Abstract
Background
This 13-week, double-blind study was conducted to confirm the efficacy and safety of paliperidone palmitate (PP), at dosing regimens approved in other countries, in Asian patients with schizophrenia.
Methods
Asian patients (aged ≥20 years) diagnosed with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-Text Revision criteria), and having a Positive and Negative Syndrome Scale (PANSS) total score of 60 to 120 were enrolled and randomized (1:1) to a PP or placebo group. Patients received PP intramuscularly at recommended doses: initiation dose 150 mg equivalent (eq) PP on day 1 and 100 mg eq PP on day 8 (deltoid); and a monthly maintenance dose of 75 mg eq PP on days 36 and 64 (deltoid or gluteal). The change from baseline to week 13 in PANSS total scores (primary endpoint), Clinical Global Impression-Severity (CGI-S) scores, and PANSS Marder factor scores and subscales, and responder rate at week 13 were evaluated. Safety was also assessed.
Results
The PANSS total score (P<0.0001, least-squares mean change from baseline to week 13: PP, −3.5; placebo, +6.2), CGI-S score (P<0.0001), and PANSS Marder factor scores (P≤0.0025) were significantly improved at week 13 in the PP group versus placebo. More treatment responders (≥30% decrease in PANSS total score) were in the PP group (22.8%) versus placebo (8.5%). Insomnia (PP 17.0% versus placebo 15.2%), injection site pain (13.2% versus 6.7%), nasopharyngitis (12.6% versus 6.1%), psychiatric symptoms (11.3% versus 26.2%), and extrapyramidal symptoms (10.1% versus 4.9%) were the most frequently occurring treatment-emergent adverse events.
Conclusion
PP is efficacious for Asian patients with schizophrenia at the dosing regimen approved in other countries, with a similar safety and tolerability profile.
Introduction
Improving quality of life for patients with schizophrenia remains an ultimate goal of clinicians. Given the chronic nature of this psychotic illness, a treatment approach that offers immediate and prolonged control of symptoms and behavior, with minimal adverse events, is of clinical interest. Notably, the primary symptoms of schizophrenia are actually conducive to patients failing to adhere to the daily-dosing regimen recommended for oral medications, that eventually has devastating consequences, such as an increased risk of psychotic relapse and rehospitalization.Citation1 Long-acting injectables offer a prolonged therapeutic advantage over oral antipsychotics by enhancing patient adherence to treatment, thereby mitigating the risk of relapse, hospitalization, and suicide secondary to treatment nonadherence.Citation2,Citation3
Paliperidone palmitate (PP), a long-acting injectable antipsychotic, is approved for the acute and maintenance treatment of schizophrenia in adults in the US,Citation4 European Union, and several other countries (including the People’s Republic of China, Korea, and other Asian countries).Citation5 The efficacy and safety profiles of PP are well determined but predominantly in White patients, with the next largest race represented being Blacks.Citation6–Citation9 Asians have generally accounted for a very small proportion (≤5%) of these study populations. However, race and ethnic differences can influence treatment response, as well as the type and extent of adverse events that follow antipsychotic treatment.Citation10–Citation12 Recently, a 13-week open-label study by Li et al confirmed the efficacy and safety of PP specifically in Chinese patients with acute schizophrenia.Citation13 This is the first placebo-controlled, double-blind study designed to confirm the efficacy and safety of PP in Asian patients with schizophrenia at the dosage regimen (including the initial loading dose administration method) approved in other countries.
Materials and methods
Study population
Asian patients (men and women, aged ≥20 years), diagnosed with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-Text Revision [DSM-IV-TR]) for at least a year before screening and having a Positive and Negative Syndrome Scale (PANSS) total score of 60 to 120 at screening and baseline were enrolled in this study. The patients were required to have documented tolerability to either a risperidone or a paliperidone formulation before baseline or to have demonstrated tolerability following oral tolerability testing conducted during the screening period. Key exclusion criteria were: a primary active DSM-IV-TR I diagnosis other than schizophrenia and a DSM-IV-TR diagnosis of active substance dependence within 3 months before screening (except nicotine and caffeine).
The independent ethics committee or institutional review board at each study site approved the protocol, and the study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki and consistent with Good Clinical Practices and applicable regulatory requirements. This study is registered at ClinicalTrials.gov: NCT01299389.
