Abstract
Objective
Neurocognitive disorders commonly occur following cardiac surgery. However, the underlying etiology of these disorders is not well understood. The current study examined the association between perioperative glucose levels and other risk factors and the onset of neurocognitive disorders in adult patients following coronary artery bypass and/or valvular surgery.
Methods
Adult patients who underwent their first cardiac surgery at a large tertiary care medical center were identified and those with neurocognitive disorders prior to surgery were excluded. Demographic, perioperative, and postoperative neurocognitive outcome data were extracted from the Society for Thoracic Surgery database, and from electronic medical records, between January 2004 and June 2009. Multiple clinical risk factors and measures associated with insulin resistance, such as hyperglycemia, were assessed. Multivariable Cox competing risk survival models were used to assess hyperglycemia and postoperative neurocognitive disorders at follow up, adjusting for other risk factors and confounding variables.
Results
Of the 855 patients included in the study, 271 (31.7%) had new onset neurocognitive disorders at follow-up. Age, sex, New York Heart Failure (NYHF) Class, length of postoperative intensive care unit stay, perioperative blood product transfusion, and other key factors were identified and assessed as potential risk factors (or confounders) for neurocognitive disorders at follow-up. Bivariate analyses suggested that new onset neurocognitive disorders were associated with NYHF Class, cardiopulmonary bypass, history of diabetes, intraoperative blood product use, and number of diseased coronary vessels, which are commonly-accepted risk factors in cardiac surgery. In addition, higher first glucose level (median =116 mg/dL) and higher peak glucose >169 mg/dL were identified as risk factors. Male sex and nonuse of intra-operative blood products appeared to be protective. Controlling for potential risk factors and confounders, multivariable Cox survival models suggested that increased perioperative first glucose measured in 20 unit increments, was significantly associated with the onset of postoperative neurocognitive disorders at follow-up (hazard ratio [HR] =1.16, P<0.001) and that women had an elevated risk for this outcome (HR =4.18, P=0.01).
Conclusion
Our study suggests that perioperative hyperglycemia was associated with new onset of postoperative neurocognitive disorders in adult patients after cardiac surgery, and that men tended to be protected from these outcomes. These findings may suggest a need for the revision of clinical protocols for perioperative insulin therapy to prevent long-term neurocognitive complications.
Introduction
Patients undergoing cardiac surgery are at higher risk for developing postoperative neurocognitive disorders,Citation1 but the etiology of these outcomes remains unclear. Published studies have suggested multifactorial associations in the development of postoperative neurocognitive disorders, including older age, educational level, preoperative cognitive function, diabetes mellitus,Citation2–Citation4 cardiac dysrhythmia,Citation5 cardiopulmonary bypass surgery,Citation1,Citation6,Citation7 post-surgery body temperature and rewarming time,Citation8 jugular venous oxygen desaturation,Citation9,Citation10 acid-base management strategies,Citation11 microembolic load during surgical bypass,Citation12 and postoperative intubation time.Citation13,Citation14 Clinical trials of perioperative brain-protection protocols have included hypothermia,Citation15 minimizing aortic manipulation,Citation16 increasing perfusion pressure,Citation17,Citation18 epiaortic ultrasound guided aortic cannulation during cardiopulmonary bypass,Citation19 intra-aortic filters to prevent gaseous embolism,Citation12 and use of several pharmacological agents.Citation4,Citation20–Citation24 However, none of these preventive measures have proven to be effective.
Stress-induced hyperglycemia is a common perioperative phenomenon that may be caused by temporary insulin resistance from high physiological and psychological stress levels. Perioperative insulin therapy improves overall clinical outcome in patients after cardiac surgery,Citation25 and is frequently given due to the detrimental effects of hyperglycemia. However, the association between perioperative insulin resistance and the onset of postoperative neurocognitive disorders after cardiac surgery, and the role of perioperative insulin therapy in neuroprotection, are not well understood.
In animal studies, diabetic rats have significantly lower scores on cognitive tests.Citation26 Clinical data suggest that patients with type II diabetes mellitus tend to have poorer long-term neurocognitive performance compared to non-diabetic controls,Citation27 and one of the causal mechanisms for this association is thought to be insulin resistance. Data from in vitro studies indicate that the insulin signaling pathway is critical for maintaining long-term potentiation in hippocampal neurons, which are likely important in learning and memory.Citation28,Citation29 Moreover, activation of the insulin signaling pathway may reduce neuronal programmed cell death under nutritional deprivation, ischemia, and traumatic injury;Citation30–Citation32 thus, this pathway may serve a neuroprotective function during physiologic stress. Animal studies have also demonstrated that insulin therapy improved neurological outcome in global and focal brain ischemia.Citation33,Citation34 A recent clinical trial showed that intranasal insulin therapy may improve cognitive function in elderly patients with early dementia.Citation35 The potential benefits of perioperative glucose-insulin-potassium therapy have also been under investigation among patients with myocardial infarction and those undergoing cardiac surgery.Citation36,Citation37 These data suggest that insulin signaling pathway dysfunction may be an important mechanism in neurocognitive deterioration after neurological injuries. Thus, evidence suggests that maintaining normal insulin signaling pathway function during perioperative stress may provide important neuroprotec-tive effects.
