 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Patients with non-lesional or bilateral temporal-lobe epilepsy (TLE) are often excluded from surgical treatment. This study investigated focus lateralization in TLE to understand identification of the affected hemisphere with regard to non-lesional or bilateral affection and postsurgical outcome. A total of 24 TLE patients underwent presurgical evaluation with magnetic resonance imaging (MRI), proton magnetic resonance spectroscopy (1H-MRS), video-electroencephalogram (video-EEG), and/or intracranial EEG (icEEG), and they were classified as MRI-positive or negative, unilateral or bilateral TLE cases. In patients with positive-MRI, MRI and 1H-MRS indicated high (100%) concordant lateralization to EEG findings in unilateral TLE, and moderate (75%) concordance to icEEG findings in bilateral TLE; whereas in patients with negative-MRI, 1H-MRS indicated moderate (60%–75%) concordance to EEG and/or icEEG in unilateral TLE, and relatively low (50%) concordance to icEEG in bilateral TLE. Ninety point nine percent of patients with unilateral TLE and 41.7% of patients with bilateral TLE (including 50% of MRI-negative bilateral TLE) became seizure-free. The MRS findings were not correlated with seizure outcome, while non-seizure-free patients had an insignificantly higher percentage of contralateral N-acetyl aspartate (NAA) reduction compared with seizure-free patients, indicating the relatively low predictive value of 1H-MRS for surgical outcome. Further, EEG and icEEG findings were significantly correlated with seizure outcome, and for patients with positive MRI, MRI findings were also correlated with seizure outcome, indicating the predictive value of these modalities. The results suggested that a multimodal approach including neuroimaging, EEG, and/or icEEG could identify seizure focus in most cases, and provide surgical options for non-lesional or bilateral TLE patients with a possible good outcome.
Introduction
Around 30% of patients with partial seizures are resistant to antiepileptic drugs and may need surgical treatment. Temporal lobe epilepsy (TLE) is the most common type of epilepsy. Hippocampal sclerosis (HS) is the most common cause of TLE in adult patients,Citation1,Citation2 while malformations of cortical development (MCD) is more commonly seen in pediatric patients.Citation3 Epilepsy surgery opens the possibility of complete seizure control and brings the hope of seizure-free outcome. Over decades, epilepsy surgery has improved gradually and approached 60%–90% seizure-free outcome in patients with TLE and 40%–60% in extratemporal lobe epilepsy (ETLE).Citation4
Correct lateralization of the affected hemisphere with precise localization of the epileptic foci is a prerequisite for good surgical outcome, but it remains a challenge, especially for non-lesional epilepsy, bilateral TLE, and ETLE. Neuroimaging such as magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), and positron-emission tomography (PET) can detect lesions and other structural or functional abnormalities, and is thus important in focus lateralization and localization. Concordance of different imaging modalities in localization of seizure focus increases the confidence of correct hemispheric lateralization in TLE.Citation5–Citation7 However, when the clinical, electrophysiological and neuroimaging findings are discordant or the focus lateralization and localization are unclear, intracranial electroencephalogram (icEEG) with deep electrodes is needed to further localize the seizure focus.Citation6–Citation8 IcEEG monitoring with surgically implanted electrodes is critical for the assessment of patients with drug-resistant epilepsy (especially in challenging cases) in presurgical evaluation. It has good sensitivity and spatial specificity, but limited spatial sampling, and requires additional surgical procedure, which increases patient discomfort, immobility, risks for complications, and medical costs.Citation9 Neuroimaging is useful in planning icEEG implantation, and concordant neuroimaging findings may minimize the need for invasive icEEG.Citation10
Nevertheless, patients with drug-resistant bilateral TLE (ie, seizures originating from both temporal lobes) are still often excluded from surgical treatment.Citation11,Citation12 This is because surgery is only performed in one hemisphere to avoid severe functional deficits (due to bilateral resection), and it is often difficult to identify the side of seizure origin (ie, the epileptic focus in one hemisphere that initiates most seizures and causes the most severe seizure).Citation13 In addition, poor focus lateralization and/or localization often lead to unfavorable seizure outcome in bilateral TLE.Citation14 Furthermore, apart from bilateral TLE, focus lateralization and localization in non-lesional TLE is also difficult due to the absence of imaging guidance for resection. Therefore, how to improve focus lateralization and localization in TLE (especially in challenging cases such as non-lesional or bilateral TLE) remains a challenge.
This study investigated presurgical focus lateralization in 24 TLE patients with neuroimaging (MRI and proton MRS [1H-MRS]), video-EEG, and/or icEEG. The patients were classified as MRI positive or negative, unilateral or bilateral TLE cases. Ictal video-EEG and/or icEEG findings were used as the “gold standard” for focus lateralization comparison, surgical outcomes were compared between different subgroups of patients, and the relationship between presurgical findings and seizure outcome was explored.
