Abstract
Aim
Inconsistent results continue to be reported in studies that examine the neural correlates of cognitive behavioral therapy (CBT) in patients with panic disorder. We examined the changes in regional cerebral blood flow (rCBF) associated with the alleviation of anxiety by CBT in panic patients.
Methods
The change in rCBF and clinical symptoms before and after CBT were assessed using single photon emission computed tomography and various clinical measures were analyzed.
Results
Fourteen subjects who completed CBT showed significant improvements in symptoms on clinical measures, including the Panic and Agoraphobic Scale and the Anxiety Sensitivity Index-Revised. After CBT, increased rCBF was detected in the left postcentral gyrus (BA 43), left precentral gyrus (BA 4), and left inferior frontal gyrus (BA 9 and BA 47), whereas decreased rCBF was detected in the left pons. Correlation analysis of the association between the changes in rCBF and changes in each clinical measure did not show significant results.
Conclusion
We found changes in the rCBF associated with the successful completion of CBT. The present findings may help clarify the effects of CBT on changes in brain activity in panic disorder.
Introduction
Panic disorder is characterized by the presence of recurrent panic attacks along with the following symptoms: persistent thoughts about the possibility of future attacks; worry about the implications and consequences of an attack; and changes in behavior because of previous attacks.Citation1 Unexpected paroxysmal bursts of severe anxiety, especially when complicated by agoraphobia, affect an individual’s functioning in a progressive, disabling pattern and reduce quality of life.Citation2 Several psychological factors such as anxiety sensitivity,Citation3 conditioned fear of internal cues,Citation4 and catastrophic misappraisals of bodily sensationsCitation5 seem to play an important role in the onset and maintenance of panic disorder.Citation2
Two broad categories of treatment have been accepted because of their effectiveness in treating panic disorder: pharmacotherapy and cognitive behavioral therapy (CBT). Pharmacotherapy is a very effective treatment modality for panic disorder. Selective serotonin reuptake inhibitors (SSRIs) are now considered to be the first-line drug, and benzodiazepines such as alprazolam and clonazepam have also proved effective for the treatment of panic disorder in controlled trials.Citation6–Citation8 However, pharmacotherapy alone seems to result in considerable relapse, regardless of the maintenance of an adequate dose,Citation9,Citation10 and may be related to significant problems with long-term medication use, such as dependence and withdrawal symptoms.Citation11 CBT is also an effective treatment in panic disorder, with 75% of patients achieving panic-free end states.Citation12 In contrast to pharmacotherapy, CBT maintains its effect after the end of treatment and has a distinct advantage over pharmacotherapy in preventing relapse.Citation12,Citation13
Various hypotheses of neural mechanisms underlying panic disorder have been proposed to understand the effectiveness of treatments. Patients with panic disorder have a sensitive fear network that is centered in the amygdala, which is modulated by both thalamic input and prefrontal projections, and itself projects to several areas that are related to autonomic and behavioral responses.Citation14 Serotonergic drugs would act by desensitization of the fear network via projection from the raphe nuclei to various brain areas, such as the locus coeruleus for arousal, brain stem for respiratory activation, hypothalamus for activation of the HPA axis, periaqueductal gray region for freeze/flight response, and possibly directly to the amygdala, inhibiting the excitatory pathway from both the thalamus and prefrontal cortex.Citation14 CBT is thought to reduce phobic avoidance by deconditioning the contextual fear learned at the level of the hippocampus and decreasing cognitive misattributions by strengthening the ability of the medial prefrontal cortex to inhibit the amygdala.
Although many studies have been performed to further understanding of the neurobiology of panic disorder,Citation15–Citation17 only three studies investigated the neural correlates of CBT in patients with panic disorder.Citation18–Citation20 Possible interpretation of these findings is that panic disorder is associated with hyper-reactivity in emotion-generative regions, possibly because of an overgeneralization of fearful stimuli, and hypo-reactivity in emotion-regulatory regions, reflecting a failure to control learned fear.Citation21 From this perspective, CBT might be normalizing both emotion-generative and emotion-regulatory responses.Citation21 However, the finding from each study provides only modest support for this hypothesis, and neural mechanism underlying the effects of CBT still remains unclear.
