Abstract
Vigabatrin, the first therapeutic agent to be approved by the Food and Drug Administration for the treatment of infantile spasms, as well as for adjunctive use in the treatment of refractory complex partial epilepsy, represents an important advance for patients with difficult-to-manage epilepsy. This review summarizes the complex history, chemistry, and pharmacology, as well as the clinical data leading to the approval of vigabatrin for infantile spasms in the US. The long path to its approval reflects the visual system and white matter toxicity concerns with this agent. This review provides a brief description of these concerns, and the regulatory safety monitoring and mitigation systems that have been put in place to enhance benefit over risk.
Keywords:
Introduction
Vigabatrin, an irreversible inhibitor of gamma aminobutyric acid (GABA) transaminase (GABA-T) was specifically designed to be a suicide substrate for this enzyme and thereby augment tissue GABA concentrations for the treatment of epilepsy. It was first licensed as an antiepileptic agent in the UK and the Republic of Ireland in 1989. By the late 1990s, it had been accepted into mainstream clinical practice in the care of adult and pediatric patients in over 40 countries.Citation1 In the US, vigabatrin has experienced a stuttering regulatory course. In 1998, the Food and Drug Administration (FDA) notified the manufacturer, Hoechst Marion Roussel, that vigabatrin was “not approvable” based on the data submitted up until that time, in the light of the then emerging concerns about vigabatrin. These concerns included reports of severe, persistent visual field defects noted in association with the use of vigabatrin.Citation2,Citation3 Interestingly, the development of vigabatrin in the US had been delayed for several years due to earlier concerns about white matter toxicity (intramyelinic edema) encountered in experimental animals,Citation4 then resumed when such toxicity could not be demonstrated in humans.Citation1 After years of having being shelved and after renewed efforts by a different company (Ovation Pharmaceuticals, now Lundbeck), vigabatrin received FDA approval in 2009 for the treatment of infantile spasms, as well as for refractory complex partial seizures.
The indication for infantile spasms can be considered quite remarkable because vigabatrin is the first FDA-approved treatment for this very difficult to treat disorder. Adrenocorticotropic hormone (ACTH), the legacy treatment for infantile spasms, has never been formally approved for that use in the US and is now entering regulatory hearings at the US FDA. As described here, infantile spasms represents a rare and distinctive disorder of multiple etiologies and presents many challenges to the conduct of an adequately powered controlled study.
Infantile spasms represent a devastating childhood epileptic syndrome, and has been described as one of the “catastrophic epilepsies of infancy”.Citation5 What makes infantile spasms such a form of devastating epilepsy is the combination of extremely difficult to control seizures and its association with mental retardation. The seizures involve violent flexion or extension spasms that occur in clusters. This clinical presentation, accompanied by a distinctive high-voltage chaotic electroencephalogram (EEG) pattern called hypsarrhythmia and developmental delay, is called West syndrome. Hypsarrhythmia is the characteristic interictal EEG pattern, consisting of a triad of features, including high amplitude and disorganized or “chaotic” background and multifocal independent spike discharges. Infantile spasms has an incidence of 0.25–0.42 per 1000 live births and a prevalence of 0.14–0.52 per 1000 children. Infantile spasms are slightly more common in males.Citation6 This syndrome represents the response of the brain to a severe insult during a specific developmental window, and can result from hypoxic-ischemic encephalopathy, infections, focal cortical dysplasia, intraventricular hemorrhage, tuberous sclerosis, Down syndrome, trauma, Aicardi syndrome, neonatal hypoglycemia, Ohtahara syndrome, and pyridoxine deficiency. When a specific etiology is not apparent, the syndrome is classified as cryptogenic. Advances in genetic testing have shown that mutations within the aristaless-related homeobox gene on chromosome Xp22.13 is associated with an X-linked infantile spasms syndrome.Citation7 The cyclin-dependent kinase-like or serine-threonine kinase 9 gene located on the Xp22.3 chromosome is also a cause of X-linked infantile spasms. Mutations in the lissencephaly-1 and the doublecortin genes are related to classical lissencephaly, double cortex syndrome, and infantile spasms.Citation8
Epileptic spasms have a variety of clinical and electrographic forms. The typical clinical spasms is a flexor spasms that is a rapid flexion of the neck, trunk, and extremities lasting less than two seconds, which is frequently followed by a sustained tonic phase for up to 10 seconds.Citation9 On occasions, spasms can be extensor, with a rapid extension of the neck and arching of the back, or a mixture of both flexion and extension postures. The intensity of spasms can vary, even within a cluster, from brief eye rolling or subtle head nodding to a violent shock-like event. On occasion, children will become upset or cry in between spasms. Spasms typically occur in clusters, most commonly when waking from sleep. Electrographically, there are a variety of EEG patterns which can accompany the spasms, but the most common is a generalized medium to high amplitude slow wave, followed by an electrodecrement and attenuation.Citation6 Children with symptomatic infantile spasms can have focal findings both clinically and electrographically.
