Abstract
Background
This study aims to determine the effectiveness of blonanserin (BNS) on the cognitive and social functions of patients with schizophrenia compared with risperidone (RIS) during acute-phase (8-week) treatment.
Methods
A total of 39 schizophrenia inpatients were included in this study. The subjects received either BNS (N=20) or RIS (N=19), and the clinical responses were evaluated periodically. The concomitant use of mood stabilizers was not allowed. Efficacy was assessed with the Positive and Negative Syndrome Scale for schizophrenia. Cognition was assessed using the Brief Assessment of Cognition in Schizophrenia, Japanese-language version. Social function was assessed using the Life Assessment Scale for the Mentally Ill.
Results
For both groups, each assessment exhibited a decrease in the mean change from baseline on the Positive and Negative Syndrome Scale. The depression subscale was significantly improved in the BNS group compared with the RIS group at 8 weeks after administration. BNS improved verbal fluency and executive function (cognitive function) and daily living and work skills (social function). Compared with the RIS group, BNS was observed to improve daily living.
Conclusion
BNS may improve psychotic symptoms, cognitive function, and daily living in patients with acute-phase schizophrenia. BNS may be superior to RIS in the improvement of daily living.
Introduction
Cognitive improvements in patients with schizophrenia are strongly associated with quality of life and independent living, whereas the successful treatment of positive symptoms has not been demonstrated to significantly improve employment status or social relationships.Citation1 A number of studies have claimed cognitive benefits from treatment with various atypical antipsychotics; however, the pattern and degree of cognitive improvement differ from drug to drug.Citation2–Citation6 The study of social cognition in schizophrenia has increased rapidly during the past decade. Schizophrenia patients exhibit impairments in both low- and high-level social cognitive processes,Citation7–Citation10 and their impaired social cognition is consistently related to functional outcome.Citation11,Citation12
Blonanserin (BNS) was developed in Japan as a novel antipsychotic drug,Citation13,Citation14 and it was approved for the treatment of schizophrenia in Japan and Korea.Citation15 BNS has a high affinity for the dopamine D2,3 and serotonin 5-HT2A receptors, but low affinity for the D1,4,5, adrenergic α1,2, β, 5-HT1A, 5HT2B, 5HT2C, 5HT3–7, histamine H1, and muscarinic M1 receptors.Citation14,Citation15 A preclinical study demonstrated that BNS increased the extracellular levels of dopamine and norepinephrine in the prefrontal cortex.Citation16 In a recent meta-analysis, the effect of BNS was demonstrated to be equal to that of haloperidol and risperidone (RIS) in primary endpoints and superior to haloperidol in improving negative symptoms in patients with schizophrenia.Citation17 Moreover, BNS improved verbal fluency and executive function with first-episode schizophrenia.Citation18 However, to our knowledge, no study has evaluated the effects of BNS on the cognitive and social functions of patients with acute-phase schizophrenia. Furthermore, in the absence of direct comparisons with RIS, it remains difficult to reach a final verdict on the potential additional therapeutic benefits of BNS. Therefore, we examined BNS’s effectiveness on cognitive and social function in acute-phase schizophrenia by comparing it with that of RIS.
Methods
Subjects
Thirty-nine inpatients (18 males and 21 females) were included in this study. Twenty were receiving BNS treatment and 19 were receiving RIS treatment as a control group. All of the patients met the diagnostic criteria for schizophrenia based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Test Revision.Citation19 Patients with concomitant medical illness (for example, diabetes, high blood pressure, hypothyroidism, or a chronic respiratory condition) were eligible for participating in the study if their condition had been stable for at least 3 months and they had been receiving standard therapy for the condition for at least 1 month.
Patients were excluded for any untreated or unstable clinically significant medical condition, or for any clinically significant laboratory or physical examination abnormality or thyroid function abnormality. A history of seizures, recent drug or alcohol abuse, or any principal psychiatric condition other than schizophrenia were reasons for exclusion, as was a suicide attempt in the current psychotic episode. Patients were excluded if they had received RIS or BNS for the current psychotic episode or electroconvulsive therapy during the previous 6 months. In addition, patients were excluded if they required concomitant therapy with drugs approved for the treatment of memory deficits. Patients who did not tolerate or respond to RIS or BNS during a previous psychotic episode were ineligible. Additionally, patients who had failed more than one adequate trial of antipsychotic treatment for the current psychotic episode were excluded.