Methods
Subjects
A total of 24 consecutive patients with drug-resistant epilepsy who were admitted to the Department of Functional Neurology and Neurosurgery, Beijing Haidian Hospital during 2010 (July)–2012 (April) were included in this study. All patients were diagnosed as having TLE and underwent presurgical evaluation, and of these, 23 underwent surgery. Surgical outcomes were evaluated with Engel classificationCitation15 during patients’ postoperative visits, and the patients’ follow-up lasted for 1.5–3.0 years. This study was approved by the Institutional Review Board at the Capital Medical University.
EEG recordings
The simultaneous registration of continuous video-EEG monitoring was performed using the Nicolet system (Natus Medical Incorporated, San Carlos, CA, USA). Simultaneous documentation of behavior via a split-screen technique was used over a period of 4–264 hours (0.17–11.00 days) for all patients, and over 200 hours (8.3 days) for most patients. Technical details were: number of EEG/polygraphic channels, 32; sampling rate, 256 Hz. EEG electrodes were placed according to the international 10/20 system of EEG. EEG analysis was performed using continuous 24-hour video-EEG monitoring for most patients. EEG analysis was performed without knowledge of imaging results. Interictal epileptiform discharges and ictal EEG findings were counted, and unilateral or bitemporal activity was reported by EEG specialists. Based on the frequency of unilateral or bitemporal EEG activity (together with icEEG findings if available), unilateral or bitemporal TLE was determined with the following rules. Briefly, if ictal EEG activity was bitemporal, then the patient was considered as having bitemporal TLE; if ictal EEG activity was unilateral, but interictal or ictal icEEG activity was bilateral, then the patient was still considered as having bilateral TLE; otherwise, unilateral TLE.
Structural MRI
MRI acquisition was performed using a 1.5T clinical whole body MRI scanner (Siemens Essenza, Erlangen, Germany) at Beijing 466 Hospital, with a standard head coil. T1-weighted MRI images were acquired using FLASH 2D sequence (19 axial slices, repetition time [TR] =201 ms, echo time [TE] =4.76 ms, flip angle =70°, matrix =256×256, field of view [FOV] =194×230, slice thickness =5 mm, and scan time =91 seconds). T2-weighted MRI images were acquired using Spin Echo sequence (19 axial slices, TR =4500 ms, TE =107 ms, flip angle =70°, matrix =256×256, FOV =201×230, slice thickness =5 mm, and scan time =87 seconds).
MRI images were analyzed by neuroradiologists at Beijing 466 Hospital. The criteria for a diagnosis of HS included the presence of unilateral atrophy and high T2 signal intensity in the hippocampus.Citation5 A patient with lesional MRI findings was considered MRI-positive; a patient with non-lesional MRI findings, MRI-negative.
Single voxel 1H-MRS
Single voxel 1H-MRS was carried out by the same 1.5T Siemens Magnetom Essenza MR scanner with a standard head coil. Transversal and coronal T2-weighted images in three orthogonal planes were used as localizer. The voxel comprised the major part of the hippocampus and covered the visible lesion of the hippocampus (if it existed). The voxel size was 3.375 cm3 (1.5 cm ×1.5 cm ×1.5 cm). Water suppression was achieved by using chemical shift selective (CHESS) pulses before the “point resolved spectroscopy” (PRESS) localization: TR=4500 ms; TE=107 ms; averages=192; vector size=1024; scan time=16 minutes 30 seconds.
The 1H-MRS data was analyzed with the software provided by Siemens on the Siemens MRI scanner. Resonance intensities were computed by integrating the peak areas of three metabolites: N-acetyl aspartate (NAA), creatine (Cr), and choline (Cho). Ratios of NAA/(Cho + Cr) and NAA/Cr were calculated for each patient.
To measure the degree of asymmetry, the asymmetry index (AI) was computed for 1H-MRS as follows:
where (right – left) means the difference between the voxels in the right and left hippocampus. An AI threshold of 12% was applied to identify significant NAA reduction and Cho or Cr increase ipsilateral to the seizure focus.Citation16 Hemispheric lateralization was based on significant asymmetry of NAA reduction in any of the three NAA measures, ie, AI of {[NAA], [NAA/Cr], or [NAA/(Cho + Cr)]} ≥12%.
IcEEG
When the findings of noninvasive tests were disconcordant and non-localizing (without clear seizure focus/onset/origin), presurgical icEEG was performed. In total, icEEG monitoring was performed for 12 patients presurgery to lateralize and localize the seizure focus. According to each patient’s condition, up to four subdural 2×8 contact strips were placed in the bilateral temporal and/or frontal (or parietal) regions.
In addition, intraoperative icEEG monitoring was performed at the beginning of and throughout epilepsy surgery for every patient (who underwent surgery) to further localize and confirm the epileptogenic zone. If intraoperative icEEG monitoring confirmed the epileptogenic zone identified by other modalities (presurgical MRI, MRS, EEG, or icEEG) or detected new seizure focus nearby, then the resection region was determined by the intraoperative icEEG findings, and resection was performed accordingly to remove the seizure focus as completely as possible.