Study medication
PP was provided as 75, 100, and 150 mg equivalent (eq) injectable suspensions (PP 75, 100, and 150 mg eq equate to 117, 156 and 234 mg, respectively, of PP). To maintain blinding, the placebo for each PP dose was supplied in a matching prefilled syringe; both PP and placebo were wrapped in blinded fashion (using labeling) to prevent visibility of the content.
Study design
This 13-week, double-blind study, conducted in Asia (47 sites in Japan, 12 sites in Korea, 11 sites in Taiwan) between September 27, 2010 and March 17, 2012, consisted of a 2-week screening period, a 13-week double-blind phase, and a post-observation follow-up phase (after week 13 up to week 21, ). The patients were randomized (1:1) to receive PP or placebo on day 1. The dosing regimen for PP corresponded to the approved initiation and maintenance regimen: a PP initiation dose of 150 mg eq on day 1 and 100 mg eq at week 1 (day 8), both given intramuscularly in a deltoid muscle; and a monthly PP maintenance dose of 75 mg eq administered intramuscularly at week 5 (day 36) and 9 (day 64) in a deltoid or gluteal muscle. Patients assigned to the placebo group received injections matching the PP injections on the same days.
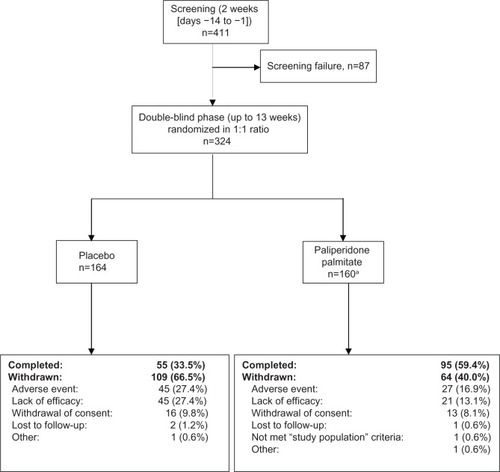
Figure 1 Patient disposition (full analysis set).
Abbreviation: PP, paliperidone palmitate.
Assessments
Efficacy
The primary endpoint was the mean change in PANSS total scores from baseline (day 1) to endpoint (week 13). Secondary endpoints were: change from baseline to the endpoint in Clinical Global Impression-Severity (CGI-S) scores and five PANSS Marder factor scores and subscales; and responder rate (proportion of patients with a ≥30% reduction in PANSS total scores) at endpoint. Both the PANSS and CGI-S assessments were performed on days 1 and 4, and weeks 1, 2, 3, 5, 9, and 13 by a trained rater. The same rater was required to perform these assessments at each visit.
Safety
Safety assessments included evaluation of treatment-emergent adverse events (as per Medical Dictionary for Regulatory Activities, version 14.1), clinical laboratory parameters, electrocardiograms, vital sign measurements, and examination of weight, waist circumference, and body mass index. The Drug-Induced Extrapyramidal Symptoms ScaleCitation14 was used to evaluate the severity of drug-induced extrapyramidal symptoms and the Columbia-Suicide Severity Rating ScaleCitation15 was used to measure the spectrum of suicidal ideation. Injection site reactions and injection pain examinations were performed.
Pharmacokinetics
Blood samples (4 mL, at each data collection point) were taken from a peripheral vein using collection tubes on days 1, 4, 8, 15, 22, 36, 64, and 92 (on days 1, 8, 36, and 64, the samples were collected before administration of the study drug). Plasma concentrations of paliperidone were determined using liquid chromatography coupled to a tandem mass spectrometry method with a quantification limit of 0.1 ng/mL at the Department of Bioanalysis, Janssen Research and Development, LLC, Raritan, NJ, USA.
Statistical analysis
Based on previous studies,Citation6,Citation7,Citation9 a total of 146 patients per group were to be enrolled to achieve a study power of 90% at a two-sided significance level of 0.05, with an assumption that the difference in mean change from baseline to endpoint in PANSS total score between the PP and placebo groups was −8.0 and the standard deviation was 21. A total of 308 patients were planned to be enrolled.
