13134
- Tharyan P, Adams CE. Electroconvulsive therapy for schizophrenia. Cochrane Database Syst Rev. 2005. 2000(2):Cd000076 doi:10.1002/14651858.CD000076.pub2..
- Saito S. Anesthesia Management for Electroconvulsive Therapy. J Anes. 2005;19(2):142–149. doi:10.1007/s00540-004-0288-7
- Kadiyala PK, Kadiyala LD. ECT: a new look at an old friend. Current Opinion Anaes. 2018;31(4):453–458. doi:10.1097/ACO.0000000000000615
- Shahin O, Gohar SM, Ibrahim W, et al. Brain-Derived neurotrophic factor (BDNF) plasma level increases in patients with resistant schizophrenia treated with electroconvulsive therapy (ECT). International J Clini Prac. 2022;26(4):370–375. doi:10.1080/13651501.2022.2035770
- Kato N. Neurophysiological mechanisms of electroconvulsive therapy for depression. Neuro Res. 2009;64(1):3–11. doi:10.1016/j.neures.2009.01.014
- Pfleiderer B, Michael N, Erfurth A, et al. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res Neuroim. 2003;122(3):185–192. doi:10.1016/S0925-4927(03)00003-9
- Njau S, Joshi SH, Espinoza R, et al. Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression. J Psychiatry Neurosci. 2017;42(1):6–16. doi:10.1503/jpn.150177
- Xia M, Wang J, Sheng J, et al. Effect of Electroconvulsive Therapy on Medial Prefrontal γ-Aminobutyric Acid Among Schizophrenia Patients. J ECT. 2018;34(4):227–232. doi:10.1097/YCT.0000000000000507
- Baldinger P, Lotan A, Frey R, Kasper S, Lerer B, Lanzenberger R. Neurotransmitters and Electroconvulsive Therapy. J ECT. 2014;30(2):116–121. doi:10.1097/YCT.0000000000000138
- Yatham LN, Liddle PF, Lam RW, et al. Effect of electroconvulsive therapy on brain 5-HT2 receptors in major depression. Br J Psychiatry. 2018;196(6):474–479. doi:10.1192/bjp.bp.109.069567
- Landau AM, Phan J-A, Iversen P, et al. Decreased in vivo α2 adrenoceptor binding in the Flinders Sensitive Line rat model of depression. Neuropharmacology. 2015;91:97–102. doi:10.1016/j.neuropharm.2014.12.025
- Lillethorup TP, Iversen P, Wegener G, Doudet DJM, Landau AM. α2-adrenoceptor binding in Flinders-sensitive line compared with Flinders-resistant line and Sprague-Dawley rats. Acta Neuropsychiatr. 2015;27(6):345–352. doi:10.1017/neu.2015.24
- Kobayashi K, Imoto Y, Yamamoto F, et al. Rapid and lasting enhancement of dopaminergic modulation at the hippocampal mossy fiber synapse by electroconvulsive treatment. J Neurophysiol. 2017;117(1):284–289. doi:10.1152/jn.00740.2016
- Rush G, O’Donovan A, Nagle L, et al. Alteration of immune markers in a group of melancholic depressed patients and their response to electroconvulsive therapy. J Affective Disorders. 2016;205:60–68. doi:10.1016/j.jad.2016.06.035
- Freire TFV, Rocha NSD, Fleck MPA. The association of electroconvulsive therapy to pharmacological treatment and its influence on cytokines. J Psychiatr Res. 2017;92:205–211. doi:10.1016/j.jpsychires.2017.05.004
- Hestad KA, Tonseth S, Stoen CD, Ueland T, Aukrust P. Raised Plasma Levels of Tumor Necrosis Factor α in Patients With Depression:Normalization During Electroconvulsive Therapy. J ECT. 2003;19(4):183–188. doi:10.1097/00124509-200312000-00002
- Luccarelli J, McCoy TH, Seiner SJ, Henry ME. Changes in seizure duration during acute course electroconvulsive therapy. Brain Stimulation. 2021;14(4):941–946. doi:10.1016/j.brs.2021.05.016