The focus of the current study is to identify risk factors for new onset neurocognitive disorders after cardiac surgery, with a focus on assessing the association between perioperative insulin resistance, as indexed by perioperative hyperglycemia, and the onset of postoperative neurocognitive disorders. Our hypothesis is that perioperative hyperglycemia is associated with postoperative neurocognitive disorders and that good perioperative glucose control is important for improving neurocognitive outcome. As we note (to follow), the primary risk factor of interest in the current study is glucose level, which we use as a proxy measure for insulin resistance. We hypothesize that glucose level is associated with postoperative neurocognitive outcome, controlling for other risk factors and potential confounders.
Methods
Study population
This is a single medical center, retrospective longitudinal study, with an average patient follow-up time of approximately 4 years. Patients who underwent first-time cardiac surgery from January 2004 to June 2009 at Geisinger Medical Center (Danville, PA, USA) were eligible for the study. Patients who received on or off pump coronary artery bypass graft (CABG) and/or valve repair or replacement under general anesthesia were included in the study. Those who had repeated open sternotomy for CABG and/or valve surgery were excluded from the current study. The study population was restricted to those patients who were 18 years or older at the time of surgery and who had first time open sternotomy for CABG and/or valve surgery only. The study was also restricted to patients who had no prior history of neurocognitive disorders in their medical records, as defined below. The purpose of this neurocognitive disorder restriction was to reduce potential bias at the index date (time of surgery), because patients with prevalent neurocognitive disorders would be more likely to have further recurrences. In addition, this exclusion would potentially reduce confounding related to clinicians’ clinical decision-making associated with their patient care approach. For this study, patients’ CABG surgery information was retrospectively extracted from the Society for Thoracic Surgery (STS) database maintained at Geisinger Medical Center. This database also includes all cardiac surgery information as well as the presence of key cardiovascular disease measures, such as number of diseased vessels, percent aortic occlusion, and the New York Heart Failure (NYHF) classification. These were recorded by clinicians in the STS registry as being present at the time of surgery. The STS National Database has more than 250 instructional participants in the US and the Adult Cardiac Surgery Database now contains more than 4.5 million surgical records and represents an estimated 94% of all adult cardiac surgery centers across the US (http://www.sts.org/national-database).
Since blood insulin level was not routinely monitored, perioperative (ie, the closest postoperative time point) measurement of glucose was used as a surrogate marker for insulin resistance. In this case, perioperative glucose is defined as the glucose measurement results obtained on the same day of surgery. All non-STS surgery data were extracted from Geisinger’s electronic health records (EHR) database. Since information pertaining to the start and end of general anesthesia, aortic cross clamping, and hypothermic cardiopulmonary bypass were not always recorded in the electronic records, the timing of some of these measurement results could not be determined. For our analyses, we assessed each of the following as insulin resistance proxies: first available intra or postoperative glucose, peak glucose, median glucose, and glucose range. Glucose results were all taken within the admission stay for the surgery. In order to control for disease burden, we also assessed key comorbidities for each patient at the time of surgery, which were recorded by clinicians in the STS registry as present at the time of surgery. These comorbidities included infectious endocarditis, immunosuppresive therapy, peripheral vascular disease, cerebrovascular disease, hypertension, and diabetes. The onset of postoperative neu-rocognitive disorder – the study outcome – was identified from data in the EHR at follow-up. The study outcome was the date of the first International Classification of Diseases, Ninth revision, clinical modification (ICD-9 CM) diagnosis code for stroke, cerebrovascular accident, transient ischemic attack, Alzheimer’s disease, dementia, memory deficit, coma, encephalopathy, or adult onset neurological conditions after surgery. The first date for any of these disorders was then taken as the date of neurocognitive disorder onset. The study end date was June 30, 2009. This study was approved by the Institutional Review Board of Geisinger Health System (Danville, PA, USA).
