Abstract
Improving medication adherence is critical to improving outcomes in patients with schizophrenia. A long-acting injectable (depot) antipsychotic is one of the most effective methods for improving treatment adherence and decreasing rehospitalization rates in patients with schizophrenia. Until recently, only three second-generation antipsychotics were available in a long-acting injectable formulation (risperidone, paliperidone, and olanzapine). In this respect, the emergence of long-acting aripiprazole injection (ALAI), approved by the US Food and Drug Administration for the treatment of schizophrenia in 2013, is timely. ALAI is a lyophilized powder of aripiprazole, and the aripiprazole molecule is unmodified. The initial and target dosage of ALAI is 400 mg once monthly, but it could be reduced to 300 mg if adverse reactions occur with 400 mg. When first administering ALAI, it is recommended to continue treatment with oral aripiprazole (10–20 mg/day) or another oral antipsychotic for 2 weeks in order to maintain therapeutic antipsychotic concentrations. The primary clearance route for ALAI is hepatic, ie, cytochrome P450 (CYP)2D6 and CYP3A4, so dose adjustment is required in poor CYP2D6 metabolizers. The efficacy of ALAI was demonstrated in three studies. A randomized controlled trial that formed the basis for approval of ALAI in the treatment of schizophrenia showed that ALAI significantly delayed time to impending relapse when compared with placebo (P<0.0001, log-rank test). An open-label, mirror study demonstrated that total psychiatric hospitalization rates were significantly lower after switching from oral antipsychotics to ALAI. Another randomized controlled trial presented in poster form suggested that ALAI 400 mg was comparable with oral aripiprazole 10–30 mg in preventing relapse. ALAI was generally well tolerated during both short-term and long-term studies. Its tolerability profile, including extrapyramidal symptoms and clinically relevant metabolic parameters, was similar to placebo. However, insomnia, headache, anxiety, akathisia, weight gain, injection site pain, and tremor need clinical attention. These studies suggest that ALAI is a viable treatment option for patients with schizophrenia, but direct head-to-head comparisons between ALAI and other long-acting injectable antipsychotics are needed to elucidate its risk–benefit profile.
Introduction
Schizophrenia affects approximately 1% of the population, and is a devastating illness with a chronic impact on social and occupational functioning and activities of daily living.Citation1,Citation2 Therefore, maintenance treatment with antipsychotics is a core feature of its long-term management.Citation3 Nonadherence to antipsychotic medication is one of the most important risk factors for relapse and hospitalization in patients receiving treatment for schizophrenia.Citation4,Citation5 A study showed that the median time to all-cause discontinuation of medication was less than 4 months for the majority of oral second-generation antipsychotics.Citation6 Another study showed that more than 75% of patients with schizophrenia eventually became nonadherent within 2 years of being discharged from hospital.Citation7 Nonadherence also largely contributes to an increased cost burden for patients. Up to 50% of direct medical costs of psychiatric hospitalization were attributed to nonadherence with antipsychotic medication.Citation8,Citation9
For these reasons, strategies to reduce hospitalization by improving medication adherence are becoming the focus of treatment in patients with schizophrenia.Citation10,Citation11 Depot antipsychotics are shown to be associated with a significantly lower risk of nonadherence and rehospitalization than oral antipsychotics.Citation1,Citation6 Since the introduction of a second-generation antipsychotic long-acting injection (risperidone) in 2003, only three second-generation antipsychotics have been available as a long-acting injectable formulation (risperidone, paliperidone, and olanzapine).Citation12–Citation14 In this respect, the emergence of a once-monthly aripiprazole depot injection or aripiprazole long-acting injection (ALAI) is a timely development. In 2013, ALAI was approved for the treatment of schizophrenia by the US Food and Drug Administration.Citation15 The purpose of this paper is to provide a concise review regarding the clinical usefulness of once-monthly ALAI in patients with schizophrenia.
Data search
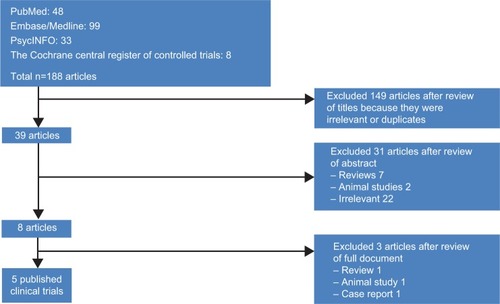
We conducted a data search on April 1, 2014, using the key terms “aripiprazole” with “depot”, “once monthly”, “long-acting injection”, and/or “long-acting injectable” in the following databases: PubMed, Embase/Medline, PsycINFO, and Cochrane Library. The studies searched were verified for publication in English peer-reviewed journals, but we did not utilize date constraints. Reference lists from the identified articles and reviews were also used to find additional studies.