Histopathological examination
Specimens obtained from epilepsy surgery were sent to the Department of Pathology, Beijing Haidian Hospital for histopathological examination after the surgery. The histopathological findings were used to compare with the neuroimaging results and to understand the histopathological alterations and the causes of abnormalities that MRI, 1H-MRS, and EEG indicated (or failed to indicate).
Statistical analysis
Presurgical findings of different modalities were compared with the seizure focus (detected by icEEG) or the surgical site, and scores of correct lateralization were assigned to each patient for each modality. Spearman correlation between the presurgical findings (eg, the lateralization scores of EEG, MRI, MRS including AIs of MRS metabolites, and icEEG), and the surgical outcome was computed by SPSS 21.0 (IBM Corporation, Armonk, NY, USA).
Results
Of the 24 patients, 16 had seizure focus in the temporal lobe alone, the other eight patients had seizure foci in the temporal lobe as well as extratemporal regions (). Based on EEG and/or icEEG findings, 12 patients were classified as unilateral and 12 as bilateral TLE cases. Of the patients with unilateral TLE, seven were MRI-positive with reported temporal lobe abnormality (eg, HS), and five were MRI-negative. Of the patients with bilateral TLE, four were MRI-positive and eight were MRI-negative. IcEEG was performed in 12 patients, including two unilateral MRI-negative, three bilateral MRI-positive, and seven bilateral MRI-negative TLE patients. A total of 23 patients underwent surgery, and one patient (patient 14) did not (due to possible improvement by further antiepileptic drug treatment).
Table 1 Patient characteristics and subgrouping
shows the 1H-MR spectrum and MRI of individual patients. MRI and 1H-MRS both indicated concordant lateralization to EEG findings in 100% (7/7) of patients with unilateral MRI-positive TLE, and they indicated concordant lateralization to icEEG findings in 75% (3/4) of patients with bilateral MRI-positive TLE. In addition, 1H-MRS indicated concordant lateralization to EEG and/or icEEG findings in 60% (3/5) ∼75% (3/4) of patients with unilateral MRI-negative TLE, and concordant lateralization to icEEG findings in 50% (4/8) of patients with bilateral MRI-negative TLE. The 60%–75% concordance between 1H-MRS and EEG/icEEG in unilateral MRI-negative TLE was due to one patient (case 16) who was also diagnosed with ETLE (EEG and icEEG found abnormal epileptiform discharges in both the left frontal and temporal regions, with more abnormal electrical discharges in the left frontal region). If case 16 was counted as unilateral MRI-negative TLE, the 1H-MRS and EEG/icEEG concordance was 60%; otherwise, 75%.
Figure 1 1H-MR spectrum and MRI of individual patients. (A) 1H-MR spectrum and MRI of patient 13 (31-year-old female; seizure history, 20 years; right [NAA/Cho + Cr] =0.48, left [NAA/Cho + Cr] =0.42; seizure focus in the left temporal region; left HS; surgical outcome, Engel class I). (B) 1H-MR spectrum and MRI of patient 7 (38 year old male; seizure history, 28 years; right [NAA/(Cho + Cr)] =0.26, left [NAA/(Cho + Cr)] =0.35; seizure focus in the right temporal region; right HS; surgical outcome, Engel class II). (C) 1H-MR spectrum and MRI of patient 20 (24-year-old female; seizure history, 22 years; right [NAA/Cho + Cr] =0.56, left [NAA/Cho + Cr] =0.48; seizure focus in the bilateral temporal regions; right HS; surgical outcome, Engel class II).
![Figure 1 1H-MR spectrum and MRI of individual patients. (A) 1H-MR spectrum and MRI of patient 13 (31-year-old female; seizure history, 20 years; right [NAA/Cho + Cr] =0.48, left [NAA/Cho + Cr] =0.42; seizure focus in the left temporal region; left HS; surgical outcome, Engel class I). (B) 1H-MR spectrum and MRI of patient 7 (38 year old male; seizure history, 28 years; right [NAA/(Cho + Cr)] =0.26, left [NAA/(Cho + Cr)] =0.35; seizure focus in the right temporal region; right HS; surgical outcome, Engel class II). (C) 1H-MR spectrum and MRI of patient 20 (24-year-old female; seizure history, 22 years; right [NAA/Cho + Cr] =0.56, left [NAA/Cho + Cr] =0.48; seizure focus in the bilateral temporal regions; right HS; surgical outcome, Engel class II).](/cms/asset/d0b2d3a5-5312-45b8-9907-680616ebe8ee/dndt_a_56404_f0001_b.jpg)
In addition, patients with unilateral TLE achieved good outcome: 90.9% (11/12) were seizure-free (Engel class I) (among them, 85.7% [6/7] with MRI-positive and 100% [5/5] with MRI-negative were seizure-free), and the rest of the patients had Engel class II outcome. However, in patients with bilateral TLE, 41.7% (5/12) became seizure-free (including one [25%] MRI-positive and four [50%] MRI-negative patients); and all (4/4, 100%) MRI-positive and six (6/8, 75%) MRI-negative patients had Engel class I or II outcome. Although 25% (2/8) of patients with MRI-negative bilateral TLE attained unfavorable outcomes (Engel class III or IV), 50% (4/8) of them did become seizure-free ().