Statistical methods
We first present baseline characteristics and comorbidities of the 855 eligible patients. Statistical distributions were assessed for each variable, stratified by postoperative cognitive disorder status. For continuous variables the Student’s t-test was used, if normally distributed; otherwise, Mann–Whitney–Wilcoxon or Kruskal–Wallis tests were used. Chi-square test was used for categorical variables. Continuous variables were summarized using means and standard deviation, if normally distributed, otherwise we used the median with interquartile range (IQR; 25th–75th percentiles). Next, we evaluated the association of each variable with postoperative neurocognitive event, using a bivariate Cox proportional hazard model. For patients with multiple glucose measurements, we took the first glucose level as a predictor since it was the one closest to the pre-operation value. We used different strategies to deal with missing data, dependent on measurement level. For variables with more than 30% missing data, the variable was not used in the analysis. Otherwise, we treated missing as a category, if the variable was categorical, and as an indicator measure, if the variable was continuous, with an interaction term for the missing indicator and the variable included in the model for the latter.
For the final model, we included the candidate variables selected from the analyses described above at a significance level of 0.10 or less. An age interaction effect with each of the other variables was tested in the model, followed by sex interaction effect on the variables. Postoperative neurocognitive disorder was the primary outcome of interest, with all-cause mortality included as a competing risk in the model. We utilized this competing-risk proportional hazard model to estimate the cause-specific hazard for onset of postoperative neurocognitive disorders. The advantage of this competing risk method was that the model accounts for the failure time from the competing risk while estimating the hazard ratio, which avoids overestimating the hazard of the disease outcome of interest.Citation38 All statistical tests were two-sided with a P-value of less than 0.05 considered as a cut-off for statistical significance. Data analysis was performed using the SAS (version 9.3) statistical package (SAS Institute Inc., Cary, NC, USA).
Results
Among 855 eligible patients, 271 (31.7%) developed postoperative cognitive disorders, and 22 (2.6%) patients died during the follow-up period, which averaged about 4 years in this study (). Higher percentage of female patients developed postoperative cognitive dysfunction than males (P=0.005). The average age at surgery was not significantly different for those who had postoperative cognitive disorders compared to those who did not (P=0.36), and while the average age of patients in the mortality group was 3 years older at surgery, this difference was also not significant (P=0.36). However, the mortality group exhibited relatively more severe cardiovascular disease symptoms: 94.7% were in the class 3 or higher heart failure group, compared to 72.6% for the event group, and 63.6% for the no-event group (P=0.006). In terms of the number of diseased coronary vessels, the event group appeared to have more coronary-vessel disease than the no-event group (57.9% versus 50.4% had three diseased vessels), while the mortality group appeared to have less coronary vessel disease (38.1% had three diseased vessels; P=0.08). In addition, the mortality group had the highest percentage of aortic occlusion (77.3% compared to 30.6% for the event group and 41.8% for the no-event group; P<0.001). For surgery-related procedures, the mortality group had the highest percentage of cardiopulmonary bypasses (90.9% compared to 41.7% for the event group and 48.2% for the no-event group; P<0.001), and the highest percent of blood product use (59.1% versus 27.7% for the event group and 31.1% for the no-event group; P=0.009). However, the three groups had a similar prevalence for most major comorbidities, except immunosuppressive therapy (IMT) and cerebrovascular disease (CEVD): the mortality group had the highest prevalence of IMT (5% compared to 0% in the event group and 0.5% in the no-event group; P=0.006), while CEVD was more prevalent in the event-group (7.8% compared to 4.1% for the no-event group and 0% for the mortality group; P=0.04). In terms of perioperative glucose measurements, when we compared the first glucose (preoperative measure), median, peak, and range of repeated measurements, it appeared that the mortality group always had the highest glucose level (median of each of the above measurements was about 20 units higher than the two other groups), followed by the event group, which was typically about 4 units higher than the no-event group (P<0.05).
Table 1 Patient characteristics by postoperative neurocognitive outcome results among those with no prior cognitive disorders (N=855)
The multivariate hazard ratios (HRs) are presented in , representing the adjusted hazard of developing postoperative cognitive disorders over time. Patient characteristics and common risk factors were adjusted in the model. As discussed, the hazard for postoperative neurocognitive disorders was significantly greater for women compared to men (HR =4.18, 95% confidence interval [CI]: 1.33–13.14; P=0.01) and older age also presented as an increased hazard for females (HR =1.04, 95% CI: 1.03–1.05; P<0.001). Higher heart failure classification (HFC) was associated with a higher hazard (HFC =4 versus 1; HR =4.95, 95% CI: 2.41–10.13; P<0.001), as well as utilization of intraoperative blood products (HR=2.04, 95% CI: 1.77–2.35; P<0.001). Several comorbidities, such as diabetes and infectious endocarditis were significantly associated with study outcome (P-values <0.01). Notably, male patients with cardiopulmonary bypass (CPB) were at about a 15 times higher risk for postoperative cognitive disorders than female patients (HR =15.6, 95% CI: 11.2–21.9, P<0.001).