The present study was an uncontrolled investigation to examine changes in regional cerebral blood flow (rCBF) associated with the alleviation of anxiety symptoms by CBT using single photon emission computed tomography (SPECT) in patients with panic disorder. We hypothesized that there would be increases of activity in regions of the upstream circuitry from the amygdala, including the prefrontal cortex, as an adaptive modification after CBT. It was also hypothesized that the activity would decrease in any area of “panic neurocircuitry” (amygdala, insulae, thalamu, pons, etc), which may be soothed during recovery after CBT. In addition, we expected to find significant correlations between changes in the psychometric measures and the rCBF in these brain regions.
Methods
Subjects
The eligible subjects were patients between the ages of 18 and 65 who met DSM-IV criteriaCitation1 for panic disorder on the Mini International Neuropsychiatric Interview (MINI).Citation22,Citation23 The exclusion criteria were as follows: any comorbidity of the current psychiatric disorder, including major depressive disorder, bipolar disorder, schizophrenia, social phobia, obsessive-compulsive disorder, post-traumatic stress disorder, or generalized anxiety disorder; current suicide ideation or suicide attempt history; serious trauma history; any neurological disorder such as stroke, epilepsy, or brain tumor; current serious or unstable medical disease such as ischemic heart disease or cancer, or use of prescribed medication for more than 6 months; alcohol and other substance abuse within 6 months prior to the baseline; pregnancy or breast-feeding; and left handedness.
Nineteen participants fulfilling inclusion and exclusion criteria were enrolled from the Mettaa Institute, located in Seoul, South Korea, which specializes in CBT for anxiety disorder. Of a total of 19 participants, 5 subjects dropped out of the study (2 with withdrawal of informed consent and 3 with follow-up loss), and finally 14 participants completed the study protocol and were included in the analysis (). Ten of them were women (71.4%), and the mean (standard deviation) age and duration of illness was 32.4 (9.0) years and 16.4 (15.4) months, respectively. Eleven subjects were being treated with medication, including SSRIs and benzodiazepine, prior to enrollment, and their medication was maintained without change during the study period.
Table 1 Demographic and clinical characteristics of subjects who completed the full session of cognitive behavior therapy (n=14)
This study was approved by the Institutional Review Board of St Mary’s Hospital, Catholic University of Korea. All participants signed a written informed consent form after receiving a full explanation of the procedure. All research was undertaken in accordance with the latest version of the Declaration of Helsinki.
Psychometric measures
Subject assessments were performed at baseline and at the end of CBT by a psychology staff member who was blinded to the study and trained in use of the scale. The severity of the panic disorder was measured using the Panic and Agoraphobic Scale (PAS).Citation24 The PAS is a 13-item scale that was developed to assess the severity of panic disorder and agoraphobia. It consists of five divisions of symptoms to determine the severity of the panic disorder: 1) frequency of panic attacks; 2) agoraphobic avoidance; 3) anticipatory anxiety; 4) family, social, and employment impairments; and 5) health worries. Each item is rated on a five-point scale (0–4), with higher scores representing greater severity. A total score (0–52) representing the panic severity is obtained by adding all of the item scores. The PAS and the Korean version of the PAS showed good psychometric reliability and validity.Citation24,Citation25 The Cronbach’s alpha for the Korean version of the PAS was 0.86.Citation25
The Anxiety Sensitivity Index-Revised (ASI-R) was used to assess the fear of anxiety related with the sensation that arises from the belief that these symptoms have harmful physical, psychological, or social consequences.Citation26 The ASI-R was developed by Taylor et al and retains the same instructions and response format as the ASI.Citation26 The ASI-R has 36 items to measure fear of cardiovascular, respiratory, gastrointestinal, publicly observable, dissociative, neurological, and cognitive dyscontrol anxiety symptoms. Each item is rated on a five-point scale (0–4) and the total score ranges from 0–144. The ASI-R and the Korean version of the ASI-R have demonstrated good psychometric properties.Citation26,Citation27 The internal consistency coefficient for the Korean version of the ASI-R was 0.92.Citation27