Figure 2 Surgical outcome of unilateral or bilateral MRI-positive or negative TLE (n=23).
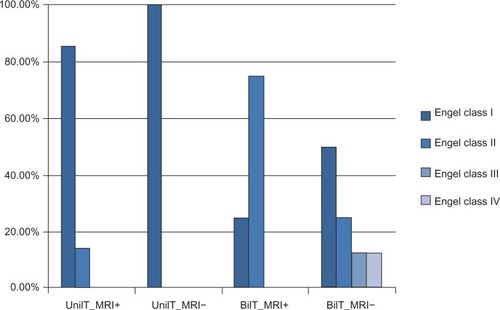
Correlation results are summarized in . For all patients who underwent surgery (n=23), EEG lateralization score was significantly (P<0.05) correlated with the seizure outcome, and for those who underwent presurgical icEEG (n=12), icEEG lateralization score was significantly correlated with the outcome. For patients with positive MRI (n=11), only MRI lateralization score was significantly correlated with the seizure outcome, while for those with negative-MRI (n=12), EEG lateralization finding (potentially together with icEEG finding, P<0.1) was significantly (P<0.05) correlated with the outcome.
Table 2 Spearman correlation between presurgical (and pathological) findings and surgical outcome
Further, compared with non-seizure-free patients, the seizure-free patients had a lower percentage of contralateral NAA reduction (40% [6/15] versus 62.5% [5/8], the difference was not significant), and the individual asymmetry levels of individual 1H-MRS measures were not correlated with seizure outcome. In addition, the ipsilateral NAA/(Cho + Cr) values in non-seizure-free or seizure-free patients were not significantly lower than those of contralateral values, and the ipsilateral NAA/(Cho + Cr) values of non-seizure-free patients were not lower than those of seizure-free patients.
Discussion
Focus lateralization in TLE is still challenging especially in non-lesional and bilateral TLE. This study showed that: 1) 1H-MRS had high concordant lateralization to video-EEG and/or icEEG findings in MRI-positive unilateral TLE (100%), moderate concordance in MRI-positive bilateral TLE (75%) and MRI-negative unilateral TLE (60%–75%), and low concordance in MRI-negative bilateral TLE (50%); 2) a higher percentage of patients with unilateral TLE than that of those with bilateral TLE (90.9% versus 41.7%) became seizure-free, the worst outcome was seen in patients with MRI-negative bilateral TLE; and 3) EEG and icEEG lateralization findings (together with MRI findings in MRI-positive patients) were associated with seizure outcome, and there was a lower percentage of contralateral NAA reduction in the seizure-free patients than in those with non-seizure-free outcome.
Focus lateralization with a multimodal approach in challenging TLE cases
Up to 30% of patients with TLE have normal MRI findings.Citation13 The results of this study suggest that the 1H-MRS has a much lower accuracy of focus lateralization in MRI-negative TLE than in MRI-positive TLE (60%–75% versus 100%). In MRI-negative unilateral TLE, the moderate concordance (60%–75%) of 1H-MRS to EEG findings (or 25%–40% disconcordance) requires more evidence from neuroimaging or icEEG for hemispheric lateralization. In this study, ictal icEEG findings largely confirmed the ictal EEG findings in MRI-negative unilateral TLE. In addition, other neuroimaging modalities such as PET may be considered to aid focus lateralization in MRI-negative TLE.
Compared with unilateral TLE, focus lateralization in bilateral TLE remains a bigger challenge. Cendes et al reported that 1H-MRS correctly lateralized 86% in 100 consecutive (mixed) TLE cases, but 54% with bilaterally abnormal TLE.Citation17 In patients with negative-MRI, this study indicated that 1H-MRS was still helpful for focus lateralization in unilateral TLE (60%–75%), but not in bilateral TLE (50%), which is consistent with the findings of two other studies.Citation5,Citation18 Because of the difficulty in identifying the side of seizure originCitation13 and the often unfavorable outcome,Citation12 patients with bilateral TLE are often excluded from surgical treatment.Citation11,Citation14 Presurgical icEEG is critical for focus lateralization and localization in bilateral TLE. In this study, icEEG monitoring was performed in most (83.3%) patients with bilateral TLE: among them, 75% (3/4) were MRI-positive and 87.5% (7/8) were MRI-negative. Overall, 100% (4/4) of patients with MRI-positive bilateral TLE attained good outcomes (Engel class I or II), while patients with MRI-negative bilateral TLE attained varied outcomes: 75% (6/8) had good outcomes, and 25% (2/8) had bad outcomes (Engel class III or IV).