- Jarineshin H, Kashani S, Fekrat F, et al. Seizure Duration and Hemodynamic State During Electroconvulsive Therapy: sodium Thiopental Versus Propofol. Global J Health Sci. 2015;8(2):126–131. doi:10.5539/gjhs.v8n2p126
- Nishihara F, Saito S. Adjustment of anaesthesia depth using bispectral index prolongs seizure duration in electroconvulsive therapy. Anaes Inten Care. 2004;32(5):661–665. doi:10.1177/0310057X0403200509
- Kranaster L, Hoyer C, Janke C, Sartorius A. Bispectral Index Monitoring and Seizure Quality Optimization in Electroconvulsive Therapy. Pharmacopsychiatry. 2013;46(04):147–150. doi:10.1055/s-0032-1331748
- Zahavi GS, Dannon P. Comparison of anesthetics in electroconvulsive therapy: an effective treatment with the use of propofol, etomidate, and thiopental. Neuropsychiatr Dis Treat. 2014;10:383–389. doi:10.2147/NDT.S58330
- Mahmoodiyeh B, Modir H, Shayganfard M, Abdus A, Almasi-Hashiani A. Efficacy of ketamine, propofol, and dexmedetomidine for anesthesia in electroconvulsive therapy in treatment-resistant major depressive disorder patients: a double-blind randomized clinical trial. Med Gas Res. 2023;13(3):112. doi:10.4103/2045-9912.350860
- Kumar A, Sharma D, Mani R. A comparison of propofol and thiopentone for electroconvulsive therapy. J Anaesthesiol Clin Pharmacol. 2012;28(3):353. doi:10.4103/0970-9185.98337
- Hussain Mir A, Shah NF, Din MU, Langoo SA, Reshi FA. Effectiveness of sodium thiopentone, propofol, and etomidate as an ideal intravenous anesthetic agent for modified electroconvulsive therapy. Saudi J Anaesth. 2017;11(1):26–31. doi:10.4103/1658-354X.197339
- Butterfield NN, Graf P, Macleod BA, Ries CR, Zis AP. Propofol reduces cognitive impairment after electroconvulsive therapy. J ect. 2004;20(1):3–9. doi:10.1097/00124509-200403000-00002
- Dannon P, Zahavi G. Comparison of anesthetics in electroconvulsive therapy: an effective treatment with the use of propofol, etomidate, and thiopental. Neuropsychiatr Dis Treat. 2014;1:383.
- Ingram A, Schweitzer I, Ng CH, Saling MM, Savage G. A Comparison of Propofol and Thiopentone Use in Electroconvulsive Therapy: cognitive and Efficacy Effects. J ECT. 2007;23(3):158–162. doi:10.1097/yct.0b013e318070d1e9
- Kavakbasi E, Stoelck A, Wagner NM, Baune BT. Differences in Cognitive Adverse Effects and Seizure Parameters Between Thiopental and Propofol Anesthesia for Electroconvulsive Therapy. J ect. 2023;39(2):97–101. doi:10.1097/YCT.0000000000000893
- Zivot JB. The absence of cruelty is not the presence of humanness: physicians and the death penalty in the United States. Philos Human Med. 2012;7(1):13. doi:10.1186/1747-5341-7-13
- Lammeren A, Dols A, van de Ven PM, et al. Etomidate and Seizure Duration in Electroconvulsive Therapy. J ECT. 2013;29(2):101–105. doi:10.1097/YCT.0b013e31827a7ebb
- Hoyer C, Kranaster L, Janke C, Sartorius A. Impact of the anesthetic agents ketamine, etomidate, thiopental, and propofol on seizure parameters and seizure quality in electroconvulsive therapy: a retrospective study. European Arch Psych Clini Neuro. 2013;264(3):255–261. doi:10.1007/s00406-013-0420-5
- Ninke T, Groene P. Electroconvulsive therapy: recent advances and anesthetic considerations. Current Opinion Anaes. 2023;36(4):441–446. doi:10.1097/ACO.0000000000001279
- Rosa MA, Rosa MO, Belegarde IMT, Bueno CR, Fregni F. Recovery after ECT: comparison of propofol, etomidate and thiopental. Rev Bras Psiquiatr. 2008;30(2):149–151. doi:10.1590/S1516-44462008005000010
