Abstract
Decompressive craniectomy is an established procedure to lower intracranial pressure and can save patients’ lives. However, this procedure is associated with delayed cognitive decline and cerebral hemodynamics complications. Studies show the benefits of cranioplasty beyond cosmetic aspects, including brain protection, and functional and cerebrovascular aspects, but a detailed description of the concrete changes following this procedure are lacking. In this paper, the authors report a patient with trephine syndrome who underwent cranioplasty; comprehensive cognitive and cerebral hemodynamic evaluations were performed prior to and following the cranioplasty. The discussion was based on a critical literature review.
Introduction
Decompressive craniectomy (DC) is a treatment option for refractory intracranial hypertension that involves removing a portion of the skull to alleviate swelling.Citation1 Harvey Cushing was the first to describe this technique, and for decades it has been mainly used in the treatment of ischemic stroke and traumatic brain injury.Citation2–Citation5 Despite the reduction of morbidity and mortality associated with this surgical technique, many patients experience early- and late-onset complications such as herniation through the edges of the craniectomy (51%) resulting in brain injury (6% to 58%),Citation6 subdural hygroma (16% to 62%),Citation6–Citation8 hydrocephalus (2% to 29%),Citation9,Citation10 motor impairment,Citation9,Citation11 infection,Citation10 and trephine syndrome (TS) (26%).Citation7
Patients with large skull defects are particularly at risk of developing TS, also known as “sinking skin flap syndrome” or “trephined motor syndrome”. This syndrome is characterized by headache, dizziness, alterations in behavior and mood, seizures, fatigue, motor deficits, and language disturbances.Citation12–Citation14 To date, little is known about the pathophysiological mechanisms underlying this syndrome; however, some authors have hypothesized that abnormal brain pulsatility,Citation15 the effect of atmospheric pressure over the bone defect,Citation13,Citation16–Citation18 changes in cerebrospinal fluid and venous drainage dynamics,Citation19–Citation22 and changes in blood flow and metabolism may all contribute.Citation23–Citation33
Case reports and clinical series published to date suggest improvement in neurological deficits, cognitive function, and brain hemodynamics after bone replacement (cranioplasty),Citation34–Citation40 suggesting potential benefits of this procedure in respect to cognitive aspects. However, little is known about cognitive changes after cranioplasty. The purpose of this paper is to report a comprehensive description of cognitive and hemodynamic changes seen in a patient after cranioplasty.
Materials and methods
Clinical assessment protocol
A patient clinically diagnosed with TS was selected to a prospective protocol. Demographic data, clinical signs, and symptoms were recorded after the completion of a systematic questionnaire regarding the most common symptoms in patients with skull defects. The battery of neuropsychological tests consisted of logical memory and visual reproduction (subtests of the Wechsler Memory Scale – Revised); the Rey Audio-Verbal Learning Test (RAVLT); the Controlled Oral Word Association Test (COWAT-FAS); the Verbal Fluency Test – Animal Category, the Victoria Stroop Test (VST); digits, cubes, arithmetic, sequence of numbers and letters; the Working Memory Index from the Wechsler Adult Intelligence Scale (WMI-WAIS); the Wechsler Memory Scale (WMS) and Wechsler Adult Intelligence Scale; the Trail Making Test A (TMTA) and B (TMTB); the Pfeffer Outpatient Disability Scale (POD); the Rey Complex Figure Test; the Beck Depression Inventory (BDI); the Beck Anxiety Inventory (BAI); the Beck Hopelessness Scale (BHS); the Lipp’s Inventory of Stress Symptoms for adults (ISSL); and the mini-mental state examination (MMSE). The modified Rankin Scale (mRS) and Barthel Index (BI) were recorded to evaluate global functional status. All neuropsychological tests, and the mRS and BI were performed 1 day before cranioplasty. These assessments were repeated 6 months after cranioplasty.
In order to minimize the effects of cumulative learning due to retesting of RAVLT, List A and B, and the recognition test, the words used in the preoperative assessment were replaced by others in the postoperative evaluations. The words used in the tests after surgery were similar with respect to preoperative testing in terms of size (number of letters) and frequency of use by the population. The patient did not undergo a cognitive rehabilitation program in the period between preoperative and postoperative clinical assessments.
