Abstract
Background
Previous functional magnetic resonance imaging (fMRI) studies have shown abnormal functional connectivity in regions involved in emotion processing and regulation in pediatric bipolar disorder (PBD). Recent studies indicate, however, that task-dependent neural changes only represent a small fraction of the brain’s total activity. How the brain allocates the majority of its resources at resting state is still unknown. We used the amplitude of low-frequency fluctuation (ALFF) method of fMRI to explore the spontaneous neuronal activity in resting state in PBD patients.
Methods
Eighteen PBD patients during the mania phase and 18 sex-, age- and education-matched healthy subjects were enrolled in this study and all patients underwent fMRI scanning. The ALFF method was used to compare the resting-state spontaneous neuronal activity between groups. Correlation analysis was performed between the ALFF values and Young Mania Rating Scale scores.
Results
Compared with healthy controls, PBD patients presented increased ALFF in bilateral caudate and left pallidum as well as decreased ALFF in left precuneus, left superior parietal lobule, and bilateral inferior occipital gyrus. Additionally, ALFF values in left pallidum were positively correlated with Young Mania Rating Scale score in PBD.
Conclusion
The abnormal resting-state neuronal activities of the basal ganglia, parietal cortex, and occipital cortex may play an important role in the pathophysiology in PBD patients.
Introduction
Childhood-onset bipolar disorder (BD) has been shown to be associated with shorter periods of euthymia, higher rates of psychosis, more recurrences, and greater likelihood of suicide attempts and violence than adult-onset BD.Citation1,Citation2 A follow-up study involving 24-year olds noted that pediatric BD (PBD) patients or subsyndromal youth BD patients showed significant impairment in psychosocial functioning and had higher mental health treatment utilization.Citation3 Childhood-onset BD cases are more treatment resistant and have a worse prognosis than adult-onset BD,Citation4 which poses a great treatment challenge for clinicians and a heavy burden on affected families. Thus, there is a pressing need for greater neurobiological understanding of PBD.
Toward that end, structural magnetic resonance imaging (MRI) studies have reported abnormalities in affect-processing regions, including abnormalities in prefrontal cortex, amygdale, superior temporal gyrus, and hippocampal volumes in PBD.Citation5,Citation6 Other studies have shown that abnormalities converge in the prefrontal white matter and, in particular, prefrontal and temporal associative tracts are known to be involved in emotion in PBD.Citation7,Citation8
Most functional MRI (fMRI) studies concerning PBD have also found abnormal affect-processing circuitry, which may cause emotional dysregulation and sociocognitive difficulties. Previous studies have shown reduced activation of right rostral ventrolateral prefrontal cortex together with increased activity in anterior cingulate, amygdala, and paralimbic cortex in PBD patients compared to healthy controls using a variety of cognitive tasks.Citation9,Citation10 Task-related changes in neural activation, however, only represent a small fraction (perhaps <5%) of the brain’s total activity.Citation11,Citation12 Intrinsic activity consumes more of the brain’s energy than external stimuli.Citation13 Thus, knowing to what the brain allocates the majority of its resources is essential for understanding neural mechanisms associated with PBD, but this is something that remains unclear in the present day.
Resting-state intrinsic activity may be measured by the amplitude of low-frequency fluctuations (ALFF) of the blood oxygenation level-dependent (BOLD) fMRI signal.Citation14,Citation15 The ALFF approach may reflect spontaneous neuronal activity and physiological states in the region and help to determine abnormal activity within the entire brain.Citation16 That is to say, this method could be a more direct index reflecting the amplitude of regional spontaneous neuronal activity and can be used to locate specific, impaired brain regions in the resting state, as has been demonstrated by Zang et al.Citation17 Another advantage of the ALFF approach is that ALFF may help to avoid potential bias induced by the selection of the “seed” voxels in functional connectivity analysis. Indeed, since Zang et alCitation17 demonstrated, ALFF has been used in many psychiatric studies to reveal regional brain activity alteration.Citation17–Citation19
In this study, we first adopted the ALFF method to examine the hypothesis that activity alterations occur in affect-processing regions in PBD patients in resting state, then the ALFF values of the brain regions that showed significant difference between the patients and controls were correlated with the Young Mania Rating Scale (YMRS) scores.