Since false localization rate by MRI, MRS, or EEG alone may be high, a multimodal imaging approach including MRI, MRS, EEG, and PET/single-photon emission computed tomography (SPECT) is useful to increase the diagnostic yield in challenging TLE cases such as non-lesional or bilateral TLE.Citation5–Citation7 Concordant neuroimaging findings could not only guide the placement of icEEG electrodes, but also help avoid the need for invasive icEEG monitoring. For example, due to limited spatial sampling of icEEG, false negative icEEG findings have been reported,Citation6 and concordant neuroimaging and EEG findings might guide the surgery and minimize the need for presurgical icEEG in such cases.
Focus lateralization and localization in ETLE is another challenge. Eight (8/24, 33.3%) patients in this study had unilateral seizure foci in both the temporal and extratemporal regions. Three of those (3/8, 37.5%) were MRI-positive and could be correctly lateralized by 1H-MRS. In the rest of those with negative-MRI, only one (1/5, 20%) was correctly lateralized by 1H-MRS. Such accuracy was much lower than that in patients with seizure focus in the temporal region alone. Seizure foci in both the temporal and extratemporal regions might cause widespread neuron loss and a more diffuse distribution of 1H-MRS abnormality. Mueller et al studied the extrahippocampal 1H-MRS abnormalities in TLE,Citation20,Citation21 and found that 1) extrahippocampal NAA/(Cr + Cho) reductions had a bilateral frontotemporal distribution in TLE with mesial temporal sclerosis (MTS) and a more diffuse distribution in TLE without MTS; and 2) the inter-individual variability and non-focal and inhomogeneous features of extrahippocampal NAA/(Cr + Cho) reductions reduced their value in seizure focus lateralization.Citation21
To lateralize and localize seizure focus in challenging TLE, long-term extensive video-EEG monitoring and icEEG are helpful. Video-EEG could increase the diagnostic yield of focus lateralization and localization,Citation22 and is successful in focus localization in 60%–90% of cases.Citation23,Citation24 Intensive 24-hour high-resolution video-EEG monitoring has even been proposed as the “gold standard” for focus localization,Citation23,Citation24 and such “gold standard” has been used in a number of studies.Citation5,Citation18 Cendes further suggested that when the interictal EEG and hippocampal atrophy coincide, ictal EEG recording might not be mandatory in TLE focus lateralization and localization.Citation17 However, the results of this study indicated that video-EEG is good for focus localization, but it might not be sufficient as the “gold standard,” and icEEG is still needed in difficult cases such as nonlesional or bilateral TLE.
Taken together, a multimodal approach using neuroimaging, EEG and/or icEEG is needed in presurgical assessment, and it provides surgical options for patients with challenging TLE cases.
The sensitivity and predictive value of 1H-MRS
1H-MRS has been increasingly used as a noninvasive “biopsy” tool for detection of metabolite hippocampal abnormalities in TLE presurgical evaluation. NAA reduction indicates neuronal loss or dysfunction; Cr increase indicates increased astrocytes and glial cells; and Cho increase may reflect cell membrane damage, myelin breakdown, and gliosis.Citation13
The AI threshold (12%) used in this study to identify metabolite abnormalities and lateralize the affected hemisphere was the same as that in Knowlton et al,Citation16 where 61% (1H-MRS sensitivity for focus lateralization in MRI-negative unilateral TLE) was obtained, which is comparable to the sensitivity (60%–75%) (in MRI-negative unilateral TLE) obtained in this study and other studies (eg, 55%–65% by Connelly et al).Citation25 If relaxing the lateralization criteria by, eg, lowering the AI threshold, or adding Cr or Cho increase (ipsilateral to the seizure focus) to the lateralization criteria to capture metabolite abnormalities, 1H-MRS might be more sensitive in hemispheric lateralization, but we agree with Knowlton et al that a conservative 1H-MRS lateralization criteria is needed, even at the expense of sensitivity.Citation16
Since 1H-MRS has been widely used for seizure focus lateralization,Citation5,Citation11,Citation17,Citation25,Citation26 MCD abnormality detection,Citation27 and brain tumor grading,Citation19,Citation28,Citation29 it is natural to expect that 1H-MRS is sensitive enough to detect HS and even differentiate between HS, MCD, and tumor. However, the results of this study did not support this. Chernov et al also reported that 1H-MRS had limited usefulness for differentiation of HS and low-grade brain tumor.Citation11 Nevertheless, Vermathen et al demonstrated that hippocampal NAA was able to discriminate neocortical TLE (including MCD) from mesial TLE (including HS).Citation30 Hammen et al demonstrated that multi-voxel 1H-MRS was able to distinguish between mesial TLE and lateral neocortical TLE.Citation31,Citation32 In addition, NAA reduction before surgery was found to correlate with the degree of HS, reflecting neuronal loss.Citation33,Citation34