Cerebral hemodynamic assessment protocol
The patient was studied with transcranial Doppler ultrasonography (TCD) coupled to a 2-MHz transducer (DWL Doppler Box™, Compumedics Germany GmbH, Singen, Germany) to assess cerebral blood flow velocity from both middle cerebral arteries before and 15 days after cranioplasty. The measurements were conducted while the patient was at rest, in the supine and sitting positions.
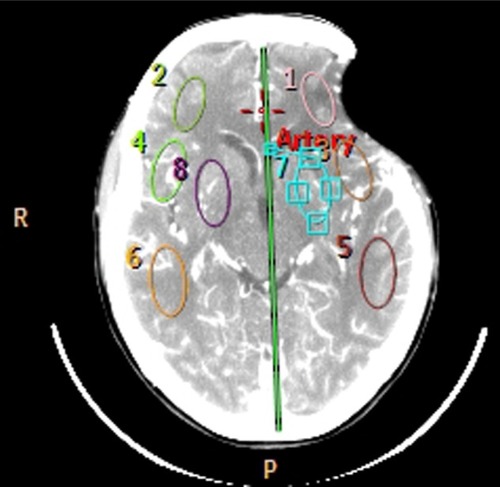
Computed tomography (CT) brain perfusion was also performed before and 30 days after cranioplasty. The parameters of cerebral blood flow (CBF), cerebral blood volume, and mean transit time in four symmetrical brain regions of interest in each hemisphere were evaluated (). An Ingenuity FlexCitation32 CT scanner (Philips Healthcare, DA Best, the Netherlands) with 32 channels was used. The scan was based on continuous acquisition at 80 kVp 200 mA for a duration of 40 seconds, allowing a total of 240 images to be obtained; contrast enhancement medium was administered at a rate of 5 mL/second. The CT data were processed using Philips iSite PACS software (Philips Healthcare).
Ethical considerations
This case study was approved by the ethics committee of our institution (CAPPESQ – University of São Paulo Medical School) and informed consent was obtained from the patient or his caregiver.
Literature review
We systematically searched the PubMed database up to September 2013 using the following search terms and combinations: “cranioplasty”, “trephined syndrome”, “sinking skin flap syndrome”, “neurological deficits”, “cerebral decompression”, “brain decompression”, and “decompressive craniotomy”. Reference lists of recovered articles were examined for additional suitable papers. The “Related Articles” feature in PubMed was also used for all selected studies to maximize the probability of locating additional relevant studies. The inclusion criteria for the review was any study including case reports and case series describing clinical improvements after cranioplasty such as improvement of symptoms related to TS or even reversal of neurological deficits and cognitive disturbances. We excluded studies describing only cerebral hemodynamic changes and papers in which interventions other than cranioplasty had been performed (). Two observers (RLA and WSP) independently reviewed the results of the PubMed search and selected the articles to be included in the analysis; discrepancies were resolved by consensus. We found 1,996 articles, and after matching the inclusion criteria, 29 articles were selected. All studies were case series or case reports.
Table 2 Summary of case series reported
Case report
A 44-year-old woman with a history of antiphospholipid antibody syndrome (coumarin user) presented with traumatic brain injury after falling to the ground. Upon admission, she scored 12 points on the Glasgow Coma Scale, and a CT scan depicted a massive left frontotemporal subdural hematoma and brain midline shift. The patient underwent a craniotomy to remove the clot and was discharged 2 weeks later. She was conscious and oriented, and exhibited no motor deficit except for a slight difficulty in speaking. Four weeks later, she developed a wound infection. She was treated with antibiotics and the affected bone had to be removed.