Methods
Subjects
A total of 36 right-handed, 12–18-year-old subjects were recruited, including 18 PBD patients and 18 normal controls. Patients were recruited at the Mental Health Institute of the Second Xiangya Hospital, Changsha, People’s Republic of China. Healthy controls were recruited from a local middle school. The study protocol was approved by the ethics committee of the Second Xiangya Hospital. Oral and written informed consent was obtained from parents and children. The PBD (n=18) inclusion criteria were meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IV-TR) criteria for BD, including at least one episode meeting full DSM-IV-TR criteria for hypomania (≥4 days) or mania (≥7 days).Citation20 All participants were evaluated by two qualified psychiatrists using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL), and the Wechsler Abbreviated Scale of Intelligence for Chinese Revised (WASI-CR) was administered as an overall measure of their cognitive ability.Citation21 All participants also completed the Young Mania Rating Scale (YMRS) and Mood and Feelings Questionnaire (MFQ) to evaluate mood of the patient within 24 hours of scanning.Citation22 According to Wood et alCitation22 and Rich et al,Citation23 manic state should be YMRS >26 and MFQ <18; hypomanic state should be YMRS >12 but <18 and MFQ <18.
Exclusion criteria were left-handedness, IQ <80, pervasive developmental disorder, neurological illness, metal implants, history of skull fracture or head trauma with loss of consciousness longer than 5 minutes, and substance abuse.
Data acquisition
Functional imaging was performed on a 3.0 T Siemens Trio scanner (Siemens, Munich, Germany). During the resting-state fMRI scanning, subjects were asked to lie supine with their heads kept still and to keep their eyes closed without thinking anything systematically nor falling asleep. Resting-state functional images were acquired with 33 axial slices, repetition time 2,000 milliseconds, echo time 30 milliseconds, slice thickness 4.0 mm, gap 0.4 mm, flip angle 90°, field of view 240 mm × 240 mm, and matrix 64 × 64, lasting for 8 minutes. Structural images were acquired by using three-dimensional magnetization-prepared rapid gradient-echo sequence (repetition time 2,300 milliseconds; echo time 2.98 milliseconds; inversion time 900 milliseconds; field of view 256 mm × 256 mm; flip angle 90°; matrix 256 × 256).
Image analysis
Spatial preprocessing of fMRI was conducted using the Statistical Parametric Mapping package (v 8; Wellcome Trust Centre for Neuroimaging, London, UK). For the MRI signal to reach a steady state and the subjects to get used to the scanner noise, we discarded the first 10 volumes of the resting-state images. Then, the remaining images were preprocessed by performing the following steps: slice timing; realignment; spatial normalization to the standard Montreal Neurological Institute echo-planar imaging template in the Statistical Parametric Mapping package; and resampling to 3 mm cubic voxels, followed by spatial smoothing with 4 mm full-width at half-maximum Gaussian kernel. Participants were excluded if their head motion was >1.5 mm maximum or 1.5° of any angular motion throughout the course of the scan.Citation24
ALFF analysis
The ALFF was performed using the Resting-State fMRI Data Analysis Toolkit (version 1.8; The State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing, People’s Republic of China). The time series for each voxel was filtered (band-pass filtering: 0.01–0.08 Hz) to remove the effect of low-frequency drifts and high-frequency noise. Then, the filtered time series was transformed to a frequency domain using a fast Fourier transform (FFT) (parameters: taper percent 0, FFT length shortest) and the power spectrum was then obtained by square-rooted FFT and averaged across 0.01–0.08 Hz at each voxel. This averaged square root was taken as the ALFF. Then, a mask made by the Montreal Neurological Institute template to ensure matching with the normalization step was used to remove the tissues outside the brain. For standardization purposes, the ALFF of each voxel was divided by the global mean ALFF value to standardize data across subjects.Citation17
Correlation analysis
Brain regions showing significant ALFF alteration between the PBD patients and healthy controls were treated as the regions of interest (ROIs). The ALFF values of the voxels in the ROIs were averaged to present the ALFF of the ROIs. Pearson’s correlation analysis was performed between the ALFF values of the ROIs and the YMRS scores of the patients to evaluate the relation between the ALFF of a brain region and manic severity.