Historically, the first-line treatment of infantile spasms has been ACTH. The availability of vigabatrin in much of Europe, and the finding that it was often efficacious in the treatment of infantile spasms, resulted in its adoption as the preferred agent, because of its relative ease of use. In Europe and many other countries, vigabatrin is considered the initial drug of choice. The European expert opinion statement from 2007 recommended vigabatrin as the treatment of choice for patients with tuberous sclerosis and other symptomatic etiologies.Citation10 In the US, where vigabatrin was not FDA-approved until very recently, ACTH has been considered the initial drug of choice, even though it awaits regulatory approval at this time.
Many additional agents and treatments have been tried as second and third options, including valproic acid, pyridoxine, zonisamide, topiramate, levetiracetam, intravenous immunoglobulin, and the ketogenic diet, each with a handful of uncontrolled open-label studies or retrospective case series. Resective surgery for some symptomatic cases (in particular focal cortical dysplasia) has been successful.
In 2004 the “Practice Parameter: Medical Treatment of Infantile Spasms” was released by the American Academy of Neurology and the Child Neurology Society. Their conclusions on vigabatrin included “1. Vigabatrin is possibly effective for the short-term treatment of infantile spasms (level C, class III and IV evidence), 2. Vigabatrin is also possibly effective for the short-term treatment of infantile spasms in the majority of children with tuberous sclerosis (level C, class III and IV evidence), and 3. Serious concerns about retinal toxicity in adults suggest that serial ophthalmologic screening is required in patients on vigabatrin. However, data are insufficient to make recommendations regarding the frequency or type of screening that would be of value in reducing the prevalence of this complication in children (level U, class IV studies)”.Citation11
Vigabatrin was finally approved by the FDA on August 21, 2009 with indications for refractory complex partial seizures in adults and infantile spasms in children aged one month to two years. All health care providers using vigabatrin will need to enroll in the SHARE (Support, Help and Resources for Epilepsy) program and complete the Risk Evaluation and Mitigation Strategy (REMS) course. This was specifically designed to reduce the risk of vision loss, and includes baseline and regular vision monitoring, frequent assessments of effectiveness, and education. It is suggested to discontinue the medication if patients fail to show substantial improvement in seizures within three months. Vigabatrin is now the only medication in the US with an FDA approval for infantile spasms.Citation12
Pharmacology
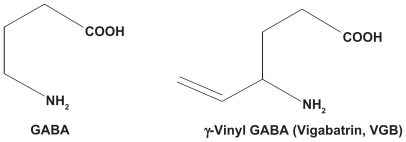
The chemical structure of vigabatrin is 4-amino-6-hexenoic acid (γ-vinyl GABA). It was designed specifically to achieve irreversible inhibition of GABA-T,Citation13 which is present in neurons and glia and causes oxidative deamination of GABA to succinic semialdehyde.
Unlike several other inhibitors of GABA-T which also display some activity against the enzyme responsible for the synthesis of GABA (glutamic acid decarboxylase) vigabatrin is selective in its antagonism of GABA degradation. Citation14 The structures of GABA and vigabatrin are shown in . The obvious structural similarity is responsible for the ability of vigabatrin to interact with GABA-T. The presence of the vinyl moiety in the structure of vigabatrin renders it a mechanism-based suicide substrate which is covalently (and irreversibly) bound to the enzyme. Only new enzyme synthesis can restore GABA-T activity. Thus, even a single dose produces a lasting, dose-dependent inhibition of brain GABA-T in experimental animals, and elevated GABA levels are seen for at least 24 hours.Citation13 After a dose of 1500 mg/kg of vigabatrin in mice, GABA levels increased to a maximum of 650% of control in four hours,Citation15 Levels of the excitatory amino acids, glutamate and aspartate, reached a nadir of approximately 80% of control values in about the same duration. Noninvasive measurement of brain GABA levels in humans by the application of 1H magnetic resonance spectroscopy has permitted the demonstration of 2.5- to 3-fold elevations in GABA levels in the brains of epileptic patients treated with standard doses of vigabatrin.Citation16,Citation17