All of the subjects who participated in this study were inpatients. Treatment compliance for all of the subjects was confirmed by a nurse.
Study design
Twenty patients were recruited to the present study and assigned to the acute-phase schizophrenia. Patients were administered BNS monotherapy for 8 weeks. The daily doses of the drug were individually adjusted according to the patient’s clinical status, and no additional drugs, except lorazepam, were permitted during the study period.
The clinical improvement of the patients was evaluated using the Positive and Negative Syndrome Scale (PANSS)Citation20 on days 0, 28 (4 weeks), and 56 (8 weeks). Cognitive function and social functions were measured using the Brief Assessment of Cognition in schizophrenia, Japanese language version (BACS-J) and the Life Assessment Scale for the Mentally Ill (LASMI) on days 0 and 56 (8 weeks).
To exclude possible learning effects on BACS-J, 19 patients with acute-phase schizophrenia as a RIS group. The RIS group’s dosage and additional drugs were performed on the same conditions as BNS group. The raters were blinded about the treatment status.
Patients with at least a 30% or more decrease in their baseline PANSS scores were defined as responders, whereas those with less than a 30% decrease were regarded as nonresponders. The protocol for this study was approved by the Ethics Committee of the University of Occupational and Environmental Health (Kitakyushu, Fukuoka, Japan). All participants provided their consent to participate after being informed of the study’s purpose.
Cognitive functions, social functions, intelligence test, and clinical assessment
The primary outcome measures were the changes in cognitive functions and social functions from baseline to the endpoint. The secondary outcome measures were changes in psychiatric symptoms and the severities of the psychopathologies. The cognitive functions were assessed by trained psychiatrists using the BACS-J.Citation21 The BACS-J has established reliability and validity and is designed to measure cognitive function in schizophrenia.Citation21,Citation22 The metric includes brief assessments of verbal memory, working memory, motor speed, verbal fluency, attention and processing speed, and executive function. The primary measures from each BACS-J subtest were standardized by creating z-scores (the mean of healthy controls was set to 0, and the standard deviation was set to 1). All of the data from the healthy controls were obtained from a study by Kaneda et al,Citation23 and a composite score was calculated by averaging all of the z-scores for the six primary measures. The influence of age was adjusted using age-matched cohorts of controls to calculate the BACS-J z-scores for each schizophrenia patient in the present study.
We assessed functional outcomes in this study. The LASMI was developed to assess disability in daily life or community functioning,Citation24–Citation26 and it is one of the most commonly used scales to evaluate community functioning in Japan. The LASMI is composed of the following five categories: 1) daily living; 2) interpersonal relations; 3) work skills; 4) endurance and stability; and 5) self-recognition. Each category consists of several items, with each item being rated on a 5-point scale (responses range from no problem =0 to a serious problem =4). Lower scores indicate higher degrees of independent living in the community. The mean score for each category was calculated by dividing the total score for that category by the number of items. The LASMI scores were assigned based on observations of patient behavior and information from the patients and their families.
Statistical analysis
Differences between the RIS and BNS groups in terms of demographic and baseline characteristics were assessed using independent samples t-tests and the chi-square test.
This study’s primary aim was to clarify the effects of RIS and BNS on cognitive and social function, as measured by the BACS-J and the LASMI. A repeated measures analysis of covariance was performed for each cognitive and social variable with the baseline data serving as the covariate. For the primary analysis, the between-subjects factor was the group (RIS group and BNS group) and the within-subjects factor was time (day 0 and day 56). The effects of group, time, and group-by-time (interaction effect) were examined. Additionally, we used a Bonferroni correction for multiple comparisons of the BACS-J and LASMI data. In a secondary analysis, within-group improvements in cognitive performance and social function over time were evaluated using paired t-tests. All statistical tests were two-tailed, and a P-value <0.05 was considered significant. Effect size (Cohen’s d) was calculated as the within-group differences between the mean values divided by the pooled standard deviation.