Statistical analysis
Two-sample t-test was applied to compare the results between PBD patients and healthy controls. Monte Carlo simulation was utilized for the correction for multiple comparisons using the Resting-State fMRI Data Analysis Toolkit (version 1.8). A corrected significance level of P<0.05 was obtained by a combined threshold of P<0.001 for each voxel and an extent threshold of >6 voxels. The independent-sample t-test was used to compare the demographic data using SPSS software (v 11.5; IBM Corporation, Armonk, NY, USA).
Results
Subjects
PBD patients and healthy subjects completed the whole study and did not show any statistical differences in age, sex, or Full-Scale IQ. The demographic and clinical data are presented in . No subject had fallen asleep nor was excluded due to head motion greater than 1.5 mm or any angular motion greater than 1.5° during scanning.
Table 1 Participant demographic data in PBD (n=18) versus typically HC (n=18) subjects
ALFF: PBD patients versus control subjects
As shown in and , compared to the controls, the PBD group showed significant ALFF decrease in left precuneus, left superior parietal lobule, and bilateral inferior occipital gyrus, and increase in the bilateral caudate and left pallidum.
Figure 1 Brain regions with increased/decreased amplitude of low-frequency fluctuation (ALFF) in pediatric bipolar disorder patients are superimposed on a T1 template.
Abbreviations: L, left; R, Right.
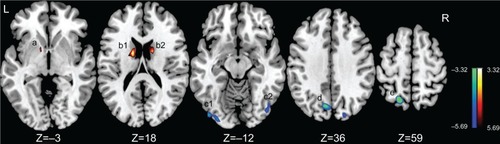
Table 2 Brain regions showing increased/decreased ALFF in PBD versus HC subjects
Correlations between ALFF values and YMRS scores
As shown in and , significant positive correlation was found between the ALFF value of the left pallidum (r=0.518, P=0.028) and the YMRS score in the PBD group.
Figure 2 Scatter plots showing significant positive correlation between total Young Mania Rating Scale scores and regional amplitude of low-frequency fluctuation (ALFF) values in the left pallidum in pediatric bipolar disorder patients (P<0.05).
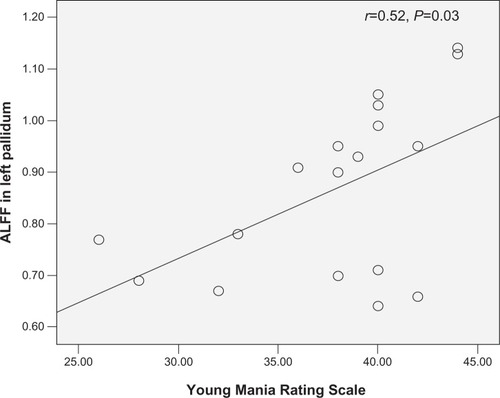
Table 3 Brain regions with significant between-group differences in ALFF and YMRS
Discussion
To the best of our knowledge, this is the first resting-state fMRI study to examine PBD during mania state using the ALFF method. In order to advance early detection and effective intervention, it is important for us to have a good understanding of the early natural history of BD. In fact, childhood and adolescence are crucial developmental periods. Frontal and parietal lobes attain an adult level of development at around 12 years of age and the temporal lobe at around 16 years, whereas cortical white matter refinements continue until near age 20.Citation25 In addition, PBD that is less influenced by psychological and social factors may represent a more homogeneous subtype of BD than adult-onset BD in genetic studies. That is to say, PBD may be closer in nature to BD disease. Only three neuroimaging studies have evaluated PBD using resting-state fMRI method.Citation26–Citation28 All of these studies reported abnormalities in affect circuits, such as abnormal activities in prefrontal cortexCitation26,Citation27 and superior temporal gyrusCitation28,Citation29 and abnormalities in cognitive circuits, such as abnormal activities in anterior cingulate cortexCitation28,Citation29 and caudate.Citation26,Citation27
Two key findings are present in our study. First, the PBD group displayed significant ALFF increase in the bilateral caudate and the left pallidum, and there was a strong positive association between the ALFF value of left pallidum and YMRS score. Caudate nucleus and pallidum are major components of the basal ganglia. Investigators have reported increased activity in the caudate both in adult BD and childhood BD during mania state.Citation29–Citation32 A task-independent spontaneous resting-state functional connectivity (RSFC) study in PBD involved youth BD patients (n=15) at euthymic state, showing decreased resting-state functional connectivity between the right