- Gurel SC, Ozden HC, Karahan S, Ayhan Y. The superiority of ketofol and etomidate against propofol or thiopental anesthesia for ECT. Asian J Phsych. 2022;72:103090. doi:10.1016/j.ajp.2022.103090
- Wojdacz R, Święcicki Ł, Antosik-Wójcińska A. Comparison of the effect of intravenous anesthetics used for anesthesia during electroconvulsive therapy on the hemodynamic safety and the course of ECT. Psychiatria Polska. 2017;51(6):1039–1058. doi:10.12740/PP/75635
- Wang N, Wang XH, Lu J, Zhang JY. The Effect of Repeated Etomidate Anesthesia on Adrenocortical Function During a Course of Electroconvulsive Therapy. J ECT. 2011;27(4):281–285. doi:10.1097/YCT.0b013e3182182be0
- Chawla N. Anesthesia for Electroconvulsive Therapy. Anes clini. 2020;38(1):183–195. doi:10.1016/j.anclin.2019.10.007
- Millischer V, Pramhas S, Wiedermann I, et al. Comparison of etomidate and methohexital as anesthetic agents for continuation and maintenance electroconvulsive therapy: a retrospective analysis of seizure quality and safety. J Affective Disorders. 2023;330:33–39. doi:10.1016/j.jad.2023.02.085
- Fond G, Bennabi D, Haffen E, et al. A Bayesian framework systematic review and meta-analysis of anesthetic agents effectiveness/tolerability profile in electroconvulsive therapy for major depression. Sci Rep. 2016;6(1):19847. doi:10.1038/srep19847
- Vaidya PV, Anderson EL, Bobb A, Pulia K, Jayaram G, Reti I. A within-subject comparison of propofol and methohexital anesthesia for electroconvulsive therapy. J ect. 2012;28(1):14–19. doi:10.1097/YCT.0b013e31823a4220
- Bryson EO, Ahle GM, Liebman LS, et al. Dosing and effectiveness of ketamine anesthesia for electroconvulsive therapy (ECT): a case series. Australas Psychiatry. 2014;22(5):467–469. doi:10.1177/1039856214545547
- Rybakowski JK, Bodnar A, Krzywotulski M, et al. Ketamine Anesthesia, Efficacy of Electroconvulsive Therapy, and Cognitive Functions in Treatment-Resistant Depression. J ECT. 2016;32(3):164–168. doi:10.1097/YCT.0000000000000317
- Kelkar V, Gaddam N, Kulkarni S, Joshi P, Bhale P. A comparative study of propofol, thiopentone sodium, and ketofol as induction agents for electro convulsive therapy. J Anaesthesiol Clin Pharmacol. 2021;37(4):1.
- Delgado-Herrera L, Ostroff RD, Rogers SA. Sevoflurane:Approaching the Ideal Inhalational Anesthetic. A Pharmacologic, Pharmacoeconomic, and Clinical Review. CNS Drug Rev. 2001;7(1):48–120. doi:10.1111/j.1527-3458.2001.tb00190.x
- Rasmussen KG, Spackman TN, Hooten WM. The Clinical Utility of Inhalational Anesthesia With Sevoflurane in Electroconvulsive Therapy. J ECT. 2005;21(4):239–242. doi:10.1097/01.yct.0000180469.30712.90
- Toprak HI, Gedik E, Begeç Z, Oztürk E, Kaya B, Ersoy MO. Sevoflurane as an Alternative Anaesthetic for Electroconvulsive Therapy. J ECT. 2005;21(2):108–110. doi:10.1097/01.yct.0000166633.73555.28
- Aoki N, Suwa T, Kawashima H, et al. Sevoflurane in electroconvulsive therapy: a systematic review and meta-analysis of randomised trials. J Psychiatr Res. 2021;141:16–25. doi:10.1016/j.jpsychires.2021.06.030
- Milne B. Nitrous oxide (laughing gas) inhalation as an alternative to electroconvulsive therapy. Med Hypotheses. 2010;74(5):780–781. doi:10.1016/j.mehy.2009.11.021
- Lee K, Sparkle T. Use of Nitrous Oxide to Facilitate Induction for Electroconvulsive Therapy: a Case Report. Am J Case Rep. 2020;1:21.