The Brief Bodily Sensations Interpretations Questionnaire (BBSIQ) is a shortened version of the Bodily Sensations Interpretation Questionnaire that was developed to evaluate the catastrophic misinterpretation of sensations by patients with panic disorder.Citation28 The BBSIQ consists of two subscales that contain seven ambiguous event items of panic body sensations and external events, respectively. The BBSIQ has two scales that yield a ranking and belief score for each item. The ranking scores are established from the order of negative responses among the three alternative response items: one reflects the catastrophic misinterpretation of patients, and the other two are either both neutral or they are neutral or positive. The belief scores represent the likelihood that interpretations are true for the patients on a scale from zero (not likely at all) to eight (extremely likely). The BBSIQ and the Korean version of the BBSIQ have shown good psychometric reliability and validity.Citation28,Citation29 The Cronbach’s alpha for the Korean version of the BBSIQ was within the range of 0.78 and 0.89, excluding the ranking score for the external event (Cronbach’s α =0.56).Citation29
The Anxiety Control Questionnaire (ACQ) is a 30-item self-report instrument that is designed to assess perceived control over emotional reactions and external threats.Citation30 Participants are instructed to respond to a six-point Likert-type scale (0= “strongly disagree” to 5= “strongly agree”) by indicating the degree of agreement with a particular statement (eg, “I am usually able to avoid a threat quite easily”). The ACQ score is the sum of all responses, with the total score (0–150) representing perceived control. The ACQ and the Korean version of the ACQ have shown good psychometric properties.Citation30,Citation31 The internal consistency coefficient for the Korean version of the ACQ is 0.83.Citation31
Cognitive behavioral therapy
The CBT provided to subjects was manual-guided group therapy which was based on the work of Barlow et al.Citation12 The CBT was given to groups of 8–12 patients for approximately 3 months in a 2 hours/week format. It consisted of several components that included psychoeducation (sessions 1–2), breathing retraining and muscle relaxation training (sessions 3–5), cognitive restructuring (sessions 6–8), and interoceptive and in vivo exposure (sessions 9–12). The CBT was provided, with the assistance of one or two other psychologists, by a psychiatrist specialized in CBT and internationally certified by the Academy of Cognitive Therapy.
Single photon emission computed tomography (SPECT) image acquisition
The SPECT was scheduled to be performed within the week before the beginning of CBT and within the week after the ending of CBT. Images were taken using a dual-head gamma camera (E-CAM, Siemens AG, Munich, Germany) with a low-energy fan-beam collimator 40 minutes after 740 MBq of technetium-99m-ethyl cysteinate dimer (Tc-99m-ECD) was infused intravenously. Post-CBT treatment brain perfusion SPECTs were performed under the same conditions.
Image processing and statistical analysis
The software for image manipulation included Matlab version 6.5 (Mathworks, Inc., Natick, MA, USA) and Statistical Parametric Mapping 2 (SPM2: Institute of Neurology, University College of London, London, UK). Each SPECT scan was then spatially normalized using a 12-parameter affine warp and sinc-linear interpolation to the SPECT template brain from the Montreal Neurological Institute (reformatted to a 16-bit image having 79 × 95 × 68 voxels, each 2 × 2 × 2 mm in size). These images were spatially smoothed using a Gaussian filter of 16 mm full width at half maximum. Normalized rCBF values were calculated by dividing the CBF at each voxel by the global CBF in each individual. As no comparisons reached significance when the corrected P-value (family-wised error) for multiple comparisons was made, the uncorrected P-value threshold and voxel of interest (VOI) analysis were applied. A paired t-test model was fitted, and a t-statistic image was constructed and then thresholded at t>3.14, corresponding to an uncorrected P<0.01 in conjunction with a cluster filter of 100 voxels in the reformatted template imaging space. In the exploratory analyses, a statistical probabilistic anatomic map (SPAM) of the International Consortium for Brain Mapping was applied to objectively draw VOIs.Citation32 A SPAM consists of 98 VOIs in a single image, in which each voxel has the probability of belonging to a specific VOI. After spatial normalization, the counts of each SPECT image were normalized using proportional scaling, with the mean counts of the cerebellum set at 50. The normalized counts were multiplied by the probability of the SPAM and were determined as the count of each VOI. The cerebral lobar counts were then calculated by averaging the counts of the VOIs that had been reclassified into each lobe. We assessed the intercorrelations between the clinical measures and the rCBF for regions that differed significantly. To compare the scores from each psychometric measure before and after CBT, the data were analyzed using a paired t-test. Statistical significance was defined at a level less than 0.05 using two-tailed tests.