superior temporal gyrus and left caudate.Citation26 The abnormal neuronal activity of the caudate shown in our findings may contribute to the abnormal connectivity between the two regions. Thus, our work is an important first step toward locating specific, impaired brain regions intrinsic to PBD in the resting state. Previous structural MRI studies have shown that adult BD and PBD are characteristic with increased volume of the pallidum.Citation33,Citation34 One fMRI study also showed increased activity in left pallidum in BD during mania state compared to healthy subjects.Citation35 Our findings are consistent with the abovementioned study. The pallidum is involved in the regulation of voluntary movement. If this brain region is damaged, movement disorders can result. This may help to explain the symptom of increased motor activity energy during mania state. Above all, PBD was characterized in the present study by increased activity in the basal ganglia, including caudate and pallidum, consistent with previous studies.Citation29–Citation34
The second key finding of the present study was a significantly decreased ALFF in the left precuneus, left superior parietal lobule, and bilateral inferior occipital gyrus of the PBD patients compared with that of the controls. The precuneus, a part of the superior parietal lobule, has been considered to be the core node of the default mode network that is activated during resting consciousness in which people do not engage intentionally in sensory or motor activity.Citation36,Citation37 In addition, precuneus and task superior parietal lobule are also involved in various executive functions, including planning, switching, and working memory.Citation38,Citation39 Investigators have reported decreased activity in precuneus and superior parietal lobule using emotionally valenced face tasks.Citation23,Citation40 A significant functional aspect of inferior occipital gyrus is that it contains the primary visual cortex. Occipital lesions may cause visual hallucinations. This may help to explain why hallucination symptoms frequently occur in pediatric mania. Further study is needed to explore this phenomenon more fully, however.
A hypothetical parietal-basal ganglia or occipital-basal ganglia model instead of frontostriatal circuitry used in previous studies may help to explain the imbalance of brain activity between parietal cortex,Citation41,Citation42 occipital cortex, and basal ganglia systems in the PBD group compared to the control group in our findings. The decreased neural activity of parietal cortex and occipital cortex may reflect that a smaller cognitive effort is required to exert effective excitatory control over basal ganglia regions in PBD. That is to say, the caudate nucleus receives inputs from the parietal and occipital cortices and sends stimulated information to the pallidum, which then excites the basal ganglia. The basal ganglia is associated with a variety of functions, including action selection, action gating, reward-based learning, motor preparation, and cognitive and emotional functions.Citation43,Citation44 Thus, the increased basal ganglia excitation in the resting state may underlie the behavioral abnormalities observed in mania, such as elevated self-esteem, being more talkative than usual, flight of thought, a reduced need for sleep, easily distracted and so on.
Limitations
There are some limitations in our study including comorbidity, psychotropic medications, and sample size. First, our study was a cross-sectional study, so it may be fruitful to investigate different episodes in the same subjects, such as depressive state or euthymic state. Second, the pharmaceutical effect is not excluded in our study, and this may be a confounding factor in interpreting the results, although recent BD research suggests that such psychotropic medications have demonstrated no significant effect on the BOLD signal in fMRI studies.Citation45 Third, the sample size in our study was small, and our findings need to be further verified with a larger sample.