Table 2 Multivariate Cox regression results showing risk factors for postoperative neurocognitive disorders
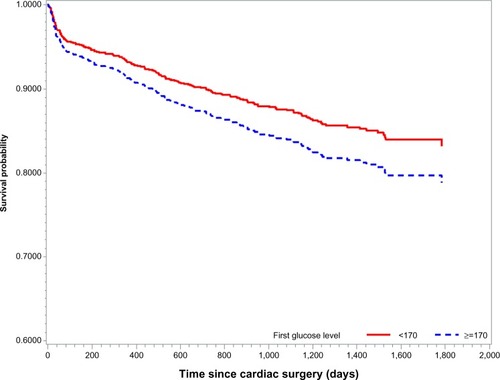
After controlling for other common risk factors, we tested the association of glucose level with postoperative cognitive disorders. Only the first glucose measurement, which was usually taken before surgery, was significantly associated with the outcome of interest: when measured by 20 unit increases in glucose level, the hazard increased by 16% (HR =1.16, 95% CI: 1.13–1.20, P<0.001). Thus, after adjusting for possible risk factors, survival analysis using Cox-proportional hazard regression with competing risks indicates that patients with first glucose level higher than 170 mg/dL have worse long-term neurocognitive outcomes compared to those who a had lower glucose level (). Furthermore, we confirmed that the estimated time point where these two glucose levels begin to be statistically significant was at 379 days post-surgery (P=0.03).
Discussion
The main research question we sought to answer in this study was whether perioperative hyperglycemia was associated with new onset of postoperative neurocognitive disorders. As was shown, our findings suggest that patients who had perioperative hyperglycemia, as indicated by increased perioperative glucose levels, tended to develop postoperative neurocognitive disorders after cardiac surgery. These findings suggest that de-novo perioperative hyperglycemia may contribute to the mechanism of negative long term neurocognitive outcome.
Hyperglycemia is associated with negative overall outcome in critical care patients, especially neurological outcome after traumatic and ischemic brain injury;Citation39,Citation40 however, the mechanism is not well defined. It is unclear if high glucose concentration itself, or the mechanisms involved in glucose dysregulation, promote a negative outcome. Glucose is essential for the survival and growth of brain cells in culture. In fact, the concentration of glucose in optimized neuronal cell cultural media is higher than physiological concentrations,Citation41 suggesting that neuronal function is better in a high glucose environment. Although an in vitro study did show evidence that high glucose concentrations induced neuronal cell death through oxidative stress and mitochondria dysfunction, it is not clinically relevant since the glucose concentrations in these experiments were much higher than that of clinical hyperglycemia.Citation42 Several animal experiments have demonstrated the memory enhancing effects of glucose through facilitating the synthesis and release of acetylcholine in brain.Citation43 In a rat model of traumatic brain injury, intentionally elevated blood glucose prior to injury did not worsen motor or cognitive function post injury, and may have been neuroprotective by reducing edema around injured lesions.Citation44 Clamped hyperglycemia increased regional cerebral oxygenation in rats subjected to cardiac arrest.Citation45 Injection of glucose into the rat hippocampus was found to reverse the memory deficit effect of gamma-Aminobutyric acid (GABA) receptor antagonist.Citation46 These research results suggest that glucose, as the obligate energetic fuel for the mammalian brain, is unlikely to be detrimental. Rather, the association between hyperglycemia and negative outcomes may be due to the malfunction of glucose regulation from insulin resistance during specific pathophysiological processes.
Perioperative hyperglycemia is commonly identified in patients with or without diagnosed diabetes mellitus.Citation47,Citation48 The mechanism could be stress induced insulin resistance, a neuroendocrine-mediated deterioration in glucose metabolism.Citation49 It is well accepted that controlling glucose is important for improving overall outcome in critical care patients, although strict insulin therapy for glucose is not recommended due to the high incidence of severe hypoglycemia.Citation50 Clinical studies showed patients with diabetes mellitus type II are subject to long term neurocognitive dysfunction, including Alzheimer’s disease later in the life.Citation51,Citation52 Clinical studies have indicated that intranasal application of insulin improved cognition in early dementia.Citation35 A study has also shown that insulin resistance generated by knocking out a molecule in the insulin signaling pathway in mice hippocampi disrupts the formation of neuronal long-term potentiation, which is critical for learning and memory.Citation29 Rats with induced type II diabetes have impaired proliferation of hippocampus neuroprogenitor cells.Citation53 The diabetic animals showed signs of deficits in motor function and spatial learning, had brain neurodegeneration similar to Alzheimer’s disease, and had evidence of inhibition of the insulin signaling pathway.Citation54,Citation55 Delivery of insulin to the rat hippocampus can enhance spatial memory which is phosphoinositide 3-kinase dependent, and the blockade of endogenous insulin signaling impairs learning and memory.Citation56 Insulin resistance is associated with worse long term neurocognitive performance in rats with brain ischemiaCitation57 and therapeutic intervention of preserving the function of the insulin signaling pathway can reduce hypoxic brain injury and improve behavioral recovery after ischemic insult.Citation58 In patients who underwent cardiac surgery, hypothermic cardiopulmonary bypass, which is known to be associated with negative postoperative neurocognitive outcome, exacerbates insulin resistance status.Citation59 Although not significant in our study, a history of diabetes was shown to be associated with postoperative cognitive decline in patients who underwent coronary artery bypass, suggesting that preoperative insulin resistance status could contribute to postoperative neurocognitive decline in patients who undergo cardiac surgery.Citation4,Citation60 Recent clinical trials found that routine perioperative application of insulin-glucose-potassium in patients who underwent aortic valve replacement experienced improved outcome; this finding was associated with activation of phosphoinositide 3-kinase/protein kinase B (Akt) and adenosine monophosphate (AMP)-activated protein kinase (AMPK), both of which have an established role in the insulin signaling pathway.Citation37 Although it was not the end point of this study, the activation of insulin signaling suggest that routine, continuous perioperative insulin therapy, in addition to its glycemic effect, may have an advantage in improving neurological outcome.