- Mirzakhani H, Guchelaar H-J, Welch CA, et al. Minimum Effective Doses of Succinylcholine and Rocuronium During Electroconvulsive Therapy: a Prospective, Randomized, Crossover Trial. Anesthesia Analg. 2016;123(3):587–596. doi:10.1213/ANE.0000000000001218
- Nazemroaya B, Ghosouri A, Honarmand A, Hashemi S. Comparison of hemodynamic changes and serum potassium levels in the use of succinylcholine and cisatracurium in electroconvulsive therapy. J Res Med Sci. 2021;26(1):106. doi:10.4103/jrms.JRMS_951_19
- Booij LH. Is succinylcholine appropriate or obsolete in the intensive care unit? Critical Care. 2001;5(5):245–246. doi:10.1186/cc1039
- Saricicek V, Sahin L, Bulbul F, Ucar S, Sahin M. Does Rocuronium-Sugammadex Reduce Myalgia and Headache After Electroconvulsive Therapy in Patients With Major Depression? J ECT. 2014;30(1):30–34. doi:10.1097/YCT.0b013e3182972bd2
- Wang X, Chen Y, Zhou X, Liu F, Zhang T, Zhang C. Effects of Propofol and Ketamine as Combined Anesthesia for Electroconvulsive Therapy in Patients With Depressive Disorder. J ECT. 2012;28(2):128–132. doi:10.1097/YCT.0b013e31824d1d02
- Shams T, El-Masry R. Ketofol-Dexmedetomidine combination in ECT: a punch for depression and agitation. Indian Jl Anaes. 2014;58(3):275. doi:10.4103/0019-5049.135037
- Gasteiger L, Heil M, Hörner E, et al. Relationship Between Anesthesia Depth and Quality of Seizures in Patients Undergoing Electroconvulsive Therapy. J ECT. 2022;38(1):62–67. doi:10.1097/YCT.0000000000000792
- Andrew D. K, Weiner RD, Dean MD, et al. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci. 2003;15(1):27–34. doi:10.1176/jnp.15.1.27
- Ikiz C, Gunenc F, Iyilikci L, et al. Effects of Propofol and Propofol-Remifentanil Combinations on Haemodynamics, Seizure Duration and Recovery during Electroconvulsive Therapy. Turk J Anaesthesiol Reanim. 2021;49(1):44–51. doi:10.5152/TJAR.2020.157
- Akcaboy ZN, Akcaboy EY, Yigitbas B, et al. Effects of remifentanil and alfentanil on seizure duration, stimulus amplitudes and recovery parameters during ECT. Acta Anaes Scand. 2005;49(8):1068–1071. doi:10.1111/j.1399-6576.2005.00766.x
- Taylor R, Wark H, Leyden J, et al. Effects of the Anaesthetic-ECT time interval and ventilation rate on seizure quality in electroconvulsive therapy: a prospective randomised trial. Brain Stimulation. 2020;13(2):450–456. doi:10.1016/j.brs.2019.12.012
- Gálvez V, Hadzi-Pavlovic D, Wark H, Harper S, Leyden J, Loo CK. The Anaesthetic-ECT Time Interval in Electroconvulsive Therapy Practice – is It Time to Time? Brain Stimulation. 2016;9(1):72–77. doi:10.1016/j.brs.2015.09.005