Results
Clinical improvement after CBT
A significant reduction of symptoms was observed in patients who completed the full sessions of CBT (n=14) (). The total and several subscale scores for the PAS, including panic attack, anticipatory anxiety, disability, and worries about health, were significantly improved after CBT. The total, as well as all subscale scores of ASI-R and BBSIQ, except the external event belief scores for BBSIQ, decreased significantly, whereas the ACQ scores increased significantly after CBT.
Table 2 Changes of clinical measures before and after cognitive behavior therapy (n=14)
Changes in regional cerebral blood flow after CBT
When the rCBF of the subjects before and after CBT were compared, significant increases in the rCBF were detected in the left postcentral gyrus (Brodmann’s area [BA] 43), left precentral gyrus (BA 4), and inferior frontal gyrus (BA 9, BA 47). Significant decreases were detected in the left pons ( and ).
Figure 1 Brain areas with changes in regional cerebral blood flow (rCBF) after 12 weekly sessions of group cognitive behavioral therapy in patients with panic disorder (n=14). (A) 3D-rendered images of rCBF changes. Red, increased rCBF; green, decreased rCBF. (B) Red, areas of increased rCBF, postcentral gyrus of left parietal lobe, precentral gyrus of left frontal lobe and inferior frontal gyrus of left frontal lobe; blue, areas of decreased rCBF, sub-gyral white matter of left limbic lobe and pons.
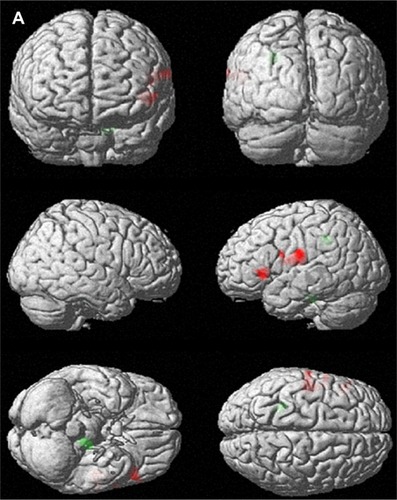
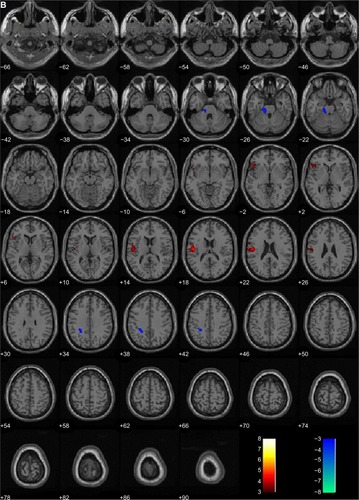
Table 3 Changes in regional cerebral blood flow in subjects after cognitive behavior therapy (n=14)
Relationship between regional cerebral blood flow and clinical measures
When correlation analysis was performed between changes in rCBF and clinical measures, no significant correlation was found between the changes in rCBF in VOIs and changes in total and subscale scores on the clinical measures.
Discussion
This study was performed to investigate neural correlates of the effects of CBT in patients with panic disorder. In the current study, we observed increased rCBF in the left inferior frontal gyrus, left postcentral gyrus, and left precentral gyrus, and decreased rCBF in the left pons after CBT. However, no significant correlation was found between changes in rCBF and in scores on the clinical measures.
Surprisingly, only a few studies using functional neuroimaging have been performed to investigate neural mechanisms underlying the effects of CBT in patients with panic disorder. Prasko et alCitation19 found that patients with panic disorder demonstrated metabolic increases in 18F-fluorodeoxyglucose (18FDG) PET (positron emission topography) after CBT in the right middle frontal gyrus, left inferior frontal gyrus, middle temporal gyrus, and insula, as well as metabolic decreases in the right inferior temporal gyrus, right inferior and superior frontal gyri, and left medial frontal gyrus. Sakai et alCitation20 investigated metabolic changes using 18FDG PET in 11 patients with panic disorder who showed improvement after the completion of CBT. They found decreases in glucose utilization in the right hippocampus, left anterior cingulate, left cerebellum, and the pons, and increases in the bilateral medial prefrontal cortices (mPFC). Recently, Kircher et alCitation18 tested this issue in a randomized controlled trial in 42 medication-free patients with panic disorder using fMRI (functional magnetic resonance imaging). They found reduced activation for the conditioned response in the left inferior frontal gyrus after CBT, and this activation reduction was correlated with reduction in agoraphobic symptoms. They also found increased connectivity between the inferior frontal gyrus and other regions including amygdalae, insulae, and the anterior cingulate cortex.