Results
A total of 20 patients in the BNS group and 19 patients in the RIS group were recruited and tested at baseline. Of these, 33 patients completed this study (BNS, number [n]=17; RIS, n=16). Baseline demographics or clinical characteristics were comparable between treatment groups (). During the acute treatment phase, three patients in the BNS group (15.0%) and three patients in the BNS group (15.8%) discontinued their participation prematurely, most of them because of adverse events (). Seventeen in the BNS and 16 in the RIS groups were retested on the cognitive measures after 8 weeks. Thus, data from these 17 patients in the BNS group and 16 patients in the RIS group were used for a more complete analysis.
Figure 1 Patient disposition (number of patients who were assigned, treated, and who completed the treatment with the reasons for discontinuation).
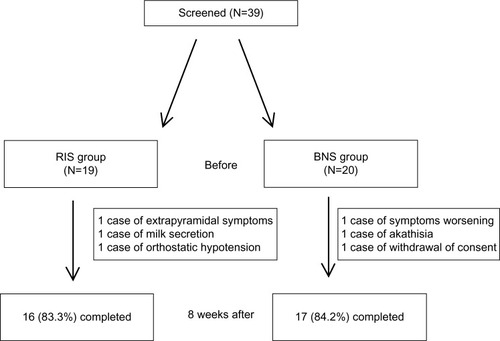
Table 1 Demographic and clinical characteristics of the patient sample at baseline
Five patients in the RIS group and 12 in the BNS group used lorazepam for sleep during the trial.
Eight weeks into treatment, the mean (standard deviation) daily doses of RIS and BNS were 3.1 (1.3) mg and 14.6 (4.0) mg, respectively.
Efficacy
Sixteen patients on RIS and 17 on BNS completed the study. Twelve (63.2%) RIS and 12 (60%) BNS patients met the criteria for being responders.
For both groups, each assessment exhibited a decrease in the mean change from baseline on the PANSS ().
Table 2 PANSS score changes for the risperidone and blonanserin groups
A significantly larger reduction was observed in the RIS group compared to the BNS group for changes in the scores on two PANSS items: the excitement and hostility scores at 4 weeks. However, these significant differences were not observed at 8 weeks. That is, the BNS group displayed a faster decrease in mean PANSS depression scores (P<0.05 for week 8).
Cognitive function change
Comparisons of the changes in the neurocognitive functions of the treatment groups are summarized in . No significant differences in the changes were observed in the six BACS-J domains measured.
Table 3 “Time × group” interaction effect on analysis of variance with BACS-J and LASMI data when compared with the BNS group
Paired t-tests demonstrated that the z-scores for verbal fluency and executive function were significantly improved after treatment with BNS (), and the z-scores for verbal fluency were significantly improved after treatment with RIS ().
Table 4 Paired t-test results on BACS-J and LASMI data for the BNS group
Table 5 Paired t-test results on BACS-J and LASMI data for the RIS group
Social function change
Comparisons of the changes in the social functions of the treatment groups are summarized in . The changes in daily living were significantly larger in the BNS group than in the RIS group. No significant differences between the RIS and BNS groups were observed for the four other domains measured.
Paired t-tests demonstrated that the daily living and work skills scores were significantly improved after treatment with BNS (), and that the work skills scores were significantly improved after treatment with RIS ().
The relationship between the RIS and BNS doses and the BACS-J scores at 8 weeks
A significant negative correlation was found between RIS dosage and the scores for motor function, attention, and processing speed at 8 weeks in the RIS group. A significant negative correlation was also found between BNS and the motor function scores ().
Table 6 The relationship between RIS and BNS doses and BACS-J scores at 8 weeks
Discussion
In this study, it is noteworthy that BNS has an improving effect on daily living when compared with RIS, although the ameliorating effect on psychiatric symptoms is comparable between the two drugs. Recently, much importance has been attached to the ameliorating effects of antipsychotic drugs, not only on psychiatric symptoms, but also on cognitive and social functions. More notably, it appears that BNS significantly improves social function. Activities of daily living are often habitual and are less influenced by antipsychotic therapy. A previous study showed that there was a positive correlation between improving executive function and improving daily living.Citation27 Although the precise mechanism behind this effect remains unknown, BNS has been shown to improve daily living by improving executive function. Furthermore, this outcome may be explained by the simple pharmacological profile and lower sedative effect of BNS, although the mechanism underlying the action of BNS has not been fully clarified. Two previous studies reported that RIS has been shown to improve daily living.Citation28,Citation29 The discrepancy between the studies could be due to differences in the sample, the sample size, or the study periods.
