Abstract
We propose the possibility of 5-hydroxytryptamine (5-HT)1A receptor involvement in mild serotonin toxicity. A 64-year-old woman who experienced hallucinations was treated with perospirone (8 mg/day). She also complained of depressed mood and was prescribed paroxetine (10 mg/day). She exhibited finger tremors, sweating, coarse shivering, hyperactive knee jerks, vomiting, diarrhea, tachycardia, and psychomotor agitation. After the discontinuation of paroxetine and perospirone, the symptoms disappeared. Another 81-year-old woman, who experienced delusions, was treated with perospirone (8 mg/day). Depressive symptoms appeared and paroxetine (10 mg/day) was added. She exhibited tachycardia, finger tremors, anxiety, agitation, and hyperactive knee jerks. The symptoms disappeared after the cessation of paroxetine and perospirone. Recently, the effectiveness of coadministrating 5-HT1A agonistic psychotropics with selective serotonin reuptake inhibitors (SSRIs) has been reported, and SSRIs with 5-HT1A agonistic activity have been newly approved in the treatment of depression. Perospirone is a serotonin–dopamine antagonist and agonistic on the 5-HT1A receptors. Animal studies have indicated that mild serotonin excess induces low body temperature through 5-HT1A, whereas severe serotonin excess induces high body temperature through 5-HT2A activation. Therefore, it could be hypothesized that mild serotonin excess induces side effects through 5-HT1A, and severe serotonin excess induces lethal side effects with hyperthermia through 5-HT2A. Serotonin toxicity via a low dose of paroxetine that is coadministered with perospirone, which acts agonistically on the 5-HT1A receptor and antagonistically on the 5-HT2A receptor, clearly indicated 5-HT1A receptor involvement in mild serotonin toxicity. Careful measures should be adopted to avoid serotonin toxicity following the combined use of SSRIs and 5-HT1A agonists.
Introduction
Serotonin toxicity (or serotonin syndrome) is the result of excessive serotonin activity caused by the administration of selective serotonin reuptake inhibitors (SSRIs), often in combination with serotonin receptor agonists.Citation1 Serotonin toxicity is characterized by myoclonus, hyperreflexia, autonomic nervous symptoms, and changes in mental status.Citation1 Given that an altered mental status, which includes agitation and anxiety, is common in depression, patients treated with SSRIs should be carefully examined for the presence of hyperreflexia. Two serotonin receptor subtypes are candidates for the underlying pathophysiology of serotonin toxicity.Citation1 However, the different effects of the stimulation of the 5-hydroxytryptamine (5-HT)1A and 5-HT2A receptors on the clinical exacerbation of serotonin toxicity have not yet been elucidated. In the present report, we suggest the possible involvement of the 5-HT1A receptor in mild serotonin toxicity without hyperthermia.
Case report
Case 1
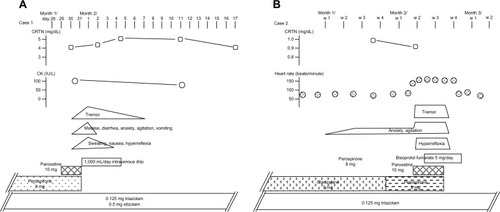
A 64-year-old woman who experienced auditory hallucinations and insomnia, was treated with hypnotic benzodiazepines and perospirone (8 mg/day; ). Perospirone had been administered for 4 months. She had developed chronic renal failure, which had continued for several years, with high serum levels of creatinine and blood urea nitrogen (). She also experienced depressed mood, diminished interest, and anhedonia; therefore, she was treated with paroxetine (10 mg/day). Eighteen hours after paroxetine was added to her ongoing perospirone treatment, she exhibited finger tremors, sweating, coarse shivering, hyperactive knee jerks, vomiting, diarrhea, tachycardia, and psychomotor agitation. After administrating paroxetine with perospirone twice, both paroxetine and perospirone were discontinued. Her symptoms gradually disappeared over a period of approximately 1 week.
Figure 1 Measured concentrations of serum CRTN, CK, side effect symptoms, and administered drugs for cases 1 and 2.
Abbreviations: CRTN, creatinine; CK, creatine phosphokinase; w, week; CYP, cytochrome P450.