Conclusion
In all, the regions that showed significant ALFF change in the PBD mania group were parts of the basal ganglia and parietal cortex and the occipital cortex system, which are important for affect-processing, decision-making, and social cognition.Citation46,Citation47 These findings support our hypothesis that the abnormality in resting-state activity of PBD mania patients is mainly located in the basal ganglia and parietal and occipital systems. Additionally, the ALFF approach may help with locating impaired regions in PBD patients in functional brain research, consistent with previous studies on other mental disorders.Citation17,Citation18
Author contributions
Dr Linyan Su designed the study. Dali Lu, Qian Xiao, Weijia Gao, Xiaoqun Liu, Xiaoling Lin, Wentao Cheng, and Lanzhu Luo collected the original imaging data. Qing Jiao, Yuan Zhong, Chuanjian Xu, and Guangming Lu managed and analyzed the imaging data, and Dali Lu wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No 81171291 and 81201077) and Key Program for Guangming Lu (grant No BWS11J063 and 10z026) and Humanity and Social Science Youth foundation of Ministry of Education (No 11YJC190039).
Disclosure
The authors report no conflicts of interest in this work.
References
- PerlisRHMiyaharaSMarangellLBSTEP-BD InvestigatorsLong-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD)Biol Psychiatry200455987588115110730
- SchulzeTGMüllerDJKraussHFurther evidence for age of onset being an indicator for severity in bipolar disorderJ Affect Disord2002682–334334512063163
- LewinsohnPMKleinDNSeeleyJRBipolar disorder during adolescence and young adulthood in a community sampleBipolar Disord200023 Pt 228129311249806
- GellerBBolhofnerKCraneyJLWilliamsMDelBelloMPGundersenKPsychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotypeJ Am Acad Child Adolesc Psychiatry200039121543154811128332
- FrazierJABreezeJLMakrisNCortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorderBipolar Disord20057655556916403181
- KalmarJHWangFChepenikLGRelation between amygdala structure and function in adolescents with bipolar disorderJ Am Acad Child Adolesc Psychiatry200948663664219454919
- MahonKBurdickKESzeszkoPRA role for white matter abnormalities in the pathophysiology of bipolar disorderNeurosci Biobehav Rev201034453355419896972
- VersaceALadouceurCDRomeroSAltered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging studyJ Am Acad Child Adolesc Psychiatry201049121249125921093774
- PavuluriMNO’ConnormmHarralESweeneyJAAffective neural circuitry during facial emotion processing in pediatric bipolar disorderBiol Psychiatry200762215816717097071
- PavuluriMNPassarottiAMHarralEMSweeneyJAAn fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorderJ Am Acad Child Adolesc Psychiatry200948330831919242292
- FoxMDRaichleMESpontaneous fluctuations in brain activity observed with functional magnetic resonance imagingNat Rev Neurosci20078970071117704812
- RaichleMENeuroscience. The brain’s dark energyScience200631458031249125017124311
- RaichleMEMintunMABrain work and brain imagingAnnu Rev Neurosci20062944947616776593
- GonçalvesSIde MunckJCPouwelsPJCorrelating the alpha rhythm to BOLD using simultaneous EEG/fMRI: inter-subject variabilityNeuroimage200630120321316290018
- LaufsHKrakowKSterzerPElectroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at restProc Natl Acad Sci U S A200310019110531105812958209
- YangHLongXYYangYAmplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRINeuroimage200736114415217434757
- ZangYFHeYZhuCZAltered baseline brain activity in children with ADHD revealed by resting state functional MRIBrain Dev2007292839116919409
- HouJWuWLinYLocalization of cerebral functional deficits in patients with obsessive-compulsive disorder: a resting-state fMRI studyJ Affect Disord2012138331332122331021
- JiaoQDingJLuGIncreased activity imbalance in fronto-subcortical circuits in adolescents with major depressionPLoS One201169e2515921949877
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text RevisionWashington, DCAmerican Psychiatric Association2000
- KaufmanJBirmaherBBrentDSchedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity dataJ Am Acad Child Adolesc Psychiatry19973679809889204677