Our present understanding of GABA receptor activation is based on its action at postsynaptic GABA(A) receptors (GABAAR), which have a specific subunit composition, and differently assembled extrasynaptic receptors. GABAAR exhibit low affinity for GABA (and thus require the high levels of GABA reached by synaptic release) and rapid inactivation (such that the receptor is ready for a new signal again). They also possess a γ-subunit that is required for allosteric interaction by benzodiazepines. The inhibition mediated by presynaptic release of GABA has been called phasic inhibition. The extrasynaptic receptors, on the other hand, have a stoichiometry that substitutes a α4 subunit (for the α1 or α2 in the synaptic receptors mediating phasic inhibition); these receptors are highly sensitive to low ambient GABA concentrations and the inhibition produced is lasting, and is called tonic inhibition. They lack a γ-subunit that confers benzodiazepine sensitivity to the synaptic receptors, and are assembled instead with a δ-subunit. Neurosteroids, related to progesterone and pregnenolone derivatives, activate extrasynaptic GABA receptors mediating tonic inhibition. This is also the postulated mechanism for ganaxalone, a synthetic neurosteroid that has shown some activity against infantile spasms.Citation18
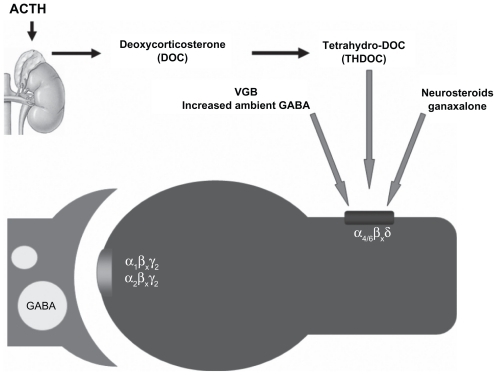
The action of vigabatrin likely augments both phasic and tonic inhibition by enhancing GABA concentration in different pools. Benzodiazepines like nitrazepam have been used to treat infantile spasms in the past. In a randomized multicenter study, nitrazepam was found to be comparable with ACTH in efficacy and better in tolerability.Citation19 The impact on tonic inhibition can be viewed as having some similarity to one of the potential actions of ACTH, which can stimulate the adrenal formation of deoxycorticosterone and its tetrahydro metabolite, which is a strong agonist at extrasynaptic receptors. This potential convergence in the mechanistic basis of seemingly unrelated compounds used to treat infantile spasms is illustrated in .
Figure 2 Schematic demonstrating a common target of action shared by many forms of therapy. Treatment with adrenocorticotropic hormone stimulates the adrenal production of tetrahydrodeoxycorticosterone which can activate extrasynaptic gamma aminobutyric acid receptors which mediate tonic inhibition. Increased ambient gamma aminobutyric acid produced by vigabatrin treatment may have a similar effect, as also the treatment with ganaxalone a neurosteroid investigational drug. The α4βxδ subunit containing extrasynaptic receptors are readily activated by modest levels of ambient gamma aminobutyric acid to produce a “lasting” current to sustain tonic activation. Adapted with permission from Auvin S, Sankar R. Antiinflammatory treatments for seizure syndromes and epilepsy. In: Rho JM, Sankar R, Stafstrom CE, editors. Epilepsy: Mechanisms, Models, and Translational Perspectives. New York, NY: CRC Press/Taylor and Francis; 2010.
In the developing brain, there may also be an important effect of ambient GABA on presynaptic GABAAR to increase the strength of GABAergic synaptic transmission. Low concentrations of GABA or zolpidem, an agonist at the benzodiazepine binding site, increased the frequency of miniature GABAergic synaptic currents,Citation20 representing a positive feedback loop. This effect was seen only in rat pups up to postnatal day 14, and has been demonstrated in the cerebellum. The presence of these presynaptic GABAAR was confirmed by immunocytochemistry.Citation20 The role of such receptors as targets for modifying spasms remains to be confirmed.
Efficacy of vigabatrin in infantile spasms
The efficacy and safety of vigabatrin for use in infants has been evaluated in numerous studies (see ). Many of the studies were retrospective reviews or add-on combination trials. However, some were open-label monotherapy trials or randomized comparative trials versus hydrocortisone and ACTH. We describe selected studies that contributed significantly to the development of vigabatrin for infantile spasms.
Table 1 Clinical studies of vigabatrin in the treatment of infantile spasms
An early large European retrospective analysis involving 250 infants evaluated vigabatrin as initial therapy for infantile spasms.Citation21 Of the initial 250 enrolled, 192 were determined to have classic spasms, based on semiology, age of onset, and EEG criteria. Hypsarrhythmia (typical or atypical) had been documented in 75% of the patients. The mean dose of vigabatrin used was 105 mg/kg. The efficacy in this survey is among the highest reported. Cessation of spasms occurred in 69% of patients with cryptogenic spasms and 60% of patients with symptomatic spasms, excluding tuberous sclerosis. Among the responders, 82% registered their response within one week after initiation of treatment. A dramatic response rate of 96% was achieved in treating infantile spasms attributed to tuberous sclerosis. This report, like many others, found a low rate of adverse effects (14%), mainly somnolence. Importantly, only two of the original 250 patients discontinued therapy because of adverse effects. Resolution of hypsarrhythmia was not specifically reported in this report.