Case 2
An 81-year-old woman who experienced persecutory delusions with mild dementia, was treated with neuroleptics (). She was admitted to a psychiatric unit, owing to increased hostility and aggression related to her delusions. The results of brain imaging tests revealed mild brain atrophy in the frontotemporal and hippocampal regions with no cerebrovascular lesions. After successful treatment of her paranoid state, the patient developed depressive symptoms, which were treated with 10 mg/day of paroxetine. The final neuroleptic dose was 8 mg/day of perospirone that had been administered for 3 weeks. Fourteen hours after the addition of paroxetine to the patient’s ongoing perospirone treatment, she exhibited tachycardia, finger tremors, prominent anxiety and agitation, and hyperactive knee jerks. The symptoms continued for days (ie, for as long as perospirone and paroxetine were coadministered), and they disappeared 3 days after the discontinuation of both drugs.
Discussion
In the present report, both patients were diagnosed as having serotonin toxicity because of the existence of tremor and hyperreflexia,Citation2 and they presented without symptoms of muscle rigidity or hyperthermia.Citation1 The possibility of neuroleptic malignant syndrome was not taken into consideration as a differential diagnosis, because the symptoms appeared just after adding paroxetine; moreover, the present cases did not exhibit severe muscle rigidity and hyperthermia.Citation3 In addition, there was no elevation of serum creatine phosphokinase in case 1. The autonomic symptoms, neuromuscular hyperactivity, and altered mental status occurred upon initiation of the serotonergic drugs, and they ceased promptly after the treatment’s discontinuation; this is characteristic of serotonin toxicity.Citation4
Paroxetine is metabolized by cytochrome P450 oxidase (CYP)2D6 in the liver and excreted in the urine. Patients with renal failure are considered to be at an increased risk of developing side effects associated with a high serum concentration of paroxetine.Citation5 The psychiatric diagnosis of Case 1 was psychotic disorder not otherwise specified, and there was no evidence of a relationship between her renal insufficiency and her psychiatric symptoms or her serotonin toxicity symptoms.
Case 2 was diagnosed as having dementia with psychotic symptoms, including persecutory delusions, anxiety, and depressive symptoms. In this case, the serotonin toxicity persisted for days because of the unfortunate oversight of a possible serotonin toxicity induced by the administered psychotropics. The increased anxiety and agitation were believed to be derived from the behavioral and psychological symptoms of dementia. Tachycardia was misdiagnosed as a result of the increased motor activity associated with her anxiety and agitation. Serum paroxetine concentrations were not evaluated in either case presented here, and the possibility remains that the paroxetine concentration was sufficiently elevated in both cases. We considered that vulnerability induced by perospirone pretreatment is most likely to have exaggerated the mild serotonin toxicity in both cases.
Perospirone is a serotonin–dopamine antagonist with unique agonistic effects on the 5-HT1A receptors,Citation6 and is metabolized by CYP3A4.Citation7 It was the second serotonin–dopamine antagonist after risperidone to be introduced to the Japanese neuroleptics market in 2001. The receptor binding profiles of perospirone (excluding 5-HT1A receptor binding) and its pharmacological properties targeting the positive and negative symptoms of schizophrenia resemble those of risperidone.Citation7 The Ki values of perospirone for the dopamine receptor D2, as well as for the 5-HT2A and 5-HT1A receptors are 1.4 nM, 0.6 nM, and 2.9 nM, respectively,Citation7 whereas those of risperidone are 3.3 nM, 0.16 nM, and 250 nM, respectively.Citation8 Data on the Ki values measured from various tissue sources can be viewed in the National Institute of Mental Health Psychoactive Drug Screening Program Ki database (http://pdsp.med.unc.edu/pdsp.php) by choosing a psychotropic drug for the test ligand. The 5-HT1A agonist activity of neuroleptics is expected to improve moodCitation9 and cognitionCitation10 in schizophrenia. Perospirone has also shown efficacy in reducing aggressive behavior in dementia patients.Citation11 To treat the symptoms of aggression and anxiety exhibited by Case 2, previously administered olanzapine was switched to perospirone 10 days before the addition of paroxetine.