Second, it should be noted that BNS may have a more potent effect on depressive symptoms than RIS in the treatment of patients with acute schizophrenia, whereas RIS manifests its effect on symptoms such as agitation and hostility early after administration. A recent meta-analysisCitation17 demonstrated that the effect of BNS is equivalent to that of RIS, which was also supported by the present study. When BNS is used in patients who exhibit strong agitation or hostility during the acute phase, the temporary use of concomitant drugs may be beneficial. In fact, when BNS is used during the acute phase, a concomitant drug reportedly increases the drug’s continuation rate.Citation30 However, the effect of RIS on depressive symptoms is poor for the first 8 weeks after administration, and if the patient remains depressed, it may be necessary to consider administering a concomitant drug.
The results of the present study suggest that both RIS and BNS have improving effects on cognitive and social function. Moreover, RIS improved verbal fluency, whereas BNS improved not only verbal fluency, but also executive function. A previous report demonstrated that the improving effect of RIS on verbal fluency is unsatisfactory.Citation3,Citation31 However, the present study’s results indicated that RIS significantly improved verbal fluency and that the effect size was moderate. BNS exerted improving effects on verbal fluency and executive function, as previously reported.Citation18 A previous study indicated that the effect size of antipsychotic drugs is small in terms of many variables related to the improvement of cognitive function.Citation31 However, in this study, this effect was relatively potent. RIS may be effective in improving work skills, whereas BNS may be effective in improving both daily living and work skills. Both drugs appear to improve patients’ social lives.
These results suggest that both RIS and BNS are effective in the treatment of schizophrenia. However, the proper use of concomitant drugs may be required, depending on the symptoms that occur during the acute stage. Moreover, both drugs are likely to improve cognitive and social function. BNS may be superior to RIS in the improvement of daily living.
This study had a relatively small sample size, was short-term (8 weeks), and open-label, but it was not double-blind. Therefore, the possibility that bias was introduced to the results cannot be ruled out and, consequently, there are limits to the conclusions that can be drawn from this study. A double-blind, randomized, controlled study on acute schizophrenia may be necessary to clarify the efficacy and safety of RIS and BNS. One must remember that other factors besides antipsychotic drugs, such as the patient’s period of illness, preillness intelligence quotient, or cognitive function levels, may influence the present study’s results.
In conclusion, RIS and BNS may improve psychotic symptoms, cognitive function, and daily living in patients with acute-phase schizophrenia. BNS may be superior to RIS in the improvement of daily living.
Author contributions
Dr Hori designed the study, performed the cognitive battery, collected the clinical data, performed the statistical analyses, wrote the first draft of the manuscript, and managed the literature searches. Dr Yoshimura and Dr Nakamura developed the study protocol and wrote the final manuscript. Dr Katsuki performed the cognitive battery. Dr Yamada, Dr Kamada, and Dr Shibata collected the clinical data. All of the authors took part in either drafting the article or revising it critically for important intellectual content, and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- MeltzerHYCognitive factors in schizophrenia: causes, impact, and treatmentCNS Spectr2004910 Suppl 11152415475873
- HarveyPDMeltzerHSimpsonGMImprovement in cognitive function following a switch to ziprasidone from conventional antipsychotics, olanzapine, or risperidone in outpatients with schizophreniaSchizophr Res2004662–310111315061242
- WoodwardNDPurdonSEMeltzerHYZaldDHA meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophreniaInt J Neuropsychopharmacol20058345747215784157
- KernRSGreenMFCornblattBAThe neurocognitive effects of aripiprazole: an open-label comparison with olanzapinePsychopharmacology (Berl)2006187331232016810506