Appleton et alCitation22 reported on a randomized, placebo-controlled, blinded study of treatment of infantile spasms using vigabatrin employing a distinctive design. In their study (n = 40, all meeting EEG criteria), the randomized, placebo-controlled phase was held for five days, after which all patients were eligible to have vigabatrin on an open-label basis. The average percent reduction in spasms in the vigabatrin-treated group was 78% compared with 26% in the placebo-treated group (P = 0.020). This study did not enroll any patients with tuberous sclerosis, a subset whose spasms have emerged to be uniquely responsive to treatment with vigabatrin. At the end of the 24-week trial period, 15 of the original 40 patients were spasm-free on monotherapy with vigabatrin, while four more were seizure-free on vigabatrin in combination with other medications. Separation between two groups was not significant because both groups had been on vigabatrin except for the first five days of the 24-week period. The study did establish that initial therapy of vigabatrin was effective, safe, and well tolerated.
The first prospective, randomized, open-label trial comparing vigabatrin and ACTH as first-line monotherapy for infantile spasms was published in 1997.Citation23 In a crossover study, 42 patients with untreated infantile spasms were randomized to vigabatrin 100–150 mg/kg/day or depot ACTH at 10 IU/day. Patients who did not respond to initial therapy by three weeks were crossed over to the other treatment arm. After the first stage of treatment, 48% of those treated with vigabatrin and 74% treated with ACTH became seizure-free (P = 0.12). During the second stage, 92% of patients who failed vigabatrin became seizure-free on ACTH, and 40% of patients who failed ACTH became seizure-free on vigabatrin (P = 0.052). Overall, 46% treated with vigabatrin and 81% treated with ACTH became seizure-free (P = 0.007). More patients in the ACTH-treated group showed normalization of the EEG, and improvements in EEG occurred earlier. Side effects (somnolence, hypotonia, irritability) were more prevalent with ACTH (37%) while the 13% adverse event rate for vigabatrin was similar to that reported by Aicardi et al.Citation21 This study also confirmed that all but one of 11 responders to vigabatrin did so within eight days of therapy, and 100 mg/kg/day seemed the optimal dose. Furthermore, this study demonstrated that vigabatrin was more effective as initial therapy in children with brain malformations or tuberous sclerosis, while ACTH was superior in children who developed infantile spasms after hypoxic-ischemic insults. It is important to note that the dose of ACTH used in this study is substantially less than what has emerged as the standard in the US (150 IU/m2/day).
Important clinical trial data for the FDA’s deliberations on the approval of vigabatrin come from the multicenter, randomized, single-masked (as to dose) study by Elterman et al.Citation24,Citation25 Initial results were published in 2001 in peerreviewed formCitation24 and the results including a larger population were presented in abstract form at the 2005 meeting of the American Epilepsy Society.Citation25 This multicenter, randomized, single-blinded study evaluated 220 subjects who had not been treated with ACTH, prednisone, or valproic acid. They were randomized into two groups, ie, low-dose (18–36 mg/kg/day) or high-dose (100–148 mg/kg/day). A strict definition of treatment response was used (ie, within the first 14 days of therapy, free of spasms for at least seven consecutive days, and absence of spasms or hypsarrhythmia on an eight-hour EEG).
Those who did not respond to the low-dose treatment in 14 days entered an open-label phase during which the dose of vigabatrin could be freely titrated according to response and/or tolerability. Only 18% were considered treatment responders by the strict definition, but 26% were considered responders if the 14-day window was extended. Eighty-eight percent of the responders remained spasms-free during the entire study. The response rate of the high-dose group (26%) was significantly higher than that of the low-dose group (11%). In addition, in concordance with other studies, patients with tuberous sclerosis (58%) had higher response rates than symptomatic (20%) or cryptogenic (37%) patients.
In a separate abstract at the same meeting, the same groupCitation26 reported their safety data for vigabatrin. Overall, vigabatrin was well tolerated, with only 5.4% of patients having adverse effects classified as related to vigabatrin that caused a discontinuation of the drug. Only 2.9% of serious adverse effects noted were classified as vigabatrin-related. There are important differences in this study, which confirmed the safety recorded by earlier studies, but achieved a lower rate of efficacy. The inclusion criteria permitted patients up to 24 months of age, and duration of spasms for up to three months, which most likely contributed to the observed lower rate of response. Further, those who responded beyond the first two weeks are not counted in the comparison between groups. Thus, the response rate of 58% among patients with tuberous sclerosis and infantile spasms compares quite well with the initial observations of Chiron et alCitation27 who studied patients during the first year of life and reported an 86% response rate for infants with tuberous sclerosis.
The United Kingdom Infantile Spasms Study compared the effect of vigabatrin with that of hormone therapy (prednisolone or tetracosactide depot) on developmental and epilepsy outcomes when the children were evaluated at 12–14 months of age.Citation28 Absence of spasms was similar in the final clinical assessment, ie, hormone 41/55 (75%) versus vigabatrin 39/51 (76%), as were the developmental outcomes determined by the Vineland Adaptive Behavior Scale (VABS). The VABS scores were hormone 78·6 (SD 16·8) versus vigabatrin 77·5 (SD 12·7). Among patients considered to be cryptogenic, hormone therapy resulted in a higher developmental outcome (hormone 88.2 [17.3] versus 78.9 [14.3]). This study excluded children with a diagnosis of, or at high risk of, tuberous sclerosis, and thus eliminated that group that is considered to represent the strongest beneficiaries of receiving therapy with vigabatrin.