Between-group comparison (placebo group versus PP group) of the changes in PANSS total scores and PANSS Marder factor scores was done using an analysis of covariance model (5% level of significance [two-sided], two-tailed 95% confidence interval) with treatment and country as factors and respective baseline score as a covariate. Similarly, CGI-S scores were investigated using the analysis of covariance model on the ranks of change from baseline, with treatment and country as factors and (nonranked) baseline score as a covariate. The last observation carried forward approach was used for missing data imputation. No adjustments were made for multiple comparisons. To evaluate the treatment-by-country interaction, least-squares means were calculated using the analysis of covariance model, with treatment, country, and treatment-by-country interaction as factors and baseline PANSS total score as a covariate. The Cochran–Mantel–Haenszel test was used to evaluate the difference in percentage of responders (as patients who showed ≥30% reduction from baseline in PANSS total score) between PP and placebo. All safety and pharmacokinetic assessments were summarized descriptively.
The full analysis set included all randomized patients who had received at least one injection of the study drug and had a baseline and at least one postbaseline PANSS total score measurement. The safety analysis set comprised of all randomized patients who received at least one injection of the study drug. The pharmacokinetic analysis set comprised of all randomized patients who received at least one injection of PP and who had at least one assessment of plasma paliperidone concentration available for pharmacokinetic analysis after drug administration.
Results
Patient position and characteristics
Of the 324 randomized patients, the majority (n=157) were from Japan, 96 were from Taiwan, and 71 were from Korea. More patients from the PP (59.4%) group than the placebo group (33.5%) completed the study (). Demographic characteristics were generally similar across groups ().
Table 1 Demographic and baseline characteristics (full analysis set)
Extent of exposure
Of the randomized patients, 164 received all four injections of the study drug (n=101 for PP and n=63 for placebo). The median duration of exposure to PP was 87.0 (range 3–100) days and to placebo was 50.0 (range 4–100) days. The mean total dose of PP administered during the study was 336.48 (range 150.0–400.0) mg eq.
Efficacy
The PANSS total scores decreased (improved) significantly (P<0.0001) from baseline to endpoint in the PP group in comparison with placebo (). The change from baseline to endpoint in CGI-S scores (P<0.0001) and all PANSS Marder factor scores (P≤0.0025) differed significantly in favor of PP versus placebo (). More patients from the placebo group (n=85/164, 51.8%) versus the PP group (n=62/158, 39.2%) were classified as having disease of “marked” severity or worse at endpoint. The percentage of treatment responders was significantly (P=0.0005) higher in the PP group (22.8%) versus placebo (8.5%). No statistically significant treatment-by-country interaction was noted (P=0.35, ).
Figure 2 Least-squares mean (±SE) changes from baseline in PANSS total score over time (LOCF) (full analysis set).
Abbreviations: LOCF, last observation carried forward; SE, standard error; PANSS, Positive and Negative Syndrome Scale; CI, confidence interval.
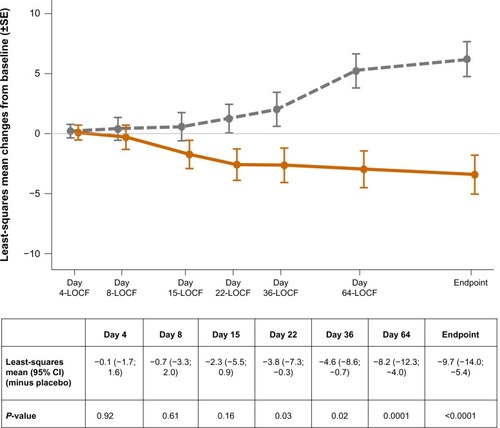
Table 2 Change in CGI-S score and PANSS Marder factor score from baseline to double-blind endpoint (LOCF, full analysis set)
Table 3 Change in PANSS total score (by country) from baseline to double-blind endpoint (LOCF, full analysis set)
Safety
The most frequent treatment-emergent adverse events in the PP group (versus placebo) were insomnia (17.0% versus 15.2%), injection site pain (13.2% versus 6.7%), nasopharyngitis (12.6% versus 6.1%), psychiatric symptoms (11.3% versus 26.2%), and extrapyramidal disorders (10.1% versus 4.9%), as shown in . The types and incidence of treatment-emergent adverse events were generally similar among patients from Japan, Korea, and Taiwan. The incidence of serious treatment-emergent adverse events was higher in the placebo group (15.2%) than in the PP group (6.3%); the most commonly reported treatment-emergent adverse events were psychiatric symptoms (7.9% versus 2.5%) and worsening of schizophrenia (1.8% versus 2.5%).