The relationship between perioperative transfusion of blood products and postoperative neurocognitive outcome is not well studied in cardiac surgery. Studies over the past three decades have demonstrated severe neurological deficits after the transfusion of packed red blood cells.Citation61–Citation64 In our study, the negative neurocognitive outcome from fresh frozen plasma and cryoprecipitate transfusions () could indicate that coagulopathy has a negative impact on the overall outcome in cardiac surgical patients. At the same time, transfusion of fresh frozen plasma may induce endothelial damage via immune activation in the central nervous system, which contributes to neurocognitive dysfunction. Further studies are warranted in order to confirm this association.
In the current study, NYHF class and longer postoperative intensive care unit (ICU) stay are predictors of postoperative neurocognitive outcome. This suggests that the overall severity of the patient’s clinical condition is associated with neurological outcome. This is not surprising given that a patient with severe cardiac dysfunction is subject to hemodynamic instability, which could reduce brain perfusion causing chronic global brain ischemia.Citation13 Patients with prolonged ICU stays frequently have a higher incidence of perioperative neurological and psychiatric complications, including ischemic and embolic stroke, and ICU delirium or psychosis.Citation65 Lastly, patients with longer postoperative ICU duration may have longer and higher dose exposures to hypnotic and sedative drugs, which may contribute to worse long term neurocognitive outcome.
In addition, our study suggests that female patients may be more vulnerable to postoperative neurocognitive disorders compared to male patients. Sex differences in neurological outcome after brain injury has been under extensive investigation recently. Although animal research suggests that female hormones may be neuroprotective, clinical findings are controversial with regard to sex effects on neurological outcome after brain injury.Citation66–Citation68 Recent clinical studies showed that female sex was associated with a reduced likelihood of returning to gainful occupation after stroke.Citation69 Hogue et al found that females had worse performance postoperatively on visuospatial tasks up to 6 weeks after cardiac surgery.Citation70 In keeping with clinical studies, our data also suggest that female patients have an increased risk for developing neurocognitive disorders post-cardiac surgery.
The current study is subject to several limitations. First, this is a single center retrospective study and all recruited patients belonged to the cardiac surgery cohort within the study time frame; we were not able to recruit normal and/or non-cardiac surgical patients as control groups due to study limitations. In addition, the types of surgery and anesthesia are two major confounding factors in this study. Second, since the follow up time is equal to or less than approximately 5 years, this may limit the ability to detect the new onset of neurocognitive disorders in some patients. The weaker association after adjusting for other significant covariates in multivariable Cox model could be explained in part by our relatively short follow-up time, leading to an underestimation of the incidence of long-term neurocognitive disorders. Moreover, the eligible cases retrospectively identified in the current study from a single medical center may have biased our study findings. Third, formal neurocognitive tests were not available for analyses in the current study, since these tests are generally neither part of the routine preoperative assessment nor were these tests done during the follow-up period. This limited our ability of identifying short and long term cognitive decline after surgery, especially in a more quantitative way. Fourth, the use of perioperative glucose level as the surrogate marker for insulin resistance status is another limitation; we were not able to directly quantify insulin resistance in the current study due to the fact that blood insulin concentration was unknown in most of the patients undergoing cardiac surgery in our study. Fifth, our perioperative glucose measures did not specifically include only fasting glucose measures; this information was not available for the current study.