Similar to previous findings, we found increased activity in the left inferior frontal gyrus (BA 9 and BA 47) after CBT. Lesions in this area are suggested to enhance fear reactivity to both the conditioned and contextual stimuli, and the efferent projections from this area are suggested to target the periaqueductal gray matter and hypothalamus, to attenuate related autonomic responses and emotional behaviors.Citation2 This region is also associated with perceptive changes in motivational value of stimuli, and activation of this region is found when participants must inhibit or select from among competing responses.Citation33 The insular cortex, which has bidirectional connections to the amygdala and prefrontal cortex and which relays information on interoceptive states to the prefrontal cortex, serves as an internal alarm center that alerts the individual to potentially distressing stimuli.Citation34 Functional neuroimaging studies have shown that activity changes develop in the insula during panic attacks induced by lactate or CCK-4.Citation35,Citation36 It has been suggested that panic disorder develops partly because panic attacks cause the conditioning of anxiety to exteroceptive and interoceptive cues.Citation4 Some portion of CBT for panic disorder focuses on eliminating this conditioning by desensitizing somatic and physical cues for panic attack.Citation37 Therefore, the increased activation in inferior frontal gyrus in the current study might reflect continuous attempts to reverse previous learning of a reinforced response to stimuli and the down-regulation of negative emotions from these exteroceptive and interoceptive cues via cognitive reappraisal.Citation38
However, there were negative findings, such as the lack of rCBF change in regions of “fear network” (amygdalae, insulae, thalamus, etc) and correlations between rCBF and symptom severity. The reason for these negative findings is not clear. Eleven of the 14 subjects in the current study were being treated with medications prior to the onset of CBT, although their medication was maintained without change during the study period. Medications, especially SSRIs, change the activity of various brain stem nuclei such as the locus ceruleus, periaqueductal gary, and hypothalamus with their treatment effects.Citation39 Besides, SSRIs may affect the central nucleus of the amygdala itself and modulate excitatory input from the thalamic and cortical pathway.Citation40 Gorman et alCitation14 suggested that there could be distinct pathways through which medication and psychotherapy can exert their therapeutic effects in panic disorder. They hypothesized that medications could reduce panic attacks by decreasing the activity of the amygdala and its ability to stimulate projection sites in the hypothalamus and brainstem. On the other hand, psychotherapy such as CBT might operate upstream from the amygdala, reducing phobic avoidance by deconditioning contextual fear learned at the level of the hippocampus and decreasing cognitive misattribution and abnormal emotional reactions by strengthening the ability to inhibit the amygdala. Prior use of medication in the current study may have influenced the limbic area independently of CBT, and may have affected the finding of no significant activity change in these areas. This result also suggests that CBT might be effective through inducing adaptive consolidation of the prefrontal cortex which exerts inhibitory control over the amygdala, rather than affecting it directly.Citation14,Citation41,Citation42 CBT is a multicomponential intervention, but because clinical presentations vary considerably between patients, it is difficult to determine which component may be responsible for observed effects.Citation18 Previous findings from intervention studies have shown the difficulty of directly linking their findings to prevailing models of underlying neural mechanisms. Prasko et alCitation19 found no significant changes in brain metabolism in limbic regions after symptom alleviations by CBT and failed to detect different neural correlates between the treatment with CBT and with antidepressants. In the study by Kircher et al,Citation18 there was a negative correlation between symptom severity and connectivity of the inferior frontal gyrus to the medial frontal cortex and anterior cingulated cortex after CBT. There was no significant interaction of group and time, suggesting that CBT might not have a specific impact on connectivity. These findings suggested that behavioral effects of treatment do not always correspond to neural findings.Citation21
This study also has other limitations. The sample size was small, which restricts the statistical power and generality of the results. The results could not be confirmed as exact treatment effects of CBT because of the lack of proper control subjects such as a waiting list and/or nonspecific nonpharmacological treatment group. Lack of a control group could further limit the interpretation of the current negative findings for the correlation analysis between rCBF and each clinical measure. Because many subjects had been treated with medication prior to the CBT sessions, and although their medication was maintained without change during the study period, our results might represent not only the added effects of medication, but also novel results arising from the combination of both treatments. Although all subjects were in a resting state and tolerated the SPECT scanning well, anxiety during scanning was not assessed, and anticipatory anxiety may have affected the results of rCBF in the current study. The uncorrected P-value threshold of 0.01 was applied in the present study. When the P-value of 0.005 was tried, no significant rCBF change was found. Fundamental limitations, such as small sample size, maintenance of medication, imaging data acquisition during resting state but not during fear conditioning paradigm, and the lower resolution power of SPECT compared to MRI or PET are suggested to limit the detectability of the current study for the impact of CBT. Although threshold P<0.01 uncorrected has been applied in several SPECT studies,Citation43,Citation44 the low statistical power might increase the risk of false positive results.