Five published clinical trials were initially identified (). One poster was found by searching scientific meetings for paper and poster presentations. To supplement these six studies, data obtained from the package information by Otsuka Pharmaceuticals were extracted online from http://www.fda.gov. The literature search and verification were handled first by one of the authors (SMW) and then independently reassessed by others (CUP and SJL). This paper aimed to provide a review of once-monthly aripiprazole depot injection in the treatment of patients with schizophrenia. Thus, all relevant studies meeting the aim of our review were selected based on consensus among the authors.
General information
A summary of general information concerning ALAI is provided in . ALAI is a lyophilized powder of aripiprazole, and the aripiprazole molecule is unmodified.Citation16,Citation17 The antipsychotic activity of ALAI is primarily from its parent drug, aripiprazole.Citation17 Its major metabolite, dehydro-aripiprazole, plays a minor role. The combination of partial agonism (agonism/antagonism) at dopamine D2 and serotonin 5-HT1A receptors and antagonism at serotonin 5-HT2A receptors is an important potential antipsychotic mechanism of aripiprazole.Citation18–Citation20 Following a single intramuscular dose of ALAI, the median time to peak plasma concentration was 5–7 days. Mean terminal elimination half-life of aripiprazole after every 4-weekly injection of ALAI 300 mg and 400 mg was 29.9 and 46.5 days, respectively. A study showed that aripiprazole and dehydro-aripiprazole concentrations increased proportionally with an increase in the dose of ALAI.Citation16
Table 1 General information on aripiprazole long-acting injection
Research showed that plasma levels of ALAI are consistent with those achieved by oral aripiprazole.Citation16,Citation17,Citation21 A study simulated aripiprazole plasma concentration-time profiles based on the pharmacokinetic parameters for a single dose of ALAI and from previous oral aripiprazole steady-state studies.Citation16 This simulation showed that minimum plasma drug concentrations during a dosing interval at steady-state (Css, min) for ALAI 300 mg and 400 mg corresponded to or was a little above the Css, min for oral aripiprazole 10 mg/day. The recommended initial and target dosage of ALAI is 400 mg once a month, which could be reduced to 300 mg once a month if adverse reactions occur with 400 mg.Citation17 The appropriate site of injection is the gluteal muscle (intramuscular), and the injection should be performed by a health care professional. After the first ALAI, it is recommended to continue treatment with oral aripiprazole (10–20 mg/day) or another oral antipsychotic for 14 consecutive days in order to maintain therapeutic antipsychotic concentrations. The primary clearance route of ALAI is hepatic, ie, via cytochrome (CYP)2D6 and CYP3A4. Therefore, dosage adjustment of ALAI is required in patients who are poor CYP2D6 metabolizers, and also in patients taking concomitant CYP3A4 inhibitors and/or CYP2D6 inhibitors for more than 14 days.Citation22 The injection schedule requires modification for missed doses.
Clinical evidence
Three studies investigated the efficacy of ALAI in the treatment of schizophrenia (). A placebo-controlled, randomized withdrawal maintenance study conducted in 2012 formed the basis of the approval of ALAI for the treatment of schizophrenia.Citation21 This multicenter study compared the efficacy of ALAI with placebo in 403 patients (aged 18–60 years). Subjects enrolled had had a diagnosis of schizophrenia for at least 3 years according to the DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision). All patients had a history of symptom exacerbation or relapse when not receiving antipsychotic treatment. Patients initially entered the oral conversion phase (phase 1) to cross-titrate their antipsychotics to oral aripiprazole monotherapy over 4 weeks. After stabilizing their symptoms using open-label oral aripiprazole monotherapy for 8 weeks (phase 2), patients meeting the criteria for stabilization then received open-label ALAI (phase 3). Thereafter, patients who remained consistently stable for more than 3 months with ALAI entered the double-blind phase (phase 4) and were randomized in a 2:1 ratio to intramuscular ALAI 400 mg (or 300 mg if not tolerant of 400 mg) or placebo (ALAI, n=269; placebo, n=134). The primary outcome measure was time to exacerbation of psychotic symptoms/impending relapse, defined as meeting any or all of the following predefined criteria during the double-blind phase (phase 4):
Table 2 Summary of clinical trials of investigating efficacy of aripiprazole once monthly in patients with schizophrenia
Clinical worsening – Clinical Global Impression-Improvement (CGI-I) score of ≥5 plus an increase of score to >4 on any of the core Positive and Negative Syndrome Scale (PANSS) items (conceptual disorganization, hallucinatory behavior, suspiciousness, or unusual thought content) with an absolute score of ≥2 on that specific item or an increase >4 on these PANSS items and an absolute increase of ≥4 on the combined score of these items since randomization
Aggravation of psychotic symptoms – hospitalization
Risk of suicide – Clinical Global Impression-Severity of Suicidality (CGI-SS) score of 4 (severely suicidal) or 5 (attempted suicide) on part 1 or a score of 6 (much worse) or 7 (very much worse) on part 2
Violent behavior – clinically significant self-injury, injuring others, or damaging the property of others.