The higher percentage of contralateral metabolite abnormalities in non-seizure-free patients (although insignificant) than in seizure-free patients found in this study suggested that 1H-MRS has a predictive value for seizure outcome. This was supported by a number of studies, with the finding that patients with unilateral metabolite alterations ipsilateral to the seizure focus had better seizure outcome than those with contralateral or bilateral metabolite alterations,Citation11,Citation35 suggesting that 1H-MRS could predict surgical outcome.Citation11,Citation13,Citation24,Citation35,Citation36 In addition, this study showed that the MRS findings were not correlated with the outcome, and the ipsilateral NAA/(Cho + Cr) values were not significantly lower than those of contralateral values in either non-seizure-free patients or seizure-free patients, suggesting that the predictive power of 1H-MRS was weak. This was different from the findings of Suhy et al,Citation35 and such difference might be due to the different patient samples, different 1H-MRS acquisition equipment and parameters, and different surgeries performed for the patients between the two studies. Further, the results of this study indicated that the predictive value of 1H-MRS was largely reduced in bilateral TLE. This is because one hemisphere in bilateral TLE often initiates most seizures, and the hemisphere with the greatest metabolite alterations may not always be the side of seizure origin.Citation13
Predictive value of other presurgical findings for surgical outcome
The significant correlation between EEG, icEEG, or MRI lateralization findings, and seizure outcome indicate the predictive value of MRI and EEG/icEEG. However, mild lesions such as mild HS and focal cortical dysplasia are difficult to identify on regular MRI. They may be missed by MRI, mis-regarded as non-lesional, and even excluded from presurgical evaluation. Thus, there are controversies on the utility of neuroimaging in predicting surgical outcome.Citation37 In this study, the fact that 8 out of 13 MRI-negative patients had HS partially reflected such limitation of MRI.
Nevertheless, the predictive value of neuroimaging for epilepsy surgical outcome has been reported by a number of studies. For example, Lerner et al,Citation38 Cossu et al,Citation39 Widdess-Walsh et al,Citation40 and Jeha et alCitation41 have shown that the complete resection of the abnormality detected by preoperative MRI is the most important predictor for favorable postoperative outcome. Functional neuroimaging modalities such as magnetoencephalography (MEG)/magnetic source imaging (MSI), PET, and ictal SPECT also have clinical value in predicting seizure-free outcome.Citation6 In addition, systematic reviews and meta-analyses have found that abnormal MRI (lesion such as HS on MRI), EEG abnormalities (well localized EEG), EEG/MRI concordance, and the presence of HS (or MTS) are positive predictors of seizure outcome, while normal MRI, non-localized EEG, and use of icEEG are negative outcome predictors.Citation37,Citation42–Citation46 These findings support the predictive value of MRI, EEG, and icEEG.
Limitations of single modality such as MRI may lead to limited predictive value for seizure outcome. Therefore, a multimodal approach including multiple neuroimaging modalities such as EEG/ESI (EEG source imaging), MRI, MRS, PET, SPECT, and MEG/MSI is needed not only for focus lateralization and localization in presurgical assessment, but also for improvement of outcome prediction. Further study on this patient data is needed to identify predictors of seizure outcome and gain better understanding of the relationship between presurgical findings and surgical outcome.
Limitations
There are several limitations in this study. First, PET and SPECT were performed for only 2 (8.3%) patients in presurgical evaluation (). This was because the local hospital (ie, Beijing Haidian Hospital) does not have a PET or SPECT scanner, and patients who need a PET or SPECT scan have to go to other hospitals (as the MRI and MRS acquisition), which is often time consuming. In such cases, the surgeons tended to use presurgical icEEG to detect the seizure focus instead, which increases the usage of icEEG in presurgical evaluation. Noninvasive neuroimaging such as PET and SPECT could be used more in the future to reduce the need for invasive icEEG and its associated risks and costs. Second, the diagnostic accuracy of MRI was not high in this study: eight out of eleven patients (72.7%) with positive MRI had HS, one (0.9%) had a tumor, and two (18.2%) had MCD (which were confirmed by histopathological examination), while in MRI-negative TLE, 8 out of 13 patients (61.5%) had HS. In other words, 38.5% of the patients with HS were not identified by MRI. The HS that eluded MRI visual inspection might be mild, while the relatively low resolution of the clinical MRI scanner used (19 slices, slick thickness =5 mm) and the experiences of the neuroradiologists who examined the MRI might also contribute to the relatively low specificity of MRI in HS identification. A high-resolution MRI scanner and quantitative MRI may be used to better reveal mild HS on MRI in the future. Third, the evaluation of surgical outcome using Engel classification in this study might be subjective and imprecise in several patients who had non-seizure-free outcomes, which might slightly influence the results associated with such outcomes in this study. This may be improved by more detailed patient follow-up over a longer period of time, which could provide more information for postsurgical assessment and make it more objective and accurate in future studies. Finally, the patients in this study were heterogeneous, and the sample sizes in the four subgroups were relatively small (n=4–8). This could be improved by recruiting more patients in each subgroup in the future.