- KeefeRSSweeneyJAGuHEffects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparisonAm J Psychiatry200716471061107117606658
- HoriHYoshimuraRKatsukiAThe cognitive profile of aripiprazole differs from that of other atypical antipsychotics in schizophrenia patientsJ Psychiatr Res201246675776122464338
- KeeKSHoranWPSaloveyPEmotional intelligence in schizophreniaSchizophr Res20091071616818805674
- KernRSGreenMFFiskeAPTheory of mind deficits for processing counterfactual information in persons with chronic schizophreniaPsychol Med200939464565418694537
- HarveyPOLeeJHoranWPOchsnerKGreenMFDo patients with schizophrenia benefit from a self-referential memory bias?Schizophr Res20111271–317117721147520
- LeeJZakiJHarveyPOOchsnerKGreenMFSchizophrenia patients are impaired in empathic accuracyPsychol Med201141112297230421524334
- FettAKViechtbauerWDominguezMDPennDLvan OsJKrabbendamLThe relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysisNeurosci Biobehav Rev201135357358820620163
- HoranWPGreenMFDeGrootMSocial cognition in schizophrenia, Part 2: 12-month stability and prediction of functional outcome in first-episode patientsSchizophr Bull201238486587221382881
- NodaYKurumiyaSMiuraYOkaMComparative study of 2-(4-ethyl-1-piperazinyl)-4-(fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b] pyridine (AD-5423) and haloperidol for their pharmacological activities related to antipsychotic efficacy and/or adverse side-effectsJ Pharmacol Exp Ther199326527457518098763
- OkaMNodaYOchiYPharmacological profile of AD-5423, a novel antipsychotic with both potent dopamine-D2 and serotonin-S2 antagonist propertiesJ Pharmacol Exp Ther199326411581658093723
- DeeksEDKeatingGMBlonanserin: a review of its use in the management of schizophreniaCNS Drugs2010241658420030420
- OhoyamaKYamamuraSHamaguchiTEffect of novel atypical antipsychotic, blonanserin, on extracellular neurotransmitter level in rat prefrontal cortexEur J Pharmacol20116531–3475721147094
- KishiTMatsudaYNakamuraHIwataNBlonanserin for schizophrenia: systematic review and meta-analysis of double-blind, randomized, controlled trialsJ Psychiatr Res201347214915423131856
- TenjinTMiyamotoSMiyakeNEffect of blonanserin on cognitive function in antipsychotic-naïve first-episode schizophreniaHum Psychopharmacol20122719010022278973
- American Psychiatric AssociationDiagnostic Criteria from DSM-IV-TRWashington, DCAmerican Psychiatric Association2000
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- KanedaYSumiyoshiTKeefeRIshimotoYNumataSOhmoriTBrief assessment of cognition in schizophrenia: validation of the Japanese versionPsychiatry Clin Neurosci200761660260918081619
- KeefeRSGoldbergTEHarveyPDGoldJMPoeMPCoughenourLThe Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive batterySchizophr Res2004682–328329715099610
- KanedaYSumiyoshiTNakagomeKThe brief assessment of cognition in schizophrenia Japanese version (BACS-J)Seishinigaku2008509913917 Japanese
- IwasakiSMiyauchiMOshimaIThe development of Life Assessment Scale for the Mentally Ill: an assessment of the reliabilitySeishin Igaku19943611391151 Japanese
- IwanamiAYamashinaMKazamatsuriHKamijimaKP300 and disability of daily life in schizophreniaProg Neuropsychopharmacol Biol Psychiatry199923342343010378227
- InadomiHTanakaGWatanabeSEfficacy of 3-year psychiatric daycare treatment in patients with schizophreniaPsychiatry Clin Neurosci200559324625215896216
- HoriHYoshimuraRKatsukiASugitaAIAtakeKNakamuraJSwitching to antipsychotic monotherapy can improve attention and processing speed, and social activity in chronic schizophrenia patientsJ Psychiatr Res201347121843184824054464
- LiebermanRPGutkindDMintzJImpact of risperidone versus haloperidol on activities of daily living in the treatment of refractory schizophreniaCompr Psychiatry200243646947312439835
- KanedaYOhmoriTImpact of risperidone medication on quality of life and gonadal axis hormones in schizophrenia male patients with acute exacerbationInt J Neuropsychopharmacol20036324725212974991
- TsutsumiYKasugaYIsakaYEffectiveness of blonanserin (BNS) in 70 in-patients with acute phase schizophreniaJapanese Journal of Clinical Psychopharmacology20111415231540 Japanese
- HarveyPDKeefeRSStudies of cognitive change in patients with schizophrenia following novel antipsychotic treatmentAm J Psychiatry2001158217618411156796