Table 4 Treatment-emergent adverse events in at least 2% of patients in any treatment group (safety analysis set)
The incidence of treatment-emergent adverse events leading to discontinuation was lower in the PP group (17.0%) than in the placebo group (29.9%); the most common treatment-emergent adverse events were psychiatric symptoms (6.3% versus 15.2%) and worsening of schizophrenia (3.1% versus 4.3%). One death (primary cause cardiac failure) occurred during the study, in the placebo group. The incidence of extrapyramidal treatment-emergent adverse events was higher in the PP group (23.3%) than in the placebo group (12.8%, ). The Drug-Induced Extrapyramidal Symptoms Scale total score decreased slightly from baseline at the 13-week endpoint in both groups. Further, the percentage of patients using anti-parkinsonian drugs was slightly higher in the PP group (29.9% for placebo versus 35.2% for PP). The number of patients experiencing treatment-emergent suicidal ideation was higher in the placebo group (n=10, 6.3%) than in the PP group (n=7, 4.7%), but none of these were serious. Fifteen patients (9.1%) in the placebo group (18 events) and 24 (15.1%) in the PP group (49 events) had injection site-related treatment-emergent adverse events.
In the PP group, the mean (standard deviation) prolactin level from baseline to endpoint remained stable in men (30.57±24.161 ng/mL at baseline and 30.72±24.096 ng/mL at endpoint) throughout the double-blind period, but increased slightly in women (68.73±50.868 ng/mL at baseline and 79.36±53.695 ng/mL at endpoint). In the placebo group, there was an overall decrease in mean prolactin levels, irrespective of sex. Only one patient had a prolactin-related treatment-emergent adverse event (irregular menstruation) during the double-blind period, and was from the placebo group.
Glucose-related treatment-emergent adverse events noted during the study were very few (four [2.4%] in the placebo group and five [3.1%] in the PP group), all of which were mild or moderate in severity.
Weight, waist circumference, and body mass index showed no clinically relevant changes in either treatment group; however, more patients had a ≥7% weight increase in the PP group (n=15, 9.4%) than in the placebo group (n=4, 2.4%).
QT prolongation was reported as an adverse event in one patient in each treatment group. Clinical laboratory parameters, electrocardiograms, and vital sign measurements indicated no clinically relevant changes in either group.
Pharmacokinetics
After the first two injections of PP (150 mg eq on day 1 followed by 100 mg eq on day 8), median paliperidone concentrations at predose on days 8 and 36 were comparable; the distributions of concentrations on days 64 and 92 were within the ranges of those on days 8 and 36. The maximum plasma concentration of paliperidone was reached on day 15 ().
Figure 3 Median (± interquartile range) plasma concentrations of paliperidone in Asian patients with schizophrenia (pharmacokinetic analysis set).
Abbreviation: BL, baseline.
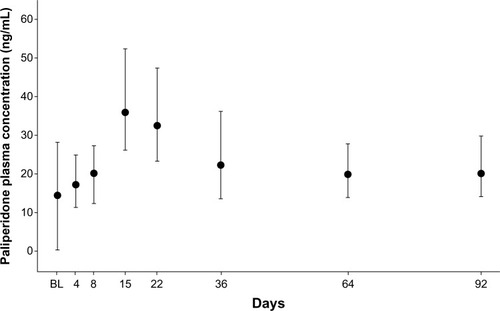
Country (Japan, Korea, and Taiwan) had no significant effect on plasma paliperidone concentrations, with the distribution of plasma paliperidone concentrations at each time point overlapping across countries throughout the doubleblind period.
Discussion
This first placebo-controlled, double-blind study conducted in Asian patients with schizophrenia supports the advantages of PP treatment in this population, and suggests that PP could be used as a potential modality for management of schizophrenia in this region. The efficacy results were generally consistent with the previous studies conducted predominantly in Whites.Citation6–Citation9,Citation16,Citation17 Further, these findings compare fairly well with results from a study by Li et al in a Chinese population with schizophrenia.Citation13 In the current study, PP treatment demonstrated significant improvement in PANSS total scores (P<0.0001) compared with matched placebo. However, the magnitude of PANSS change was apparently smaller and responder rates were lower in the PP group of this study compared with the studies reported earlier. This may in part have resulted from patients enrolled in the current study being clinically more stable, as noted by their slightly longer duration of illness (17.5 years), than the patients in the previous studies (approximately 14 years).Citation6–Citation9 Otherwise, the baseline characteristics of this study population were similar to those of the populations participating in other pivotal studies (PANSS scores of 87–91, age 39–41 years, and mostly moderate disease severity).Citation6–Citation9 As expected, PP also significantly improved CGI-S scores (P<0.0001) and the PANSS Marder factor scores in this Asian population.