Conclusion
In summary, this study investigated focus lateralization in TLE and found that 1H-MRS had relatively high sensitivity in MRI-positive unilateral TLE, moderate sensitivity in MRI-positive bilateral TLE and MRI-negative unilateral TLE, and relatively low sensitivity in MRI-negative bilateral TLE. In addition, 90.9% of patients with unilateral TLE and 41.7% of patients with bilateral TLE (50% MRI-negative bilateral TLE) attained seizure-free outcome. The MRS findings were not correlated with seizure outcome, while non-seizure-free patients had an insignificantly higher percentage of contralateral NAA reduction compared with seizure-free patients, indicating that 1H-MRS has a relatively low predictive value for surgical outcome. Further, EEG and icEEG findings were correlated with seizure outcome, and for patients with positive MRI, MRI findings were also correlated with seizure outcome, indicating the predictive value of EEG, icEEG, and MRI. Taken together, a multimodality approach including neuroimaging, EEG, and/or icEEG in surgical planning could lateralize and localize seizure focus successfully in most TLE cases, and provide surgical options for patients with non-lesional or bilateral TLE.
Acknowledgments
We are grateful to Zhao Jianxiu and Du Nansen from the Department of Radiology, Beijing 466 Hospital for their cooperation in acquiring the MRI and MRS data for the patients. We also thank Tan Bojing in the Department of Functional Neurology and Neurosurgery, Beijing Haidian Hospital for his help in providing patient information. This study was partially supported by National Natural Science Foundation (NSF) of the People’s Republic of China Grant 81071211.
Disclosure
The authors report no conflicts of interest in this work.
References
- BabbTLBilateral pathological damage in temporal lobe epilepsyCan J Neurol Sci1991186456481777886
- WolfHKZentnerJHufnagelASurgical pathology of chronic epileptic seizure disorders: experience with 63 specimens from extratemporal corticectomies, lobectomies and functional hemispherectomiesActa Neuropathol1993864664728310797
- WidjajaERaybaudCAdvances in neuroimaging in patients with epilepsyNeurosurg Focus200825E318759627
- Téllez-ZentenoJFDharRWiebeSLong-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysisBrain20051281188119815758038
- DoelkenMTRichterGStefanHMultimodal coregistration in patients with temporal lobe epilepsy – results of different imaging modalities in lateralization of the affected hemisphere in MR imaging positive and negative subgroupsAJNR Am J Neuroradiol20072844945417353311
- KnowltonRCElgavishRALimdiNFunctional imaging: I. Relative predictive value of intracranial electroencephalograpyAnn Neurol200864253418412264
- KnowltonRCElgavishRABartolucciAFunctional imaging: II. Prediction of epilepsy surgery outcomeAnn Neurol200864354118570291
- ShibasakiHIkedaANagamineTUse of magnetoencephalography in the presurgical evaluation of epilepsy patientsClin Neurophysiol20071181438144817452007
- BlountJPCormierJKimHKankirawatanaPRileyKOKnowltonRCAdvances in intracranial monitoringNeurosurg Focus200825E1818759619
- DuncanJSImaging the surgical treatment of epilepsyNat Rev Neurol2010653755020842185
- ChernovMFOchiaiTOnoYRole of proton magnetic resonance spectroscopy in preoperative evaluation of patients with mesial temporal lobe epilepsyJ Neurol Sci200928521221919647269
- HarroudABouthillierAWeilAGNguyenDKTemporal lobe epilepsy surgery failures: a reviewEpilepsy Res Treat2012201220165122934162
- HammenTKuznieckyRMagnetic resonance spectroscopy in epilepsyStefanHTheodoreWHHandbook of Clinical Neurology1073rd series Epilepsy, Part I2012399408
- HoriTYamaneFOchiaiTSelective subtemporal amygdalohip-pocampectomy for refractory temporal lobe epilepsy: operative and neuropsychological outcomesJ Neurosurg200710613414117236499
- EngelJJrVan NessPCRasmussenTOutcome with respect to epileptic seizuresEngelJJrSurgical Treatment of the Epilepsies2nd edNew YorkRaven Press1993609622
- KnowltonRCLaxerKDEndeGPresurgical multimodality neuroimaging in electroencephalographic lateralized temporal lobe epilepsyAnn Neurol1997428298379403474
- CendesFCaramanosZAndermannFProton magnetic resonance spectroscopic imaging and magnetic resonance imaging volumetry in the lateralization of temporal lobe epilepsy: a series of 100 patientsAnn Neurol1997427377469392573