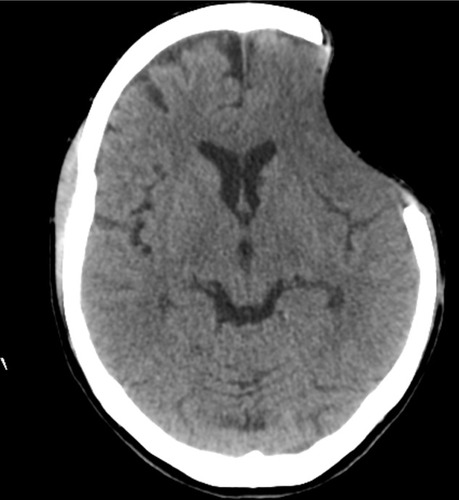
The patient was reassessed 8 months later. The bone defect was 8.2 cm × 5.3 cm in size, and the scalp had sunk 2.2 cm from the surface of the skull. A CT scan showed no shift of brain structures, but sulci were enfaced next to the bone defect (). The patient presented with headache, tinnitus, dizziness with head movements, incision discomfort, and difficulty in speech. There were no motor deficits, and she received 29 points on the MMSE. The patient scored 2 points on the mRS (ranging 0–6) and 100 points on the BI (ranging 0–100). The patient underwent cranioplasty with methyl methacrylate and was discharged uneventfully on the second postoperative day.
Figure 2 Cranial computed tomography before performing cranioplasty.
Six months after surgery, the patient reported complete resolution of symptoms, without changes in motor examination, and a subjective report of improvement in verbal fluency. Neuropsychological assessment before and after cranioplasty showed improvement in memory capacity, language, executive functions, and activities of daily living (). Postoperative MMSE showed a slight variation (down 1 point). No changes were observed in BI and mRS.
Table 1 Neuropsychological assessment before and 6 months after cranioplasty
Regarding the cerebral hemodynamic changes, postoperative TCD showed a 20% and 16% increase in the cerebral blood flow velocity in the middle cerebral artery in the supine and the sitting positions, respectively. There was no significant increment of CBF velocity on the contralateral middle cerebral artery.
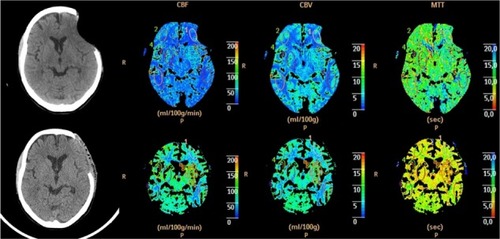
Perfusion CT study revealed an increase in CBF: 18 to 58 mL/100 g/minute ipsilateral to the cranioplasty and 19 to 70 mL/100 g/minute on the contralateral side. Interestingly, there was a reduction in the mean transit time on both sides: 14.7 to 5.2 seconds (ipsilateral to the cranioplasty) and 13.6 to 4.8 seconds (contralateral side). The increment in cerebral blood volume was less evident: 4.03 to 5.11 mL/100 g (ipsilateral to the cranioplasty) and from 4.34 to 5.46 mL/100 g (contralateral side). The values of blood pressure and heart rate were similar at the time of the tests ().
Figure 3 Perfusion computed tomography before (top) and after (bottom) surgery showing the changes in the three parameters: CBF, CBV, and MTT.
Abbreviations: CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transit time.
Discussion
The findings of this case report indicate that a skull defect can lead to an objective neuropsychological impairment, not exclusively due to the primary injury. Generally, patients submitted to DC are at risk of death and, consequently, can present with a constellation of neurological symptoms. An implication of this study is that the replacement of the bone (which had been considered merely a matter of cosmesis for decades) may actually confer functional benefits to the patient. Although the first report describing the symptoms caused by the skull defect comes from 1939 (Grant and Norcross),Citation12 researchers have not investigated the late effects of DC and cranioplasty in much detail. Grant and NorcrossCitation12 described the first clinical series of patients who underwent cranioplasty and presented with the syndrome of the trephined. Of the 83 operated patients, 43 had symptoms compatible with TS, and improvement occurred in approximately 25% of cases. The improvement in motor signs and symptoms was noted in 14% of patients. Despite the observation that there was cognitive improvement in three cases, there was no information about the variables used in cognitive assessment. Grantham and LandisCitation15 presented similar results in 1948, showing advance in 29% of TS patients and improvement of verbal fluency in some cases.