Future imaging studies should investigate functional changes in a placebo group, subjects treated only with medication, and subjects treated only with CBT, along with subjects receiving both treatments with high statistical power. Additionally, any correlations between the functional changes in the brain and different clinical measures for each treatment group and the changes by CBT for each of the drug-naïve subjects and the drug-discontinued subjects should be investigated.
Acknowledgments
This work was supported by Seoul R&BD Program (SS110008) and Korea Research Foundation. The authors thank Young-Eun Jung for her assistance with preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th edWashington, DCAmerican Psychiatric Association1994
- Roy-ByrnePPCraskeMGSteinMBPanic disorderLancet20063681023103216980119
- ReissSPetersonRAGurskyDMMcNallyRJAnxiety sensitivity, anxiety frequency and the prediction of fearfulnessBehav Res Ther198624183947307
- BoutonMEMinekaSBarlowDHA modern learning theory perspective on the etiology of panic disorderPsychol Rev200110843211212632
- ClarkDMA cognitive approach to panicBehav Res Ther1986244614703741311
- AndrisanoCChiesaASerrettiANewer antidepressants and panic disorder: a meta-analysisInt Clin Psychopharmacol201328334523111544
- BallengerJCBurrowsGDDuPontRLJrAlprazolam in panic disorder and agoraphobia: results from a multicenter trial. I. Efficacy in short-term treatmentArch Gen Psychiatry1988454134223282478
- RosenbaumJFMorozGBowdenCLClonazepam in the treatment of panic disorder with or without agoraphobia: a dose-response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose-Response Study GroupJ Clin Psychopharmacol1997173904009315990
- SimonNMSafrenSAOttoMWSharmaSGLankaGDPollackMHLongitudinal outcome with pharmacotherapy in a naturalistic study of panic disorderJ Affect Disord20026920120812103467
- ToniCPerugiGFrareFA prospective naturalistic study of 326 panic-agoraphobic patients treated with antidepressantsPharmacopsychiatry20003312113110958260
- PollackMHOttoMWTesarGECohenLSMeltzer-BrodySRosenbaumJFLong-term outcome after acute treatment with alprazolam or clonazepam for panic disorderJ Clin Psychopharmacol1993132572638376613
- BarlowDHGormanJMShearMKWoodsSWCognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trialJAMA20002832529253610815116
- LoerchBGraf-MorgensternMHautzingerMRandomised placebo-controlled trial of moclobemide, cognitive-behavioural therapy and their combination in panic disorder with agoraphobiaBr J Psychiatry199917420521210448444
- GormanJMKentJMSullivanGMCoplanJDNeuroanatomical hypothesis of panic disorder, revisedAm J Psychiatry200015749350510739407
- ErenITükelRPolatAKaramanRUnalSEvaluation of regional cerebral blood flow changes in panic disorder with Tc99m-HMPAO SPECTPsychiatry Res200312313514312850252
- GarakaniABuchsbaumMSNewmarkREThe effect of doxapram on brain imaging in patients with panic disorderEur Neuropsychopharmacol20071767268617560768
- VythilingamMAndersonERGoddardATemporal lobe volume in panic disorder – a quantitative magnetic resonance imaging studyPsychiatry Res200099758210963983
- KircherTAroltVJansenAEffect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorderBiol Psychiatry2013739310122921454
- PraskoJHorácekJZáleskýRThe change of regional brain metabolism (18FDG PET) in panic disorder during the treatment with cognitive behavioral therapy or antidepressantsNeuro Endocrinol Lett20042534034815580167
- SakaiYKumanoHNishikawaMChanges in cerebral glucose utilization in patients with panic disorder treated with cognitive-behavioral therapyNeuroimage20063321822616889985