- Haji Seyed Javadi A, Najafian E, Kayalha H, Shafikhani AA. Evaluating Factors Affecting the Time Interval Between Propofol Injection and Induction of Electro-convulsion and Relationship Between These Factors and Duration of Convulsion. Anesth Pain Medi. 2021;11(4). doi:10.5812/aapm.117442
- Gunawardane PO, Murphy PA, Sleigh JW. Bispectral index monitoring during electroconvulsive therapy under propofol anaesthesia. Br J Anaesth. 2002;88(2):184–187. doi:10.1093/bja/88.2.184
- Liu -C-C, Qian X-Y, J-X A, et al. Electroconvulsive Therapy Under General Anesthesia With Cisatracurium, Laryngeal Mask Airways, and Bispectral Index. J ECT. 2016;32(1):17–19. doi:10.1097/YCT.0000000000000251
- Gombar S, Aggarwal D, Khanna AK, Gombar KK, Chavan BS. The bispectral electroencephalogram during modified electroconvulsive therapy under propofol anesthesia: relation with seizure duration and awakening. J ect. 2011;27(2):114–118. doi:10.1097/YCT.0b013e3181df4ebb
- White PF, Rawal S, Recart A, Thornton L, Litle M, Stool L. Can the bispectral index be used to predict seizure time and awakening after electroconvulsive therapy? Anesthesia Analg. 2003;96(6):1636–1639. doi:10.1213/01.ANE.0000066018.13553.08
- Simpson KH, Halsall PJ, Carr CM, Stewart KG. Propofol reduces seizure duration in patients having anaesthesia for electroconvulsive therapy. Br J Anaesth. 1988;61(3):343–344. doi:10.1093/bja/61.3.343
- Avramov MN, Husain MM, White PF. The comparative effects of methohexital, propofol, and etomidate for electroconvulsive therapy. Anesthesia Analg. 1995;81(3):596–602.
- Nishihara F, Saito S. Pre-ictal bispectral index has a positive correlation with seizure duration during electroconvulsive therapy. Anesthesia Analg. 2002;94(5):1249–1252. (). doi:10.1097/00000539-200205000-00037
- Mayur P, Bray A, Fernandes J, Bythe K, Gilbett D. Impact of hyperventilation on stimulus efficiency during the early phase of an electroconvulsive therapy course: a randomized double-blind study. J ECT. 2010;26(2):91–94. doi:10.1097/YCT.0b013e3181c18901
- Aksay SS, Bumb JM, Janke C, Hoyer C, Kranaster L, Sartorius A. New evidence for seizure quality improvement by hyperoxia and mild hypocapnia. J ect. 2014;30(4):287–291. doi:10.1097/YCT.0000000000000109
- Haeck M, Gillmann B, Janouschek H, Grözinger M. Electroconvulsive therapy can benefit from controlled hyperventilation using a laryngeal mask. European Arch Psych Clini Neuros. 2011;261 Suppl 2:S172–176.