Role of vigabatrin in treatment of tuberous sclerosis-associated infantile spasms
Chiron et al reported on a prospective trial comparing vigabatrin with hydrocortisone as initial treatment for infantile spasms attributable to tuberous sclerosis. This randomized, crossover study utilized vigabatrin at 150 mg/kg/day or hydro-cortisone 15 mg/kg/day as initial treatment. Nonresponders were crossed over at the end of one month of treatment. All the patients who were first treated with vigabatrin (11/11) were spasms-free compared with 5/11 hydrocortisone infants (P < 0.01). All the nonresponders to hydrocortisone became spasms-free after being crossed over to vigabatrin. Mean time to disappearance of infantile spasms was 3.5 days on vigabatrin versus 13 days on hydrocortisone (P < 0.01). This study was responsible for establishing the special place of vigabatrin in the treatment of infantile spasms in patients with tuberous sclerosis. It is worth pointing out that such a high response rate is seldom seen in even so-called benign epileptic syndromes of childhood.
Vigabatrin is considered safe and effective. Retinal toxicity and white matter changes on magnetic resonance imaging (MRI) are the effects that merit separate discussion. However, the common side effects encountered commonly in the various studies include drowsiness/somnolence, nystagmus, hyperexcitability/hyperkinesia, insomnia, fever, memory impairment, depression, and confusion. Less frequently encountered are axial hypertonia, hypotonia, agitation/irritability, asthenia, laryngitis, myoclonus, diarrhea, weight gain, and vomiting. Rare cases of psychotic reactions, mild anemia, and decreased liver function tests (aspartate aminotransferase and alanine aminotransferase) have also been reported. Overall, in comparison with many other antiepileptic drugs, vigabatrin is well tolerated.Citation1,Citation21,Citation22,Citation29
Retinal toxicity
One of the most discussed and concerning adverse effects of therapy with vigabatrin is retinal toxicity leading to visual field constriction. Reports of visual field deficits have appeared in the literature since 1997.Citation30 In various studies, this effect has been documented in up to 40% of treated patients. In addition to visual field loss, abnormal funduscopic examination, including “pale disc”, mild optic nerve pallor, retinal artery narrowing, and problems with visual acuity, color discrimination, and contrast sensitivity have been reported. Electroretinography (ERG) shows bilateral retinal dysfunction with preferential cone system dysfunction. The exact pathology is still unclear, but may be two-fold. First, the increase of GABA in the retina is likely to have a role.Citation3 Second, there may be a relationship between retinal damage, vigabatrin, and exposure to light. In the rat retina, administration of vigabatrin produced a decrease in GABA-T activity to undetectable levels and a five-fold increase in GABA.Citation31,Citation32 In a study of chronic vigabatrin exposure in rats, vigabatrin for 45 days produced a decrease in visual tests, including photopic ERG, flicker response, and oscillatory potentials.Citation33 The authors described disorganization of the outer retina, as well as damage to the inner and outer cone segments, most prominent in the central areas with a decrease in numbers in up to 20% of photoreceptors. The toxicity of vigabatrin to retinal cells is likely affected by development. In the rabbit retina, the fraction of amacrine cells capable of taking up and accumulating vigabatrin was very small at postnatal day 1 and increased to near-adult levels by 10 days postnatum.Citation34
It is still not entirely clear if the toxicity is related to the cumulative dose or if retinal involvement improves after discontinuation of the drug.Citation35 There is a positive trend in one retrospective review of higher doses and longer treatment periods correlating with visual field constriction, abnormal ERG, and abnormal visual-evoked potentials (VEPs),Citation36 although these findings have not been consistent. Somewhat encouragingly for short-term treatment of infantile spasms with vigabatrin, a study of children observed that the most severe damage was not observed in the youngest patient.Citation37 More recently, a Finnish study of 16 children with a history of treatment of infantile spasms with vigabatrin tested the subjects with kinetic perimetry between six and 12 years and encountered only mild visual field loss in one patient and normal fields in 15.Citation38 Nevertheless, careful monitoring for discernible signs of retinal injury should be stressed, and has resulted in the Risk Evaluation and Mitigation Strategy (REMS) described below.