Treatment with antipsychotics is frequently associated with metabolic, cardiovascular, and extrapyramidal adverse effects that could progressively lead to nonadherence and serious physical conditions.Citation18 Thus, regular clinical monitoring of these patients is mandatory to achieve the desired treatment benefits. Overall, the safety findings of this study corroborated the earlier reports concerning PP.Citation6–Citation9,Citation16 The most common (>10%) treatment-emergent adverse events in the PP group were insomnia, injection site pain, nasopharyngitis, psychiatric symptoms, and extrapyramidal symptoms. The treatment-emergent adverse events that most frequently led to discontinuation were psychiatric symptoms and worsening of schizophrenia. The incidence of treatment-emergent adverse events related to extrapyramidal symptoms was higher (23.3%) compared with the earlier reports conducted in largely non-Asian populations (up to 6%).Citation6–Citation9,Citation16 None of these events were serious and all were manageable with anti-extrapyramidal medications. No cases of neuroleptic malignant syndrome were observed. Further, no tardive dyskinesia occurred in the PP group during this study, but one case was noted in the placebo group.
Only one patient (in the placebo group) had a prolactin-related treatment-emergent adverse event (irregular menstruation). Given that the prolactin-related treatment-emergent adverse event data were collected spontaneously, under-reporting of actual cases of sexual dysfunction cannot be excluded. Additionally, the short time period of this study limits a full assessment of prolactin-related events.
Similar to earlier reports,Citation6–Citation9,Citation16 the occurrence of glucose-related events was very low in this study. Weight-related changes noted with PP treatment were not considered to be of clinical significance. However, this is of clinical interest because weight gain might interfere considerably with compliance with antipsychotic treatment.Citation6 Minimal injection site reactions were noted, and local injection site tolerability was good.
Overall, the pharmacokinetic profile of PP in this study was consistent with the profile obtained in the western population (non-Japanese).Citation9 Initiation of treatment with a 150 mg eq dose on day 1, followed by a 100 mg eq dose on day 8, resulted in rapid attainment of therapeutic plasma paliperidone concentrations that exceeded a previously established antipsychotic efficacy threshold of 7.5 ng/mL (from the first sampling point after dosing [day 4]).Citation19–Citation21 The apparent steady state was reached by day 36. Thereafter, plasma paliperidone concentrations were maintained with 75 mg eq PP injections at 4-week intervals.
A subgroup analysis was performed to determine if efficacy, safety, and pharmacokinetic outcomes differed among patients from Japan, Korea, and Taiwan. No significant differences by country were noted in any of the aforementioned outcomes assessed in this study.
The short duration and relatively limited number of patients in this study limits any conclusions or generalizability about the efficacy and safety profile of PP in the Asian population. Further, the study was conducted only at Northeastern Asian sites (Japan, Korea, and Taiwan), and extrapolating these findings to all Asian patients would be speculative. Also, the study was not adequately powered to assess for differences in efficacy, safety, and pharmacokinetics among patients from three Asian countries; thus, interpretation of the results of subgroup analysis by country warrants caution. There was an average of four patients per study site (324 randomized patients from 70 sites), thereby preventing examination of intersite differences. Additional studies with a longer duration and larger numbers of patients that might closely simulate a clinical practice scenario are needed.
Conclusion
Overall, the results of the present study demonstrate that PP, when administered at the dosing regimen approved in other countries (150 mg eq on day 1, 100 mg eq on day 8, and monthly injections of 75 mg eq on days 36 and 64) was efficacious in these Asian patients with schizophrenia. Although rates of insomnia, extrapyramidal symptoms, and prolactin-related events were notably higher in the PP group than in the placebo group, PP was generally tolerable in the population studied, with no new or unexpected safety signals. This suggests that PP might present an efficacious treatment paradigm in Asian patients with schizophrenia.