Two serotonergic receptor subtypes have been implicated in the etiology of serotonin toxicity. When experimental animals were administered a 5-HT1A agonist, they developed tremors, forepaw treading, head-weaving and twitches, flattened body posture, hind limb abduction, the Straub tail reaction, hyperhidrosis, and defecation.Citation12 The similarities noted between the alterations in animal behavior and the symptoms of human serotonin toxicity suggest that the 5-HT1A receptor may be involved in the pathogenesis of serotonin toxicity. In another experimental animal model of serotonin toxicity, the activation of neuronal 5-HT2A receptors was identified as the cause of life-threatening hyperthermia.Citation13 Furthermore, the serotonin system has been implicated in a biphasic mechanism that controls thermoregulation. Studies in experimental animals have indicated that a slight elevation of serotonin levels decreases body temperature through neural transmission involving the 5-HT1A receptors, whereas the considerable elevation of serotonin levels induces high body temperature through 5-HT2A neural transmission.Citation14,Citation15 Therefore, it could be hypothesized that while a slight excess of serotonin causes side effects by activating the 5-HT1A receptor, a significant increase in serotonin levels can induce lethal side effects with hyperthermia, which can cause severe brain damageCitation16 through the activation of the 5-HT2A receptor. The early observationCitation17 that the affinity of endogenous serotonin for the 5-HT1A receptors (Ki =5.1 nM) is much higher than its affinity for 5-HT2A receptors (Ki =420 nM) supports this hypothesis.
Vulnerabilities to serotonin toxicity are increased by a polymorphism in the serotonin transporter or CYP, a competitive substrate of the CYP enzyme,Citation18 and by coadministered monoamine oxidase inhibitors or lithium.Citation19 The present cases underscore the importance of preventing the development of serotonin toxicity after the combined use of SSRIs and 5-HT1A agonistic drugs, even when a small amount of SSRI is administered.
Several case studies have reported the development of serotonin toxicity by the combined use of SSRIs and 5-HT1A agonist anxiolytics;Citation20,Citation21 however, high body temperatures were not observed in these studies. The exacerbation of serotonin toxicity in the present cases by adding small doses of paroxetine while administering perospirone – acting both as a 5-HT1A receptor agonist and a 5-HT2A receptor antagonist – clearly indicates the involvement of the 5-HT1A receptor in mild forms of serotonin toxicity. Although the involvement of both 5-HT1A and 5-HT2A receptors in the pathogenesis of serotonin toxicity has often been indicated,Citation22 we considered it worthwhile to pay attention to serotonin toxicity profiles under the situation of an antagonistic blockade of 5-HT2A receptors in human subjects.
Taken together, the coadministration of SSRIs and neuroleptics, like risperidone, with a potent 5-HT2A antagonistic function and a scarce 5-HT1A agonistic function would produce a lower incidence of serotonin toxicity. The coadministration of SSRIs with psychotropics possessing 5-HT1A agonist properties has received increasing attention. A recent clinical trialCitation23 indicated the effectiveness of adding a 5-HT1A partial agonist for the treatment of patients with depression who are resistant to citalopram. Mirtazapine, which acts as a 5-HT1A agonist in addition to its norepinephrine-releasing function, has been proposed to enhance the clinical effectiveness of SSRIs.Citation24 Furthermore, aripiprazole, which acts as a 5-HT1A agonist, is used to augment the clinical effectiveness of SSRI treatment.Citation25 Considering that recently available SSRIs, including vilazodoneCitation26 and vortioxetine,Citation27 have considerable 5-HT1A agonistic function, more caution is needed to detect serotonin toxicity in depression treatments.
In conclusion, the possible development of serotonin toxicity should be considered when neuromuscular toxicities including tremor, myoclonus, and hyperreflexia in the extremities are observed during the combined use of SSRIs and psychotropics with 5-HT1A agonistic properties, or during the use of SSRIs with 5-HT1A agonistic properties.
Acknowledgments
The authors would like to thank Dr Akira Monji at Saga University (Saga, Japan) for his suggestions.
Disclosure
The authors report no conflicts of interest in this work.