As part of REMS, vision testing is required at baseline, at least every three months during therapy, and at least once within three to six months after discontinuation. While ERG and VEPs are recommended, these studies are often difficult to accomplish, given the age of the patient population with infantile spasms (http://www.lundbeckshare.com/pg510_info_physicians.aspx).Citation12 Thus, periodic ophthalmologic examinations (at initiation of therapy, and subsequently, every three months while on treatment, and three to six months after discontinuation of therapy), while mandated, may or may not include ERG and VEP testing. It is also suggested that, because of the risk of permanent vision loss, vigabatrin should be withdrawn from a pediatric patient treated for infantile spasms (one month to two years of age) who fails to show substantial clinical benefit within 2–4 weeks of treatment initiation, or sooner if treatment failure becomes obvious. A recommended screening algorithm based on evidence-based review of visual function testing in patients treated with vigabatrin has been published recently.Citation39 Evaluation that includes perimetry is suggested only for children over the age of nine years.
A critical role for the amino acid taurine in mediating vigabatrin-associated retinal ganglion cell toxicity has been demonstrated in rats and mice.Citation40,Citation41 Animals treated with vigabatrin had 67% lower levels of taurine than controls. The hypothesis that the addition of taurine-rich foods or direct supplements may prevent or slow down the development of the retinal cell lesions has been proposed by the authors. In addition, this study demonstrated that limiting light exposure might minimize cellular injury. However, excessive limiting of visual stimuli in very young patients may impact adversely on neural plasticity leading to ocular cortical organization and contribute to risk for amblyopia.
White matter changes
The observation of intramyelinic edema and vacuolation of myelin of the brain in rats has been alluded to.Citation4 During the period following the initial observation, human studies in adults using MRI and VEPs reassured investigators that these findings in animals may have very limited significance for humans.
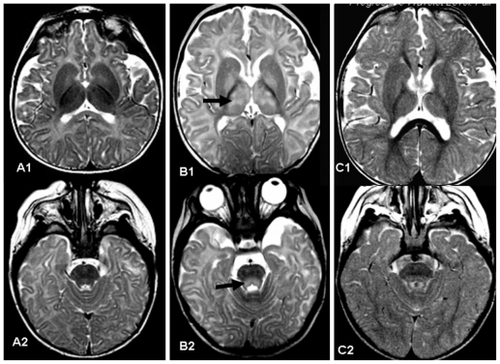
Transient MRI abnormalities unrelated to underlying cause have been seen in children and adults treated with vigabatrin. Areas of abnormality included the basal ganglia, thalamus, anterior commissure, corpus callosum, and brain-stem. Citation42 Wheless et alCitation43 reviewed 332 MRIs from 205 infants with infantile spasms and rereviewed MRIs from 668 children and adults with complex partial seizures. Abnormalities were defined as any hyperintensity on T2-weighted or fluid-attenuated inversion-recovery sequences that were diffusion restriction negative and not explained by specific pathology. Infants treated with vigabatrin were statistically more likely to have MRI abnormalities than those not treated with vigabatrin. Resolution of the MRI changes was seen in 66% of these patients. Another series involving infants (22 subjects, 34 MRIs) confirmed transient white matter signal changes.Citation44 An example of such lesions from one of our patients is shown in . It should be noted that the REMS program does not address monitoring for white matter lesions by MRI for infants treated with vigabatrin.
Figure 3 A1 and A2) show axial T2 weighted images of a normal baby aged five months. B1 and B2) show a five-month-old patient who had received vigabatrin for eight weeks. The magnetic resonance image was obtained due to abnormal eye movement. Magnetic resonance imaging shows high T2 weighted intensity in the thalamus and globus pallidus (arrow in B1) and tegmental portion of the pons (arrow in B2). Vigabatrin was discontinued. C1 and C2) Follow-up magnetic resonance imaging of the same patient three months later shows normal signal in the thalamus and pontine tegmentum.
Conclusion
Vigabatrin, the only presently approved therapy for infantile spasms in the US, has shown efficacy and tolerability, as well as relative safety (especially compared with the available options, including nontreatment),Citation45 representing a meaningful advance in the treatment of this catastrophic childhood epileptic encephalopathy. Availability of vigabatrin for infantile spasms is especially meaningful to those patients in whom it is a manifestation of tuberous sclerosis. Guidelines have been developed to monitor for visual system toxicity, and typical use will be limited to a few months, enhancing the benefit to risk ratio.
Disclosure
Dr Sankar is a member of the speakers’ bureau of Lundbeck Inc., the supplier of the Sabril brand of vigabatrin in the US. He also serves on the speakers’ bureau of Glaxo-Smith-Kline and UCB Pharma at present and is an advisor to Glaxo-Smith-Kline and NeuroTherapeutics Pharma.