The mean patient age in the ALAI and placebo groups was 40.1 years and 41.7 years, respectively. The result showed that time to impending relapse was significantly delayed in the ALAI group when compared with the placebo group in both the interim and final analyses (P<0.0001, log-rank test). Time to study discontinuation, for reasons other than study termination, from the double-blind phase was also significantly longer with ALAI than with placebo (P≤0.001, log-rank test). Since the efficacy of ALAI was demonstrated by the preplanned interim analysis, the study was terminated early. Moreover, the rate of relapse was significantly lower in the ALAI group (9.6%) when compared with the placebo group (36.8%; hazard ratio 4.72, 95% confidence interval [CI] 2.81–7.94), with a calculated number needed to treat for relapse of 4 (CI 3–5).Citation22 Clinical Global Impression-Severity (CGI-S) and PANSS total scores were maintained with ALAI but worsened significantly with placebo.
The second study used an open-label, mirror image design to investigate hospitalization rates in patients with schizophrenia treated retrospectively with oral antipsychotics followed by prospective treatment with ALAI in a naturalistic community setting.Citation23 A total of 183 patients with schizophrenia, aged 18–65 years, participated in the study. In the first phase of the study, patients were treated with oral standard-of-care antipsychotics (retrospective data) for 6 months. After 4 weeks of stabilization, the same patients were treated with ALAI 400 mg (prospective data) for 6 months. The primary endpoint was a comparison of the proportion of patients hospitalized in the latter 3 months of the retrospective oral antipsychotic treatment period and those hospitalized during the latter 3 months of the prospective ALAI 400 treatment period (months 4–6). The results showed that total psychiatric hospitalization rates were significantly lower after switching to ALAI (months 4–6) when compared with the 3-month retrospective treatment period (months −4 to −1) when the same patients were receiving oral antipsychotics (6.6% versus 28.1%, P<0.0001). Total psychiatric hospitalization rates were also lower in whole prospective 6 months after patients were switched to ALAI (hospitalization rate for months 0–6 was 14.2%) than whole retrospective 6 months while they were on oral antipsychotics (hospitalization rate for months −7 to −1, 41.5%, P<0.0001).
The third study, which was reported as a poster, is a double-blind, randomized comparison of ALAI with oral aripiprazole using a noninferiority design.Citation24 After an oral conversion phase and a stabilization phase, patients were randomized to ALAI 400 mg (with an option to decrease to 300 mg), oral aripiprazole (10–30 mg/day), and ALAI 50 mg (with an option to decrease to 25 mg). ALAI 50 mg is a subtherapeutic dosage and its purpose was for assay sensitivity. The results showed no significant difference in the relapse rate between ALAI 400 mg (7.1%) and oral aripiprazole (7.8%). Both treatment arms were significantly superior to ALAI 50 mg (21.8%; P<0.001).Citation25 In addition, time to impending relapse was also significantly delayed in the ALAI 400 mg group when compared with the ALAI 50 mg group (hazard ratio 3.2, CI 1.8–5.5; P<0.0001, log-rank test), but was similar between the ALAI 400 mg group and the oral aripiprazole group (hazard ratio 1.0, CI 0.6–1.8; P=0.99, log-rank test).Citation22
Safety and tolerability
Four studies assessed the safety and tolerability of ALAI in patients with schizophrenia.Citation16,Citation21,Citation26,Citation27 However, a long-term study by Fleischhacker et alCitation26 was an extension of the pivotal clinical trial by Kane et al.Citation21 Thus, the study by Fleischhacker et al generally contained all the safety and tolerability data reported in the study by Kane et al. The long-term study by Fleischhacker et al evaluated the safety and tolerability of ALAI throughout four study phases: oral conversion (phase 1, 4–6 weeks); oral stabilization (phase 2, 4–12 weeks); stabilization of ALAI with coadministration of oral aripiprazole (phase 3, 12–36 weeks); and a 52-week, randomized double-blind, maintenance phase (phase 4, ALAI versus placebo).Citation26 Adverse events (>5%) in any phase were insomnia, headache, anxiety, akathisia, weight gain, injection site pain, and tremor. The incidence of extrapyramidal symptoms was similar in all phases, and no significant differences between the ALAI 400 mg group and the placebo group were observed in any of the three extrapyramidal symptom scales (Abnormal Involuntary Movement Scale, Simpson Angus Scale, and Barnes Akathisia Rating Scale).Citation21,Citation26 No changes in clinically relevant metabolic parameters were noted across the study phases. Moreover, the adverse event rate for the ALAI 400 mg group was comparable with that in the placebo group in the double-blind phase.