Author contributions
All the authors contributed to the design of the study. MI was responsible for data collection. HS performed the pharmacokinetic data analysis. YS contributed to data analysis. All authors contributed to development of the manuscript, revised it critically for important intellectual content, and gave their approval of this version to be published.
Acknowledgments
We thank the study participants, without whom this study would not have been accomplished, and all the investigators for their participation. We also thank Ananya Chikramane (SIRO Clinpharm Pvt Ltd) for writing assistance and Wendy P Battisti (Janssen Research and Development LLC) for additional editorial assistance.
Disclosure
This research was funded by Janssen Pharmaceutical KK, Japan. Data from this study was presented at the 26th European College of Neuropsychopharmacology Congress, held in Barcelona, Spain, October 5–9, 2013. All authors are employees of Janssen Pharmaceutical KK. All authors met the International Committee of Medical Journal Editors (ICMJE) criteria and all those who fulfilled those criteria are listed as authors. All authors had access to the study data and made the final decision about where to publish these data and approved submission to this journal.
References
- Clinical Challenges in Schizophrenia: A Six-Part Newsletter Series to Current Psychiatr20112S1S4 Available from: http://www.currentpsychiatry.com/home/article/challenges-and-opportunities-in-schizophrenia-treatment/f5fe98b0f0d1f5ae950fb84f07e42edc.htmlAccessed November 4, 2013
- ZhornitskySStipEOral versus long-acting injectable antipsychotics in the treatment of schizophrenia and special populations at risk for treatment nonadherence: a systematic reviewSchizophr Res Treatment2012201240717122966436
- TandonRNasrallahHAKeshavanMSSchizophrenia, “just the facts” 5. Treatment and prevention. Past, present, and futureSchizophr Res201012212320655178
- Invega® Sustenna®Prescribing informationTitusville, NJJanssen Pharmaceuticals Inc2012
- KramerMLitmanRHoughDPaliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety studyInt J Neuropsychopharmacol20101363564719941696
- GopalSHoughDWXuHEfficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response studyInt Clin Psychopharmacol20102524725620389255
- NasrallahHAGopalSGassmann-MayerCA controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophreniaNeuropsychopharmacology2010352072208220555312
- PandinaGLaneRGopalSA double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophreniaProg Neuropsychopharmacol Biol Psychiatry20113521822621092748
- PandinaGJLindenmayerJPLullJA randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophreniaJ Clin Psychopharmacol20103023524420473057
- BanerjeeACross-cultural variance of schizophrenia in symptoms, diagnosis and treatmentGeorgetown Undergraduate Journal of Health Sciences201261824
- CoppolaDLiuYGopalSA one-year prospective study of the safety, tolerability and pharmacokinetics of the highest available dose of paliperidone palmitate in patients with schizophreniaBMC Psychiatry2012122622455454
- WilliamsDREarlTRCommentary: race and mental health – more questions than answersInt J Epidemiol20073675876017566003
- LiHRuiQNingXXuHGuNA comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophreniaProg Neuropsychopharmacol Biol Psychiatry2011351002100821315787
- InadaTEvaluation and Diagnosis of Drug-Induced Extrapyramidal Symptoms: Commentary on the DIEPSS and Guide to its UsageTokyo, JapanSeiwa Shoten Publishers1996
- PosnerKBrownGKStanleyBThe Columbia-Suicide Severity Rating Scale: initial validity and internal three multisite studies with adolescents and adultsAm J Psychiatry2011168121266127722193671
- GopalSVijapurkarULimPA 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophreniaJ Psychopharmacol20112568569720615933
- HoughDGopalSVijapurkarUPaliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled studySchizophr Res201011610711719959339
- CorrellCUAddressing barriers to using long-acting injectable antipsychotics and appropriately monitoring antipsychotic adverse effectsJ Clin Psychiatry201374e16
- KapurS5-HT2 antagonism and EPS benefits: is there a causal connection?Psychopharmacology (Berl)199612435398935798
- NybergSFardeLHalldinCDahlMLBertilssonLD2 dopamine receptor occupancy during low-dose treatment with haloperidol decanoateAm J Psychiatry19951521731787840348
- KarlssonPDenckerENybergSPharmacokinetics and dopamine D2 and serotonin 5-HT2A receptor occupancy of paliperidone in healthy subjects: two open-label, single-dose studiesClin Pharmacol Ther200679P74