References
- SankarRDerdiarianATVigabatrinCNS Drug Reviews19984260274
- EkeTTalbotJFLawdenMCSevere persistent visual field constriction associated with vigabatrinBMJ19973141801819022432
- KraussGLJohnsonMAMillerNRVigabatrin-associated retinal cone system dysfunction: Electroretinogram and ophthalmologic findingsNeurology1998506146189521245
- ButlerWHFordGPNewberneJWA study of the effects of vigabatrin on the central nervous system and retina of Sprague Dawley and Lister-Hooded ratsToxicol Pathol1987151431483616399
- ShieldsWDInfantile spasms: Little seizures, big consequencesEpilepsy Curr20066636916761063
- CowanLDHudsonLSThe epidemiology and natural history of infantile spasmsJ Child Neurol199163553641940138
- SherrEHThe ARX story (epilepsy, mental retardation, autism, and cerebral malformations): One gene leads to many phenotypesCurr Opin Pediatr20031556757114631200
- KatoMA new paradigm for West syndrome based on molecular and cell biologyEpilepsy Res200670SS879516806828
- FuscoLVigevanoFIctal clinical electroencephalographic findings of spasms in West syndromeEpilepsia1993346716788330577
- WhelessJWClarkeDFArzimanoglouATreatment of pediatric epilepsy: European expert opinionEpileptic Disord2007935341218077226
- MackayMTWeissSKAdams-WebberTPractice parameter: Medical treatment of infantile spasms: Report of the American Academy of Neurology and the Child Neurology SocietyNeurology2004621668168115159460
- Sabril (vigabatrin)Highlights of prescribing informationDeerfield, ILLundbeck Inc2009 Available from: http://www.lundbeckshare.com/pg510_info_physicians.aspxAccessed on 2010 Jul 29
- JungMJLippertBMetcalfBWBohlenPSchechterPJGamma-vinyl GABA (4-amino-hex-5-enoic acid), a new selective irreversible inhibitor of GABA-T: Effects on brain GABA metabolism in miceJ Neurochem197729797802591956
- SchechterPJTranierYJungMJBohlenPAudiogenic seizure protection by elevated brain GABA concentration in mice: Effects of gamma-acetylenic GABA and gamma-vinyl GABA, two irreversible GABA-T inhibitorsEur J Pharmacol197745319328923642
- BernasconiRKleinMMartinPGamma-vinyl GABA: Comparison of neurochemical and anticonvulsant effects in miceJ Neural Transm1988722132333418334
- MattsonRHPetroffORothmanDBeharKVigabatrin: Effects on human brain GABA levels by nuclear magnetic resonance spectroscopyEpilepsia199435Suppl 5S29328039467
- PetroffOARothmanDLBeharKLMattsonRHInitial observations on effect of vigabatrin on in vivo 1H spectroscopic measurements of gamma-aminobutyric acid, glutamate, and glutamine in human brainEpilepsia1995364574647614922
- KerriganJFShieldsWDNelsonTYGanaxolone for treating intractable infantile spasms: A multicenter, open-label, add-on trialEpilepsy Res20004213313911074186
- DreifussFFarwellJHolmesGInfantile spasms. Comparative trial of nitrazepam and corticotropinArch Neurol198643110711103022694
- TrigoFFChatMMartyAEnhancement of GABA release through endogenous activation of axonal GABA(A) receptors in juvenile cerebellumJ Neurosci200711142746:124521246318003823
- AicardiJMumfordJPDumasCWoodSVigabatrin as initial therapy for infantile spasms: A European retrospective surveyEpilepsia1996376386428681895
- AppletonREPetersACBMumfordJPShawDERandomized, placebo-controlled study of vigabatrin as first-line treatment of infantile spasmsEpilepsia1999401627163310565592
- VigevanoFCilioMRVigabatrin versus ACTH as first-line treatment for infantile spasms: A randomized, prospective studyEpilepsia199738127012749578521
- EltermanRDShieldsWDMansfieldKAUS Infantile Spasms Vigabatrin Study GroupRandomized trial of vigabatrin in patients with infantile spasmsNeurology2001571416142111673582
- EltermanRDCollinsSDShieldsDMansfieldKANakagawaJEfficacy of vigabatrin in subjects with infantile spasmsEpilepsia200546Suppl 814215987269
- ShieldsDCollinsSDEltermanRDNakagawaJMansfieldKAAES and safety of vigabatrin in subjects with infantile spasmsEpilepsia20054616116302891
- ChironCDulacOBeaumontDPalaciosLPajotNMumfordJTherapeutic trial of vigabatrin in refractory infantile spasmsJ Child Neurol19916Suppl 22S522S59
- LuxALEdwardsSWHancockEThe United Kingdom infantile spasms study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: A multicentre randomized trialLancet Neurol2005471271716239177
- ChironCDumasCJambaqueIMumfordJDulacORandomized trial comparing vigabatrin and hydrocortisone in infantile spasms due to tuberous sclerosisEpilepsy Res1997263893959095401
- SankarRSpenceSJVisual field defects and other ophthalmological disturbances associated with vigabatrinDrug Saf20012438540411419565