References
- BoyerEWShannonMThe serotonin syndromeN Engl J Med2005352111112112015784664
- DunkleyEJIsbisterGKSibbrittDDawsonAHWhyteIMThe Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicityQJM200396963564212925718
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV TR)Washington DCAmerican Psychiatric Association2000795798
- IsbisterGKBuckleyNAWhyteIMSerotonin toxicity: a practical approach to diagnosis and treatmentMed J Aust2007187636136517874986
- HerrKDNemeroffCBParoxetineSchatzbergAFNemeroffCBThe American Psychiatric Publishing Textbook of Psychopharmacology3rdArlington, VAAmerican Psychiatric Publishing2004259281
- OdagakiYToyoshimaR5-HT1A receptor agonist properties of antipsychotics determined by [35S]GTPgammaS binding in rat hippocampal membranesClin Exp Pharmacol Physiol2007345–646246617439416
- IshibashiTOhnoYPerospirone hydrochloride: the novel atypical antipsychotic agent with high affinities for 5-HT2, D2 and 5-HT1A receptorsBiogenic Amines200418307311
- SchotteAJanssenPFGommerenWRisperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor bindingPsychopharmacology (Berl)19961241–257738935801
- Newman-TancrediAThe importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectivesCurr Opin Investig Drugs2010117802812
- MeltzerHYSumiyoshiTDoes stimulation of 5-HT(1A) receptors improve cognition in schizophrenia?Behav Brain Res200819519810218707769
- SatoSMizukamiKMoroKTanakaYAsadaTEfficacy of perospirone in the management of aggressive behavior associated with dementiaProg Neuropsychopharmacol Biol Psychiatry200630467968316581169
- SmithLMPeroutkaSJDifferential effects of 5-hydroxytryptamine1a selective drugs on the 5-HT behavioral syndromePharmacol Biochem Behav1986246151315192942947
- NisijimaKShiodaKIwamuraTNeuroleptic malignant syndrome and serotonin syndromeProg Brain Res20071628110417645916
- Abdel-FattahAFMatsumotoKel-HadyKAWatanabeH5-HT1A and 5-HT2 receptors mediate hypo- and hyperthermic effects of tryptophan in pargyline-pretreated ratsPharmacol Biochem Behav19955223793848577805
- NisijimaKYoshinoTYuiKKatohSPotent serotonin (5-HT) (2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndromeBrain Res20018901233111164765
- SlettedalJKNilssenDOMagelssenMLøbergEMMaehlenJBrain pathology in fatal serotonin syndrome: presentation of two casesNeuropathology201131326527020880320
- DalpiazAGessiSBoreaPAGilliGBinding thermodynamics of serotonin to rat-brain 5-HT1A, 5HT2A and 5-HT3 receptorsLife Sci19955712PL141PL1467674802
- TanakaANagamatsuTYamaguchiMMyoclonus after dextromethorphan administration in peritoneal dialysisAnn Pharmacother2011451e121228393
- KalueffAVLaPorteJLMurphyDLPerspectives on genetic animal models of serotonin toxicityNeurochem Int2008524–564965817935833
- LauterbachECFluoxetine, buspirone, myoclonus, and dystoniaAm J Psychiatry19951521116977485652
- ManosGHPossible serotonin syndrome associated with buspirone added to fluoxetineAnn Pharmacother2000347–887187410928399
- OdagakiYAtypical neuroleptic malignant syndrome or serotonin toxicity associated with atypical antipsychotics?Curr Drug Saf200941849319149529
- TrivediMHFavaMWisniewskiSRSTAR*D Study TeamMedication augmentation after the failure of SSRIs for depressionN Engl J Med2006354121243125216554526
- CarpenterLLYasminSPriceLHA double-blind, placebo-controlled study of antidepressant augmentation with mirtazapineBiol Psychiatry200251218318811822997
- WorthingtonJJ3rdKinrysGWygantLEPollackMHAripiprazole as an augmentor of selective serotonin reuptake inhibitors in depression and anxiety disorder patientsInt Clin Psychopharmacol200520191115602109
- HeinrichTBöttcherHGerickeRSynthesis and structure – activity relationship in a class of indolebutylpiperazines as dual 5-HT(1A) receptor agonists and serotonin reuptake inhibitorsJ Med Chem200447194684469215341484
- Bang-AndersenBRuhlandTJørgensenMDiscovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorderJ Med Chem20115493206322121486038