- ShurickAAGrossJJEmotional reactivity and regulation in panic disorder: insights from a functional magnetic resonance imaging study of cognitive behavioral therapyBiol Psychiatry2013735623217459
- SheehanDVLecrubierYSheehanKHThe Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10J Clin Psychiatry199859Suppl 2022339881538
- YooSWKimYSNohJSValidity of Korean version of the mini-international neuropsychiatric interviewAnxiety and Mood200625055
- BandelowBAssessing the efficacy of treatments for panic disorder and agoraphobia. II. The Panic and Agoraphobia ScaleInt Clin Psychopharmacol19951073817673659
- ChoiYSEffects of Group Cognitive Behavioral Therapy on Body Sensation Interpretation, Anxiety Control and Panic/Agoraphobic Symptoms in Patients with Panic Disorder. [dissertation]SeoulCatholic University2007
- TaylorSCoxBJAn expanded anxiety sensitivity index: evidence for a hierarchic structure in a clinical sampleJ Anxiety Disord19981254634839801964
- KimJHYuBHOhKSA validation study of Korean Anxiety Sensitivity Index – Revised (ASI-R)J Korean Neuropsychiatr Assoc2004435461
- ClarkDMSalkovskisPMOstLGMisinterpretation of body sensations in panic disorderJ Consult Clin Psychol1997652032139086683
- KimSJReliability and Validity of the Korean Version of the Brief Body Sensation Interpretation questionnaire. [dissertation]ChuncheonHallym University2006
- RapeeRMCraskeMGBrownTABarlowDHMeasurement of perceived control over anxiety-related eventsBehav Ther199627279293
- ChoYRKimEJPsychometric properties of the Korean version of the Anxiety Control QuestionnaireKorean J Clin Psychol2004232503518
- ChungYAOJHKimJYKimKJAhnKJHypoperfusion and ischemia in cerebral amyloid angiopathy documented by 99mTc-ECD brain perfusion SPECTJ Nucl Med2009501969197419910418
- O’DohertyJKringelbachMLRollsETHornakJAndrewsCAbstract reward and punishment representations in the human orbitofrontal cortexNat Neurosci200149510211135651
- PaulusMPSteinMBAn insular view of anxietyBiol Psychiatry20066038338716780813
- JavanmardMShlikJKennedySHVaccarinoFJHouleSBradwejnJNeuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time pointsBiol Psychiatry19994587288210202575
- ReimanEMRaichleMERobinsENeuroanatomical correlates of a lactate-induced anxiety attackArch Gen Psychiatry1989464935002786401
- BarlowDHCognitive-behavioral therapy for panic disorder: current statusJ Clin Psychiatry199758Suppl 232369078992
- OchsnerKNRayRDCooperJCFor better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotionNeuroimage20042348349915488398
- CoplanJDPappLAPineDClinical improvement with fluoxetine therapy and noradrenergic function in patients with panic disorderArch Gen Psychiatry1997546436489236548
- StutzmannGELeDouxJEGABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioningJ Neurosci199919RC810341269
- LindenDEHow psychotherapy changes the brain – the contribution of functional neuroimagingMol Psychiatry20061152853816520823
- SchienleASchäferAStarkRVaitlDLong-term effects of cognitive behavior therapy on brain activation in spider phobiaPsychiatry Res20091729910219321317
- KimBNLeeJSShinMSChoSCLeeDSRegional cerebral perfusion abnormalities in attention deficit/hyperactivity disorder. Statistical parametric mapping analysisEur Arch Psychiatry Clin Neurosci200225221922512451463
- McGoronAJCapilleMGeorgiouMFPost traumatic brain perfusion SPECT analysis using reconstructed ROI maps of radioactive microsphere derived cerebral blood flow and statistical parametric mappingBMC Med Imaging2008841518312639