A multicenter, open-label, parallel-arm study investigated the safety and tolerability of ALAI 200 mg (n=10), 300 mg (n=15), and 400 mg (n=14).Citation16 Most treatment-emergent adverse events were classified as mild or moderate in intensity. Four patients in the ALAI 400 mg group reported injection site pain as a common treatment-emergent adverse event, but there were no reports of injection site pain in the ALAI 200 mg and 300 mg groups. Tremor, upper respiratory infection, musculoskeletal symptoms, sedation, rash, headache, and weight gain were also more common in the ALAI 400 mg group than in the ALAI 200 mg and 300 mg groups. Although the incidence of treatment-emergent adverse events tended to increase with the dosage of ALAI, the sample size was too small to evaluate its clinical significance. Four patients (10.3%) discontinued treatment due to treatment-emergent adverse events, but none receiving ALAI 400 mg withdrew from the study. Three subjects in the ALAI 300 mg group discontinued because of drug dependence, worsening of psychosis, and worsening of schizophrenia symptoms, and one patient in the ALAI 200 mg group discontinued due to worsening of psychosis.
The third study investigated the safety and tolerability of a single dose of ALAI 400 mg in patients with schizophrenia stabilized on oral atypical antipsychotics other than aripiprazole (not included in ).Citation27 In this open-label multicenter trial, 60 patients received ALAI 400 mg while taking oral olanzapine (n=3), quetiapine (n=28), risperidone (n=24), or ziprasidone (n=5). The most common treatment-emergent adverse events were injection site pain and toothache (4/60 for both, 6.7%), but overall the treatment was well tolerated. This study showed that the safety profile in patients receiving concomitant ALAI and oral antipsychotics other than aripiprazole was comparable with that reported previously in patients receiving concomitant ALAI and oral aripiprazole.Citation21 Psychotic symptoms, ie, secondary endpoint measures evaluated using PANSS total scores, CGI-S, and CGI-I scores, remained stable during the study. The mean baseline and mean changes at endpoint were 64.5 and −3.7, respectively, for PANSS total scores and 3.3 and −0.1 for CGI-S scores. The mean CGI-I score at week 1 was 3.85 and at week 4 was 3.58. This study suggests that when switching from existing oral antipsychotics to ALAI, clinicians could directly switch to ALAI without the trouble of stabilizing with oral aripiprazole.
Table 3 Safety and tolerability of aripiprazole once-monthly in patients with schizophrenia
Expert opinion
ALAI is a viable treatment option for patients with schizophrenia. The beneficial effects of ALAI in preventing relapse of schizophrenia are supported by three clinical studies. One randomized controlled trial demonstrated superior efficacy of ALAI over placebo for preventing relapse in patients with schizophrenia.Citation21 One open-label, mirror image study also showed that ALAI was superior to oral standard-of-care antipsychotics in decreasing hospitalization rates.Citation23 Another randomized controlled trial, which has been presented only as a poster at the time of writing, shows that ALAI is as effective as oral aripiprazole in preventing relapse of schizophrenia.Citation24
The safety and tolerability of ALAI has been well documented by four clinical trials. The pivotal clinical trial by Kane et al and a long-term safety study, which was an expansion of the pivotal trial, showed that ALAI was as well tolerated as placebo.Citation21,Citation26 An open-label, parallel-arm study demonstrated the safety and tolerability of three different doses of ALAI, ie, 200 mg, 300 mg, and 400 mg.Citation16 Moreover, the safety profile in patients receiving ALAI and oral antipsychotics other than aripiprazole concomitantly was comparable with that in patients receiving ALAI and oral aripiprazole concomitantly.Citation27 Psychotic symptoms assessed by PANSS total scores, CGI-S scores, and CGI-I scores, also remained stable during the same study, suggesting that clinicians could directly switch to ALAI without the trouble of stabilizing with oral aripiprazole. However, insomnia, headache, anxiety, akathisia, weight gain, injection site pain, and tremor need close monitoring when administering ALAI.