- CubellsJFBlanchardJSMakmanMHThe effects of in vivo inactivation of GABA-transaminase and glutamic acid decarboxylase on levels of GABA in the rat retinaBrain Res19874192082153676726
- NealMJCunninghamJRShahMAYazullaSImmunocytochemical evidence that vigabatrin in rats causes GABA accumulation in glial cells of the retinaNeurosci Lett19899829322710396
- DubocAHanoteauNSimonuttiMVigabatrin, the GABA-transaminase inhibitor, damages cone photoreceptors in ratsAnn Neurol20045569570515122710
- CrookDKPowDVAnalysis of the distribution of glycine and GABA in amacrine cells of the developing rabbit retina: A comparison with the ontogeny of a functional GABA transport system in retinal neuronsVis Neurosci1997147517639279003
- WildJMAhnHSBaulacMVigabatrin and epilepsy: Lessons learnedEpilepsia2007481318132717635558
- Gross-TsurVBaninEShaharEShalevRSLahatEVisual impairment in children with epilepsy treated with vigabatrinAnn Neurol200048606410894216
- IannettiPSpaliceAMassimo PerlaFVisual field constriction in children with epilepsy on vigabatrin treatmentPediatrics200010683884211015531
- GailyEJonssonHLappiMVisual fields at school-age in children treated with vigabatrin in infancyEpilepsia20095020621619215279
- SergottRCWhelessJWSmithMCEvidence-based review of recommendations for visual function testing in patients treated with vigabatrinNeuro-Ophthalmology2010342035
- JammoulFWangQNabboutRTaurine deficiency is a cause of vigabatrin-induced retinal phototoxicityAnn Neurol2009659810719194884
- JammoulFDégardinJPainDTaurine deficiency damages photoreceptors and retinal ganglion cells in vigabatrin-treated neonatal ratsMol Cell Neurosci20104341442120132888
- PearlPLVezinaLGSanetoRPCerebral MRI abnormalities associated with vigabatrin therapyEpilepsia20095018419418783433
- WhelessJWCarmantLBebinMMagnetic resonance imaging abnormalities associated with vigabatrin in patients with epilepsyEpilepsia20095019520519054414
- MilhMVilleneueveNChaponFTransient brain magnetic resonance imaging hyperintensity in basal ganglia and brain stem of epileptic infants treated with vigabatrinJ Child Neurol20092430531519258289
- SankarRWasterlainCGIs the devil we know the lesser of two evils? Vigabatrin and visual fieldsNeurology1999521537153810331674
- VlesJSHvan der HeydenAMHGGhijsATroostJVigabatrin in the treatment of infantile spasmsNeuropediatrics1993242302318232783
- AppletonREMontiel-ViescaFVigabatrin in infantile spasms – why add onLancet19933419628096297
- AppletonREA simple, effective and well-tolerated treatment regime for West syndromeDev Med Child Neurol1995371851877851675
- KwongLVigabatrin as first line therapy in infantile spasms: Review of seven patientsJ Paediatr Child Health1997331211249145354
- CovanisAThrodorouVLadaCSkiadasKLoliNThe first-line use of vigabatrin to achieve complete control of infantile spasmsJ Epilepsy199811265269
- VilleneuveNSouffletCPlouinPChironCDulacOTreatment of infantile spasms with vigabatrin as first-line therapy and in monotherapy: Apropos of 70 infantsArch Pediatr199857317389759271
- SiemesHBrandlUSpohrH-LVolgerSWeschkeBLong-term follow-up study of vigabatrin in pretreated children with West syndromeSeizure199872932979733404
- WohlrabGBoltshauserESchmittBVigabatrin as a first-line drug in West syndrome: Clinical and electrographic outcomeNeuropediatrics1998291331369706623
- CossettePRivielloJJCarmantLACTH versus vigabatrin in infantile spasms: A retrospective studyNeurology1999521691169410331702
- GranströmMLGailyELiukkonenETreatment of infantile spasms: Results of a population-based study with vigabatrin as the first drug for spasmsEpilepsia19997950957
- KooBVigabatrin in the treatment of infantile spasmsPediatr Neurol19992010611010082337
- FejermanNCersosimoRCaraballoRVigabatrin as a first-choice drug in the treatment of west syndromeJ Child Neurol20001516116510757471
- NabboutRMelkiIGerbakaBDulacOAkatcherianCInfantile spasms in Down syndrome: Good response to a short course of vigabatrinEpilepsia2001421580158311879370
- MitchellWGShahNSVigabatrin for infantile spasmsPediatr Neurol20022716116412393124
- LuxALEdwardsSWHancockEThe United Kingdom infantile spasms study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trialLancet20043641773177815541450
- AkmanCIChribogaCAFreyerRCappellJEmersonRLearyLSymptomatic infantile spasms response to antiepileptic treatment and short-term outcomeEpilepsia200546Suppl 8167
- AuvinSSankarRRhoJMSankarRStafstromCEAntiinflammatory treatments for seizure syndromes and epilepsyEpilepsy: Mechanisms, Models, and Translational PerspectivesNew York, NYCRC Press/Taylor and Francis2010