In summary, the current study suggests that postoperative neurocognitive disorders may be associated with perioperative insulin resistance in cardiac surgery, with possible contributions of age, sex, transfusion of blood products, significant cardiac dysfunction, and length of postoperative ICU stay. Further clinical and basic research are needed to develop better protocols for improving neurocognitive outcomes by way of improving dysfunctional insulin pathways in the future.
Acknowledgments
This work was funded by the Geisinger Clinic Research Fund. We would like to thank Ms Amanda Bengier and Ms Mary Ann Blosky, RN, for their assistance in data collection and project management, and Drs Alfred S Casale, Joel J Berberich, Xianren Wu and Thomas M Schieble for making this project possible.
Disclosure
The authors declare no conflicts of interest in this work.
References
- van DijkDSpoorMHijmanROctopus Study GroupCognitive and cardiac outcomes 5 years after off-pump vs on-pump coronary artery bypass graft surgeryJAMA2007297770170817312289
- KozoraEKongsSCollinsJFCognitive outcomes after on-versus off-pump coronary artery bypass surgeryAnn Thorac Surg20109041134114120868803
- KadoiYSaitoSFujitaNGotoFRisk factors for cognitive dysfunction after coronary artery bypass graft surgery in patients with type 2 diabetesJ Thorac Cardiovasc Surg2005129357658315746741
- MathewJPMackensenGBPhillips-ButeBNeurologic Outcome Research Group (NORG) of the Duke Heart CenterRandomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgeryStroke200940388088719164788
- StanleyTOMackensenGBGrocottHPNeurological Outcome Research GroupCARE Investigators of the Duke Heart CenterThe impact of postoperative atrial fibrillation on neurocognitive outcome after coronary artery bypass graft surgeryAnesth Analg200294229029511812686
- AnastasiadisKArgiriadouHKosmidisMHNeurocognitive outcome after coronary artery bypass surgery using minimal versus conventional extracorporeal circulation: a randomised controlled pilot studyHeart201197131082108821357641
- PorizkaMStriteskyMSemradMDobiasMDohnalovaAKorinekJStandard blood flow rates of cardiopulmonary bypass are adequate in awake on-pump cardiac surgeryEur J Cardiothorac Surg201139444245021237669
- GrigoreAMGrocottHPMathewJPNeurologic Outcome Research Group of the Duke Heart CenterThe rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgeryAnesth Analg200294141011772792
- KawaharaFKadoiYSaitoSGotoFFujitaNSlow rewarming improves jugular venous oxygen saturation during rewarmingActa Anaesthesiol Scand200347441942412694140
- YoshitaniKKawaguchiMSugiyamaNThe association of high jugular bulb venous oxygen saturation with cognitive decline after hypothermic cardiopulmonary bypassAnesth Analg20019261370137611375807
- VennGEPatelRLChambersDJCardiopulmonary bypass: perioperative cerebral blood flow and postoperative cognitive deficitAnn Thorac Surg1995595133113357733763
- GerrietsTSchwarzNSammerGProtecting the brain from gaseous and solid micro-emboli during coronary artery bypass grafting: a randomized controlled trialEur Heart J201031336036819541675
- BoodhwaniMRubensFDWoznyDPredictors of early neurocognitive deficits in low-risk patients undergoing on-pump coronary artery bypass surgeryCirculation2006114Suppl 1I461I46616820619
- ChengDCKarskiJPenistonCMorbidity outcome in early versus conventional tracheal extubation after coronary artery bypass grafting: a prospective randomized controlled trialJ Thorac Cardiovasc Surg199611237557648800165
- BoodhwaniMRubensFWoznyDRodriguezRNathanHJEffects of sustained mild hypothermia on neurocognitive function after coronary artery bypass surgery: a randomized, double-blind studyJ Thorac Cardiovasc Surg2007134614431450 discussion 1451–145218023662
- HammonJWStumpDAButterworthJFSingle crossclamp improves 6-month cognitive outcome in high-risk coronary bypass patients: the effect of reduced aortic manipulationJ Thorac Cardiovasc Surg2006131111412116399302
- CharlsonMEPetersonJCKriegerKHImprovement of outcomes after coronary artery bypass II: a randomized trial comparing intraoperative high versus customized mean arterial pressureJ Card Surg200722646547218039205
- SiepeMPfeifferTGieringerAIncreased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and deliriumEur J Cardiothorac Surg201140120020721168339
- GoldJPTorresKEMaldarelliWZhuravlevIConditDWasnickJImproving outcomes in coronary surgery: the impact of echo-directed aortic cannulation and perioperative hemodynamic management in 500 patientsAnn Thorac Surg20047851579158515511435