Despite the availability of these studies, more research is needed to clarify important issues. For example, clinical trials comparing the efficacy, tolerability, and safety of ALAI with that of other long-acting injectable antipsychotics are required to elaborate its risk–benefit profile. In addition, studies are also needed to confirm its efficacy and safety in pediatric and geriatric patients with schizophrenia.
Disclosure
The authors report no conflicts of interest in this work.
References
- TiihonenJHaukkaJTaylorMHaddadPMPatelMXKorhonenPA nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophreniaAm J Psychiatry201116860360921362741
- GoreishizadehMMohagheghiAFarhangSAlizadehLPsychosocial disabilities in patients with schizophreniaIran J Public Health20124111612123113186
- LeuchtSTardyMKomossaKHeresSKisslingWDavisJMMaintenance treatment with antipsychotic drugs for schizophreniaCochrane Database Syst Rev20125CD00801622592725
- NovickDHaroJMSuarezDPerezVDittmannRWHaddadPMPredictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophreniaPsychiatry Res201017610911320185182
- AcostaFJHernandezJLPereiraJHerreraJRodriguezCJMedication adherence in schizophreniaWorld J Psychiatry20122748224175171
- BitterIKatonaLZamboriJComparative effectiveness of depot and oral second generation antipsychotic drugs in schizophrenia: a nationwide study in HungaryEur Neuropsychopharmacol2013231383139023477752
- WeidenPRapkinBZygmuntAMottTGoldmanDFrancesAPostdischarge medication compliance of inpatients converted from an oral to a depot neuroleptic regimenPsychiatr Serv199546104910548829787
- WeidenPJOlfsonMCost of relapse in schizophreniaSchizophr Bull1995214194297481573
- LafeuilleMHGravelJLefebvrePPatterns of relapse and associated cost burden in schizophrenia patients receiving atypical antipsychoticsJ Med Econ2013161290129924006903
- KaneJMImproving treatment adherence in patients with schizophreniaJ Clin Psychiatry201172e2821951990
- MarcusSCOlfsonMOutpatient antipsychotic treatment and inpatient costs of schizophreniaSchizophr Bull20083417318017578893
- LoveRCConleyRJLong-acting risperidone injectionAm J Health Syst Pharm2004611792180015462250
- NussbaumAMStroupTSPaliperidone palmitate for schizophreniaCochrane Database Syst Rev20126CD00829622696377
- FramptonJEOlanzapine long-acting injection: a review of its use in the treatment of schizophreniaDrugs2010702289231321080745
- MotiwalaFBSiscoeKSEl-MallakhRSReview of depot aripiprazole for schizophreniaPatient Prefer Adherence201371181118724265550
- MallikaarjunSKaneJMBricmontPPharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallel-arm, multiple-dose studySchizophr Res201315028128823890595
- Otsuka Pharmaceutical Co LtdAbilify Maintena™ (aripiprazole) extended release injectable suspension, package insertTokyo, JapanOtsuka Pharmaceutical Co Ltd Available from: http://www.otsuka-us.com/Products/Documents/Abilify.M.PI.pdfAccessed April 23, 2014
- PaeCUA review of the safety and tolerability of aripiprazoleExpert Opin Drug Saf2009837338619505266
- BurrisKDMolskiTFXuCAripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptorsJ Pharmacol Exp Ther200230238138912065741
- JordanSKoprivicaVChenRTottoriKKikuchiTAltarCAThe antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptorEur J Pharmacol200244113714012063084
- KaneJMSanchezRPerryPPAripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled studyJ Clin Psychiatry20127361762422697189
- CitromeLNew second-generation long-acting injectable antipsychotics for the treatment of schizophreniaExpert Rev Neurother20131376778323898849
- KaneJMSanchezRZhaoJHospitalisation rates in patients switched from oral anti-psychotics to aripiprazole once-monthly for the management of schizophreniaJ Med Econ20131691792523663091
- FleischhackerWWSanchezRAripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority studyBr J PsychiatryIn press2014
- RauchASFleischhackerWWLong-acting injectable formulations of new-generation antipsychotics: a review from a clinical perspectiveCNS Drugs20132763765223780619
- FleischhackerWWSanchezRJohnsonBLong-term safety and tolerability of aripiprazole once-monthly in maintenance treatment of patients with schizophreniaInt Clin Psychopharmacol20132817117623615694
- PotkinSGRaoufiniaAMallikaarjunSSafety and tolerability of once monthly aripiprazole treatment initiation in adults with schizophrenia stabilized on selected atypical oral antipsychotics other than aripiprazoleCurr Med Res Opin2013291241125123822566