TS has been described in patients who develop progressive loss of hand strength contralateral to the skull defect in the late phase post-DC.Citation12–Citation14 In 1977, Yamaura and Makino showed that patients with sinking skin at the site of bone defects can present with progressive motor deficit, reversible in 30% of cases after cranioplasty.Citation13 They found that regardless of the underlying disease that led to DC, patients who had more prominent concavity tended to develop motor deficits that could be reversed with cranioplasty. In addition, they observed improvement in neurological deficits in 30% of patients and correlated these findings with modification of standard electroencephalogram. These authors believed that the compressive effects of atmospheric pressure on the brain parenchyma led to neurological impairment, and that the risk of TS occurrence was higher in patients with large bone defects.Citation16,Citation20,Citation34,Citation41 Our patient presented with a significant “brain compression” that was totally reversed after the cranioplasty.
With the advent of techniques for assessing cerebral circulation, studies have shown reduced local cerebral blood flow associated with the compromised cerebral hemisphere. Richaud et al,Citation21 through examinations with inhaled xenon 133, showed a 15%–30% increase in cerebral perfusion and improvement in neurological deficit in approximately eight of 15 patients evaluated. Stiver et al in 2008Citation31 showed, through CT perfusion, a possible relationship between the development of neurological deficits and delayed worsening of cerebrospinal fluid circulation by facilitating the passage of liquor into the brain parenchyma adjacent to the previous contused areas. Winkler et alCitation24 used TCD to suggest improvement of cerebrovascular reactivity after cranioplasty, and that restoration of cerebral blood flow was related to improvement in glucose metabolism in the cerebral hemispheres, including areas contralateral to the bone defect. In this study, the mean increase in the rate of glucose metabolism (12.1% on the cranioplasty side and 4.5% on the contralateral side) was associated with a positive clinical prognosis and elevated satisfaction. Of the 13 patients enrolled, seven (53%) had reversal of hemiparesis, two (15%) of hemineglect, two (15%) of aphasia, and seven (53%) showed improvement in cognitive functions (not quantified) after cranioplasty. In our case, there was an increase in cerebral blood flow demonstrated by CT perfusion study and by TCD. However, despite reports of cognitive improvement, the data provided by publications continue to be more qualitative than quantitative.
Some authors claim that cranioplasty may result in improved cognition and neurological signs and symptoms of TS.Citation12,Citation24,Citation39,Citation42–Citation47 Maeshima et alCitation26 used several scales (Word Fluency Test, Frontal Lobe Assessment Battery, Auditory Verbal Learning Test, Raven’s Colored Progressive Matrices and Revised WAIS, MMSE, and Behavioral Inattention Test) to quantify cognitive improvement, correlating these findings with cerebral perfusion data. Likewise, Agner et alCitation48 showed improvement in neurocognitive analysis of 48.3% in the Cognistat scores (Neurobehavioral Cognitive Status Examination, which independently assess different aspects of language, the ability to perform complex constructions, memory, calculations, and reasoning) and of 32.95% in the EXIT interview (Executive Interview, assesses executive functions in the bedside) after cranioplasty.
In our case, the comparative results between the two neuropsychological assessments showed significant improvement in the task of verbal fluency by phonemic category of the COWAT-FAS, having initially received a lower score than expected for the level of schooling and, at a later point in time, scoring above average. It also highlighted better scores for the task of verbal fluency (semantic category of animals), episodic memory (logical memory), audio-verbal learning (RAVLT), information processing speed (TMTA, TMTB, and VST), and visual-constructive functions (copy of Rey Complex Figure Test and cubes).
Our patient attained similar performance with respect to visual memory, logical reasoning ability, and inhibitory control, while demonstrating worsening performance on the attentional tasks (digits, TMTA, TMTB), and occupational memory index (arithmetic, digits, and sequence of numbers and letters). The capacity to perform daily activities and emotional processing showed the most promising results. Taken together, these findings show us that improvement of symptoms and signs of TS, as well as cognitive impairment, could be related to an improvement in the hemodynamic brain patterns in both hemispheres. This report served as a pilot study of a prospective cohort study that includes the entire assessments described here, aiming to evaluate patients with large and small skull defects, due to traumatic or non-traumatic causes.