- HaljanGMaitlandABuchanAThe erythropoietin neuroprotective effect: assessment in CABG surgery (TENPEAKS): a randomized, double-blind, placebo controlled, proof-of-concept clinical trialStroke20094082769277519556536
- HolinskiSClausBAlaarajNCerebroprotective effect of piracetam in patients undergoing open heart surgeryAnn Thorac Cardiovasc Surg201117213714221597409
- HudetzJAIqbalZGandhiSDKetamine attenuates post-operative cognitive dysfunction after cardiac surgeryActa Anaesthesiol Scand200953786487219422355
- SilbertBSScottDAEveredLAA comparison of the effect of high- and low-dose fentanyl on the incidence of postoperative cognitive dysfunction after coronary artery bypass surgery in the elderlyAnesthesiology200610461137114516732083
- SzalmaIKissAKardosLPiracetam prevents cognitive decline in coronary artery bypass: a randomized trial versus placeboAnn Thorac Surg20068241430143516996947
- IngelsCDebaveyeYMilantsIStrict blood glucose control with insulin during intensive care after cardiac surgery: impact on 4-years survival, dependency on medical care, and quality-of-lifeEur Heart J200627222716272416608860
- WinocurGGreenwoodCEPiroliGGMemory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesityBehav Neurosci200511951389139516300445
- SaczynskiJSJónsdóttirMKGarciaMECognitive impairment: an increasingly important complication of type 2 diabetes: the age, gene/environment susceptibility – Reykjavik studyAm J Epidemiol2008168101132113918836152
- FaivreEGaultVAThorensBHölscherCGlucose-dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesisJ Neurophysiol201110541574158021273318
- MartínEDSánchez-PerezATrejoJLIRS-2 Deficiency impairs NMDA receptor-dependent long-term potentiationCereb Cortex20122281717172721955917
- SandersonTHKumarRMurariu-DobrinACPageABKrauseGSSullivanJMInsulin activates the PI3K-Akt survival pathway in vulnerable neurons following global brain ischemiaNeurol Res200931994795819203442
- SuhSWGumETHambyAMChanPHSwansonRAHypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidaseJ Clin Invest2007117491091817404617
- ZhangXChenYIkonomovicMDIncreased phosphorylation of protein kinase B and related substrates after traumatic brain injury in humans and ratsJ Cereb Blood Flow Metab200626791592616234845
- HuiLPeiDSZhangQGGuanQHZhangGYThe neuroprotection of insulin on ischemic brain injury in rat hippocampus through negative regulation of JNK signaling pathway by PI3K/Akt activationBrain Res2005105211916018989
- VollCLAuerRNInsulin attenuates ischemic brain damage independent of its hypoglycemic effectJ Cereb Blood Flow Metab1991116100610141939378
- RegerMAWatsonGSGreenPSIntranasal insulin improves cognition and modulates beta-amyloid in early ADNeurology200870644044817942819
- Bucciarelli-DucciCBianchiMDe LucaLEffects of glucose-insulin-potassium infusion on myocardial perfusion and left ventricular remodeling in patients treated with primary angioplasty for ST-elevation acute myocardial infarctionAm J Cardiol200698101349135317134627
- HowellNJAshrafianHDruryNEGlucose-insulin-potassium reduces the incidence of low cardiac output episodes after aortic valve replacement for aortic stenosis in patients with left ventricular hypertrophy: results from the Hypertrophy, Insulin, Glucose, and Electrolytes (HINGE) trialCirculation2011123217017721200004
- FineJPGrayRJA proportional hazards model for the sub-distribution of a competing riskJ Am Stat Assoc199994496509
- BrunoABillerJAdamsHPAcute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) InvestigatorsNeurology19995222802849932944
- GoreDCChinkesDHeggersJHerndonDNWolfSEDesaiMAssociation of hyperglycemia with increased mortality after severe burn injuryJ Trauma200151354054411535907
- DennisSHJaafariNCimarostiHHanleyJGHenleyJMMellorJROxygen/glucose deprivation induces a reduction in synaptic AMPA receptors on hippocampal CA3 neurons mediated by mGluR1 and adenosine A3 receptorsJ Neurosci20113133119411195221849555
- MeyerLEMachadoLBSantiagoAPMitochondrial creatine kinase activity prevents reactive oxygen species generation: antioxidant role of mitochondrial kinase-dependent ADP re-cycling activityJ Biol Chem200628149373613737117028195
- DegrootAKornecookTQuirionRDeBowSParentMBGlucose increases hippocampal extracellular acetylcholine levels upon activation of septal GABA receptorsBrain Res20039791–2717712850573
- HillJZhaoJDashPKHigh blood glucose does not adversely affect outcome in moderately brain-injured rodentsJ Neurotrauma20102781439144820504157