- de Arriba-Arnau A, Dalmau A, Soria V, et al. Protocolized hyperventilation enhances electroconvulsive therapy. J Affective Disorders. 2017;217:225–232. doi:10.1016/j.jad.2017.04.007
- Jonker Y, Rutten DJ, van Exel ER, et al. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange During Electroconvulsive Therapy. J ECT. 2019;35(2):110–114. doi:10.1097/YCT.0000000000000556
- Vaithialingam B, Bansal S, Muthuchellappan R, Thirthalli J, Chakrabarti D, Venkatapura RJ. Comparison of hands-free Trans-nasal Humidified Rapid Insufflation Ventilatory Exchange (THRIVE) with conventional facemask ventilation technique for oxygenation in patients undergoing electroconvulsive therapy - A cross over study. Asian J Phsych. 2023;88:103734. doi:10.1016/j.ajp.2023.103734
- Dinwiddie SH, Huo D, Gottlieb O. The Course of Myalgia and Headache After Electroconvulsive Therapy. J ECT. 2010;26(2):116–120. doi:10.1097/YCT.0b013e3181b07c0a
- Karaaslan E, Akbas S, Ozkan AS, Zayman EP. Effects of preemptive intravenous paracetamol and ibuprofen on headache and myalgia in patients after electroconvulsive therapy. Medicine. 2019;98(51):e18473. doi:10.1097/MD.0000000000018473
- T-C L, Shiah IS, Sun C-J, Tzang R-F, Huang K-C, Lee W-K. Mirtazapine Relieves Post-Electroconvulsive Therapy Headaches and Nausea. J ECT. 2011;27(2):165–167. doi:10.1097/YCT.0b013e3181e63346
- Haghighi M, Sedighinejad A, Naderi Nabi B, et al. The Incidence and Predictors of Headache and Myalgia in Patients After Electroconvulsive Therapy (ECT). Anesth Pain Medi. 2016;6(3). doi:10.5812/aapm.33724.
- Andrade C, Arumugham SS, Thirthalli J. Adverse Effects of Electroconvulsive Therapy. Psychiat Clin North Am. 2016;39(3):513–530. doi:10.1016/j.psc.2016.04.004
- Andrade C. Skeletal and Dental Fractures Associated With Electroconvulsive Therapy. J Clini Psych. 2023;84(1). doi:10.4088/JCP.23f14797
- Andreas D, Maleczek M, Panjikaran B, Herkner H, Karrison T, Nagele P. Major Adverse Cardiac Events and Mortality Associated with Electroconvulsive Therapy: a Systematic Review and Meta-analysis Perioperative Medicine. Anesthesiology. 2019;130(1):83–91. doi:10.1097/ALN.0000000000002488
- Tess AV, Smetana GW. Medical evaluation of patients undergoing electroconvulsive therapy: current concepts. N Engl J Med. 2009;360(14):1437–1444. doi:10.1056/NEJMra0707755
- Sato Y, Takahashi H, Miyabe M, Toyooka H. Case of premature ventricular contraction immediately after electroconvulsive therapy in a depressive patient. Masui Japanese J Anes. 2005;54(1):46–48.
- Andrade C, Bolwig TG. Electroconvulsive Therapy, Hypertensive Surge, Blood-Brain Barrier Breach, and Amnesia. J ECT. 2014;30(2):160–164. doi:10.1097/YCT.0000000000000133
- Singh S, Jolly A. Does electroconvulsive therapy cause brain damage: an update. Indian J Psych. 2020;62(4):339. doi:10.4103/psychiatry.IndianJPsychiatry_239_19
- Lomas M, Rickard V, Milton F, Savage S, Weir A, Zeman A. Electroconvulsive therapy related autobiographical amnesia: a review and case report. Cogn Neuro. 2021;26(2):107–121. doi:10.1080/13546805.2021.1871889
- Anderson IM, McAllister-Williams RH, Downey D, Elliott R, Loo C. Cognitive function after electroconvulsive therapy for depression: relationship to clinical response. Psychological Medicine. 2020;51(10):1647–1656. doi:10.1017/S0033291720000379
- M. fraser L, O’Carroll RE, Klaus PE. The Effect of Electroconvulsive Therapy on Autobiographical Memory: a Systematic Review. J ECT. 2008;24(1):10–17. doi:10.1097/YCT.0b013e3181616c26
- Qiu Z, Zhou S, Zhang M, et al. Preventive effect of dexmedetomidine on postictal delirium after electroconvulsive therapy. European J Anaes. 2020;37(1):5–13. doi:10.1097/EJA.0000000000001113
- Kikuchi A, Yasui‐Furukori N, Fujii A, Katagai H, Kaneko S. Identification of predictors of post‐ictal delirium after electroconvulsive therapy. Psych Clin Neurosci. 2009;63(2):180–185. doi:10.1111/j.1440-1819.2009.01930.x