Conclusion
Although it is difficult to draw conclusions from a single case report, our study provides additional evidence with respect to the possible pathophysiological mechanisms involved in TS, which can be related to a global hemodynamic dysfunction. Moreover, the benefits provided by the cranioplasty suggest that skull defects can also lead, in the long term, to neuropsychological impairments. Further work needs to be done to test these hypotheses with a larger sample size. Therefore, a prospective cohort study is being prepared to address the correlation between brain hemodynamics and clinical findings in such patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- BohmanLESchusterJMDecompressive craniectomy for management of traumatic brain injury: an updateCurr Neurol Neurosci Rep2013131139224101348
- CushingHThe establishment of cerebral hernia as a decompressive measure for inaccessible brain tumors; with the description of intramuscular methods of making the bone defect in temporal and occipital regionsSurg Gynecol Obstet19051297314
- AmorimRLBor-Seng-ShuEGattásGSPaivaWde AndradeAFTeixeiraMJDecompressive craniectomy and cerebral blood flow regulation in head injured patients: a case studied by perfusion CTJ Neuroradiol201239534634922633048
- KjellbergRNPrietoAJrBifrontal decompressive craniotomy for massive cerebral edemaJ Neurosurg19713444884935554353
- KondziolkaDFazlMFunctional recovery after decompressive craniectomy for cerebral infarctionNeurosurgery19882321431473185872
- HoneybulSComplications of decompressive craniectomy for head injuryJ Clin Neurosci201017443043520181482
- StiverSIComplications of decompressive craniectomy for traumatic brain injuryNeurosurg Focus2009266E719485720
- JiangJYXuWLiWPEfficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled studyJ Neurotrauma200522662362815941372
- ChibbaroSTacconiLRole of decompressive craniectomy in the management of severe head injury with refractory cerebral edema and intractable intracranial pressure. Our experience with 48 casesSurg Neurol200768663263817765952
- YangXJHongGLSuSBYangSYComplications induced by decompressive craniectomies after traumatic brain injuryChin J Traumatol2003629910312659705
- FlintACManleyGTGeanADHemphillJC3rdRosenthalGPost-operative expansion of hemorrhagic contusions after unilateral decompressive hemicraniectomy in severe traumatic brain injuryJ Neurotrauma200825550351218346002
- GrantFCNorcrossNCRepair of cranial defects by cranioplastyAnn Surg1939110448851217857467
- YamauraAMakinoHNeurological deficits in the presence of the sinking flap following decompressive craniectomyNeurol Med Chir (Tokyo)1977171 Pt 1435374031
- StiverSIWintermarkMManleyGTMotor trephine syndrome: a mechanistic hypothesisActa Neurochir Suppl200810227327719388328
- GranthamEGLandisHPCranioplasty and the post-traumatic syndromeJ Neurosurg194851192218917349
- TabaddorKLaMorgeseJComplication of a large cranial defect: case reportJ Neurosurg19764445065081255240
- StulaDIntracranial pressure measurement in large skull defectsNeurochirurgia (Stuttg)1985284164169 German4033850
- FarringtonPRClosure of a defect of the skull with tantalumRocky Mt Med J19454284284421004113
- LangfittTWIncreased intracranial pressureClin Neurosurg1968164364714981573
- FodstadHLoveJAEkstedtJFridénHLiliequistBEffect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephinedActa Neurochir (Wien)1984701–221306741628
- RoyallDRMahurinRKGrayKFBedside assessment of executive cognitive impairment: the executive interviewJ Am Geriatr Soc19924012122112261447438
- SegalDHOppenheimJSMurovicJANeurological recovery after cranioplastyNeurosurgery1994344729731 discussion 7318008174
- KuoJRWangCCChioCChengTJNeurological improvement after cranioplasty – analysis by transcranial Doppler ultrasonographyJ Clin Neurosci200411548648915177389
- WinklerPAStummerWLinkeRKrishnanKGTatschKInfluence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolismJ Neurosurg2000931536110883905
- IsagoTNozakiMKikuchiYHondaTNakazawaHSinking skin flap syndrome: a case of improved cerebral blood flow after cranioplastyAnn Plast Surg200453328829215480019