- LennmyrFMolnarMBasuSWiklundLCerebral effects of hyperglycemia in experimental cardiac arrestCrit Care Med20103881726173220562703
- Krebs-KraftDLParentMBHippocampal infusions of glucose reverse memory deficits produced by co-infusions of a GABA receptor agonistNeurobiol Learn Mem200889214215217728160
- DunganKHallCSchusterDOseiKDifferential response between diabetes and stress-induced hyperglycaemia to algorithmic use of detemir and flexible mealtime aspart among stable postcardiac surgery patients requiring intravenous insulinDiabetes Obes Metab201113121130113521767340
- VerhoevenJJHokken-KoelegaACden BrinkerMDisturbance of glucose homeostasis after pediatric cardiac surgeryPediatr Cardiol201132213113821082177
- PeiDChenTWKuoYLThe effect of surgical stress on insulin sensitivity, glucose effectiveness and acute insulin response to glucose loadJ Endocrinol Invest200326539740212906365
- FinferSChittockDRSuSYNICE-SUGAR Study InvestigatorsIntensive versus conventional glucose control in critically ill patientsN Engl J Med2009360131283129719318384
- BakerLDCrossDJMinoshimaSBelongiaDWatsonGSCraftSInsulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetesArch Neurol2011681515720837822
- WilliamsonJDMillerMEBryanRNACCORD Study GroupThe Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methodsAm J Cardiol20079912A112i122i
- LangBTYanYDempseyRJVemugantiRImpaired neurogenesis in adult type-2 diabetic ratsBrain Res20091258253319138677
- de la MonteSMTongMNguyenVSetshediMLongatoLWandsJRCeramide-mediated insulin resistance and impairment of cognitive-motor functionsJ Alzheimers Dis201021396798420693650
- TongMNeusnerALongatoLLawtonMWandsJRde la MonteSMNitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer’s diseaseJ Alzheimers Dis200917482784420387270
- McNayECOngCTMcCrimmonRJCresswellJBoganJSSherwinRSHippocampal memory processes are modulated by insulin and high-fat-induced insulin resistanceNeurobiol Learn Mem201093454655320176121
- MoreiraTCebersGPickeringCOstensonCGEfendicSLiljequistSDiabetic Goto-Kakizaki rats display pronounced hyperglycemia and longer-lasting cognitive impairments following ischemia induced by cortical compressionNeuroscience200714441169118517175109
- ZhongJZhaoLDuYWeiGYaoWGLeeWHDelayed IGF-1 treatment reduced long-term hypoxia-ischemia-induced brain damage and improved behavior recovery of immature ratsNeurol Res200931548348919500451
- AndersonREBrismarKBarrGIvertTEffects of cardiopulmonary bypass on glucose homeostasis after coronary artery bypass surgeryEur J Cardiothorac Surg200528342543016054822
- NötzoldAMichelKKhattabAASieversHHHüppeMDiabetes mellitus increases adverse neurocognitive outcome after coronary artery bypass grafting surgeryThorac Cardiovasc Surg200654530731216902877
- HeoKParkSLeeJYLeeBILeeSKPost-transfusion posterior leukoencephalopathy with cytotoxic and vasogenic edema precipitated by vasospasmCerebrovasc Dis200315323023312646784
- NagasawaHKuritaKWadaMKawanamiTKatoTBlood transfusion-induced irreversible brain damageJ Neurol2005252121541154215940385
- SmithMJLe RouxPDElliottJPWinnHRBlood transfusion and increased risk for vasospasm and poor outcome after subarachnoid hemorrhageJ Neurosurg200410111715255244
- YamadaSKoizumiAIsoHJACC Study GroupHistory of blood transfusion before 1990 is a risk factor for stroke and cardiovascular diseases: the Japan collaborative cohort study (JACC study)Cerebrovasc Dis200520316417116088111
- NuttallGAKumarMMurrayMJNo difference exists in the alteration of circadian rhythm between patients with and without intensive care unit psychosisCrit Care Med1998268135113559710093
- BrazinovaAMauritzWLeitgebJOutcomes of patients with severe traumatic brain injury who have Glasgow Coma Scale scores of 3 or 4 and are over 65 years oldJ Neurotrauma20102791549155520597653
- IversonKMHendricksAMKimerlingRPsychiatric diagnoses and neurobehavioral symptom severity among OEF/OIF VA patients with deployment-related traumatic brain injury: a gender comparisonWomens Health Issues201121Suppl 4S210S21721724143
- LeitgebJMauritzWBrazinovaAEffects of gender on outcomes after traumatic brain injuryJ Trauma20117161620162621808209
- HannerzHHolbæk PedersenBPoulsenOMHumleFAndersenLLA nationwide prospective cohort study on return to gainful occupation after stroke in Denmark 1996–2006BMJ Open201112e000180
- HogueCWLillieRHersheyTGender influence on cognitive function after cardiac operationAnn Thorac Surg20037641119112514529997