- HammenTKerlingFSchwarzMIdentifying the affected hemisphere by (1)H-MR spectroscopy in patients with temporal lobe epilepsy and no pathological findings in high resolution MRIEur J Neurol20061348249016722973
- LikavcanovaKDobrotaDLiptajTIn vitro study of astrocytic tumour metabolism by proton magnetic resonance spectroscopyGen Physiol Biophys20052432733516308427
- MuellerSGLaxerKDCashdollarNFlennikenDLMatsonGBWeinerMWIdentification of abnormal neuronal metabolism outside the seizure focus in temporal lobe epilepsyEpilepsia20044535536615030498
- MuellerSGEbelABarakosJWidespread extrahippocampal NAA/(Cr + Cho) abnormalities in TLE with and without mesial temporal sclerosisJ Neurol201125860361220976465
- SmithSJMEEG in the diagnosis, classification, and management of patients with epilepsyJ Neurol Neurosurg Psychiatry200576ii2ii715961864
- CendesFLiLMWatsonCIs ictal recording mandatory in temporal lobe epilepsy? Not when the interictal electroencephalogram and hippocampal atrophy coincideArch Neurol20005749750010768623
- StefanHPauliEEberhardtKEMRI spectroscopy, T2 relaxometry, and postoperative prognosis in cryptogenic temporal lobe epilepsyNervenarzt20007128228710795095
- ConnellyAVan PaesschenWPorterDAJohnsonCLDuncanJSGadianDGProton magnetic resonance spectroscopy in MRI-negative temporal lobe epilepsyNeurology19985161669674779
- ChangKHKimHDParkSWUsefulness of single voxel proton MR spectroscopy in the evaluation of hippocampal sclerosisKorean J Radiol20001253211752925
- WoermannFGMcLeanMABartlettPABarkerGJDuncanJSQuantitative short echo time proton magnetic resonance spectroscopic imaging study of malformations of cortical development causing epilepsyBrain200112442743611157569
- StadlbauerAGruberSNimskyCPreoperative grading of gliomas by using metabolite quantification with highspatial-resolution proton MR spectroscopic imagingRadiology200623895896916424238
- FountasKNKapsalakiEZVogelRLFezoulidisIRobinsonJSGotsisEDNoninvasive histologic grading of solid astrocytomas using proton magnetic resonance spectroscopyStereotact Funct Neurosurg200482909715305081
- VermathenPEndeGLaxerKDHippocampal N-acetylaspartate in neocortical epilepsy and mesial temporal lobe epilepsyAnn Neurol1997421941999266729
- HammenTStefanHPauliE1H-MR spectroscopy: a promising method in distinguishing subgroups in temporal lobe epilepsy?J Neurol Sci2003215212514568123
- RiedererFBittsanskyMSchmidtC1H magnetic resonance spectroscopy at 3 T in cryptogenic and mesial temporal lobe epilepsyNMR Biomed20061954455316521092
- DucCOTrabesingerAHWeberOMQuantitative 1H MRS in the evaluation of mesial temporal lobe epilepsy in vivoMagn Reson Imaging1998169699799814780
- HammenTHildebrandtMStadlbauerANoninvasive detection of hippocampal sclerosis: correlation between metabolite alterations detected by (1)H-MRS and neuropathologyNMR Biomed20082154555218035849
- SuhyJLaxerKDCapizzanoAA1H MRSI predicts surgical outcome in MRI-negative temporal lobe epilepsyNeurology20025882182311889252
- KuznieckyRHuggJHetheringtonHPredictive value of 1H MRSI for outcome in temporal lobectomyNeurology19995369469810489028
- RowlandNCEnglotDJCageTASughrueMEBarbaroNMChangEFA meta-analysis of predictors of seizure freedom in the surgical management of focal cortical dysplasiaJ Neurosurg201211651035104122324422
- LernerJTSalamonNHauptmanJSAssessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experienceEpilepsia2009501310133519175385
- CossuMLo RussoGFrancioneSEpilepsy surgery in children: results and predictors of outcome on seizuresEpilepsia200849657217645538
- Widdess-WalshPJehaLNairDKotagalPBingamanWNajmISubdural electrode analysis in focal cortical dysplasia: predictors of surgical outcomeNeurology20076966066717698787
- JehaLENajmIBingamanWDinnerDWiddess-WalshPLüdersHSurgical outcome and prognostic factors of frontal lobe epilepsy surgeryBrain200713057458417209228
- McIntoshAMWilsonSJBerkovicSFSeizure outcome after temporal lobectomy: current research practice and findingsEpilepsia200142101288130711737164
- NajmIJehiLPalminiAGonzalez-MartinezJPaglioliEBingamanWTemporal patterns and mechanisms of epilepsy surgery failureEpilepsia201354577278223586531
- EnglotDJRolstonJDWangDDSunPPChangEFAugusteKISeizure outcomes after temporal lobectomy in pediatric patientsJ Neurosurg Pediatr201312213414123768202
- Téllez-ZentenoJFHernández RonquilloLMoien-AfshariFWiebeSSurgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysisEpilepsy Res2010892–331031820227852
- ToniniCBeghiEBergATPredictors of epilepsy surgery outcome: a meta-analysisEpilepsy Res2004621758715519134