- MaeshimaSKagawaMKishidaYUnilateral spatial neglect related to a depressed skin flap following decompressive craniectomyEur Neurol200553316416815942242
- RichaudJBoettoSGuellALazorthesYEffects of cranioplasty on neurological function and cerebral blood flowNeurochirurgie1985313183188 French4033856
- SuzukiNSuzukiSIwabuchiTNeurological improvement after cranioplasty. Analysis by dynamic CT scanActa Neurochir (Wien)19931221–249538333309
- SakamotoSEguchiKKiuraYAritaKKurisuKCT perfusion imaging in the syndrome of the sinking skin flap before and after cranioplastyClin Neurol Neurosurg2006108658358515921849
- KemmlingADuningTLemckeLCase report of MR perfusion imaging in sinking skin flap syndrome: growing evidence for hemodynamic impairmentBMC Neurol2010108020831824
- StiverSIWintermarkMManleyGTReversible monoparesis following decompressive hemicraniectomy for traumatic brain injuryJ Neurosurg2008109224525418671636
- YoshidaKFuruseMIzawaAIizimaNKuchiwakiHInaoSDynamics of cerebral blood flow and metabolism in patients with cranioplasty as evaluated by 133Xe CT and 31P magnetic resonance spectroscopyJ Neurol Neurosurg Psychiatry19966121661718708684
- ErdoganEDüzBKocaogluMIzciYSirinSTimurkaynakEThe effect of cranioplasty on cerebral hemodynamics: evaluation with transcranial Doppler sonographyNeurol India200351447948114742926
- NakamuraTTakashimaTIsobeKYamauraARapid neurological alteration associated with concave deformity of the skin flap in a craniectomized patient. Case reportNeurol Med Chir (Tokyo)198020189936154265
- NgDDanNGCranioplasty and the syndrome of the trephinedJ Clin Neurosci19974334634818638981
- SchifferJGurRNisimUPollakLSymptomatic patients after craniectomySurg Neurol19974732312379068692
- KumarGSChackoAGRajshekharVUnusual presentation of the “syndrome of the trephined”Neurol India200452450450515626847
- ChieregatoAThe syndrome of the sunken skin flap: a neglected potentially reversible phenomenon affecting recovery after decompressive craniotomyIntensive Care Med200632101668166916917776
- HanPYKimJHKangHIKimJS“Syndrome of the sinking skin-flap” secondary to the ventriculoperitoneal shunt after craniectomyJ Korean Neurosurg Soc2008431515319096548
- JosephVReillyPSyndrome of the trephinedJ Neurosurg2009111465065219361266
- SarovMGuichardJPChibarroSDECIMAL investigatorsSinking skin flap syndrome and paradoxical herniation after hemicraniectomy for malignant hemispheric infarctionStroke201041356056220056926
- BijlengaPZumofenDYilmazHCreissonEde TriboletNOrthostatic mesodiencephalic dysfunction after decompressive craniectomyJ Neurol Neurosurg Psychiatry200778443043317119005
- GottlobISimonsz-TòthBHeilbronnerRMidbrain syndrome with eye movement disorder: dramatic improvement after cranioplastyStrabismus200210427127712660851
- SugiyamaKKondoTHirayamaKTobimatsuYUrushiyamaYIzumiSA case of neurological improvement and facilitation of rehabilitation after cranioplastyJpn J Rehabil Med200441104109
- ChibbaroSValleeFBeccariaKThe impact of early cranioplasty on cerebral blood flow and its correlation with neurological and cognitive outcome. Prospective multi-centre study on 24 patientsRev Neurol (Paris)20131693240248 French23084153
- Di StefanoCSturialeCTrentiniPUnexpected neuropsychological improvement after cranioplasty: a case series studyBr J Neurosurg201226682783122702390
- HoneybulSJanzenCKrugerKHoKMThe impact of cranioplasty on neurological functionBr J Neurosurg201327563664123883370
- AgnerCDujovnyMGaviriaMNeurocognitive assessment before and after cranioplastyActa Neurochir (Wien)20021441010331040 discussion 104012382131
- MokriBOrthostatic headaches in the syndrome of the trephined: resolution following cranioplastyHeadache20105071206121120561067
- de Quintana-SchmidtCClavel-LariaPAsencio-CortesCVendrell-BrucetJMMolet-TeixidoJSinking skin flap syndromeRev Neurol20115211661664 Spanish21563117
- CarotaAPintucciMZanchiFD’AmbrosioECalabreseP‘Cognitive’ sinking skin flap syndromeEur Neurol201166422722821952114