- WoodAKrollLMooreAHarringtonRProperties of the mood and feelings questionnaire in adolescent psychiatric outpatients: a research noteJ Child Psychol Psychiatry19953623273347759594
- RichBAFrommSJBerghorstLHNeural connectivity in children with bipolar disorder: impairment in the face emotion processing circuitJ Child Psychol Psychiatry2008491889618181882
- LoweMJMockBJSorensonJAFunctional connectivity in single and multislice echoplanar imaging using resting-state fluctuationsNeuroimage1998721191329558644
- PavuluriMNSweeneyJAIntegrating functional brain neuroimaging and developmental cognitive neuroscience in child psychiatry researchJ Am Acad Child Adolesc Psychiatry200847111273128818827719
- DicksteinDPGorrostietaCOmbaoHFronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorderBiol Psychiatry201068983984620739018
- XiaoQZhongYLuDAltered regional homogeneity in pediatric bipolar disorder during manic state: a resting-state fMRI studyPLoS One201383e5797823526961
- WuMLuLHPassarottiAMAltered affective, executive and sensorimotor resting state networks in patients with pediatric maniaJ Psychiatry Neurosci201338423224023735583
- O’ConnellRAVan HeertumRLLuckDSingle-photon emission computed tomography of the brain in acute mania and schizophreniaJ Neuroimaging1995521011047718935
- BlumbergHPSternEMartinezDIncreased anterior cingulate and caudate activity in bipolar maniaBiol Psychiatry200048111045105211094137
- KimPJenkinsSEConnollyMENeural correlates of cognitive flexibility in children at risk for bipolar disorderJ Psychiatr Res2012461223022024484
- PassarottiAMSweeneyJAPavuluriMNEmotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorderJ Am Acad Child Adolesc Psychiatry201049101064108020855051
- ArnoneDCavanaghJGerberDLawrieSMEbmeierKPMcIntoshAMMagnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysisBr J Psychiatry2009195319420119721106
- LiuIYHoweMGarrettAStriatal volumes in pediatric bipolar patients with and without comorbid ADHDPsychiatry Res20111941142021875781
- CaligiuriMPBrownGGMeloyMJAn fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorderPsychiatry Res2003123317118212928105
- MarieJPZittounRSikicBIMultidrug resistance (mdr1) gene expression in adult acute leukemias: correlations with treatment outcome and in vitro drug sensitivityBlood19917835865921859877
- CavannaAEThe precuneus and consciousnessCNS Spectr200712754555217603406
- SohnMHUrsuSAndersonJRStengerVACarterCSThe role of prefrontal cortex and posterior parietal cortex in task switchingProc Natl Acad Sci U S A20009724134481345311069306
- van den HeuvelOAGroenewegenHJBarkhofFLazeronRHvan DyckRVeltmanDJFrontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London taskNeuroimage200318236737412595190
- PavuluriMNPassarottiAMParnesSAFitzgeraldJMSweeneyJAA pharmacological functional magnetic resonance imaging study probing the interface of cognitive and emotional brain systems in pediatric bipolar disorderJ Child Adolesc Psychopharmacol201020539540620973710
- PavuluriMNPassarottiAMFitzgeraldJMWegbreitESweeneyJARisperidone and divalproex differentially engage the fronto-striato-temporal circuitry in pediatric mania: a pharmacological functional magnetic resonance imaging studyJ Am Acad Child Adolesc Psychiatry201251215717022265362
- CerulloMAAdlerCMLamyMDifferential brain activation during response inhibition in bipolar and attention-deficit hyperactivity disordersEarly Interv Psychiatry20093318919722640382
- ChakravarthyVSJosephDBapiRSWhat do the basal ganglia do? A modeling perspectiveBiol Cybern2010103323725320644953
- StoccoALebiereCAndersonJRConditional routing of information to the cortex: a model of the basal ganglia’s role in cognitive coordinationPsychol Rev2010117254157420438237
- PhillipsMLTravisMJFagioliniAKupferDJMedication effects in neuroimaging studies of bipolar disorderAm J Psychiatry2008165331332018245175
- CardinalTRWazlawikEBastosJLNakazoraLMScheunemannLStandardized phase angle indicates nutritional status in hospitalized preoperative patientsNutr Res201030959460020934600
- ForstmannBUWolfenstellerUDerrfussJWhen the choice is ours: context and agencyPLoS One200834e189918398450
