Abstract
Cognitive impairment is closely related to real-life functioning in patients with schizophrenia. The aim of the present study was to evaluate the effects of adjunctive treatment with donepezil on cognition in patients with chronic schizophrenia. This was a 12-week, double-blind, randomized, placebo-controlled study of donepezil as an adjunct to antipsychotic drug therapy in patients with chronic stable schizophrenia. Sixty-one subjects were randomized to receive donepezil 5 mg/day (n=31) and/or placebo (n=30). A nine-test neuropsychological assessment battery was administered at baseline and at the end of the study. At the 12-week end point, the donepezil group showed significant improvements in the Wechsler Memory Scale Third Edition Spatial Span, Brief Visuospatial Memory Test total recall and delayed recall, Trail-Making Test Part A, and Category Fluency Test-animal naming (all P≤0.018). Compared with placebo, donepezil was associated with significant improvement in several cognitive domains, including working memory, speed of information processing, and visual learning and memory (P≤0.008). The results of the present study suggest that adjunctive use of donepezil is beneficial for improving cognitive function in patients with schizophrenia.
Keywords:
Introduction
Patients with schizophrenia have significant cognitive impairment, especially in the domains of attention, executive function, memory, verbal skills, and processing speed.Citation1,Citation2 This impairment is a major determinant of their real-life functioning, so is an important treatment target.Citation3 The effects of antipsychotic medications on cognition in schizophrenia appear to be minimal.Citation3 Recent studies examining novel adjunctive treatments have yielded some encouraging results.Citation4,Citation5
Transmission of acetylcholine in the central nervous system plays a vital role in cognitive function, specifically in attention and memory.Citation6 Modulation of the alpha-7 nicotinic acetylcholine receptor has been considered a potential treatment target in Alzheimer’s disease and schizophrenia.Citation7 Acetylcholinesterase inhibitors, including donepezil, rivastigmine, and galantamine, have shown some cognitive benefit in Alzheimer’s disease and related dementias.Citation8
Several studies have investigated the effects of acetylcholinesterase inhibitors on cognition in schizophrenia.Citation5,Citation9–Citation12 Although a recent meta-analysis does not support the use of acetylcholinesterase inhibitors for mild cognitive impairment,Citation13 several previous studies in schizophrenia spectrum disorders have reported significant cognitive improvement using donepezil as adjunctive therapy.Citation5,Citation12 Many factors, including cigarette smoking, stage of cognitive impairment, and duration of illness, may influence the effects of acetylcholinesterase inhibitors on cognition.Citation14,Citation15 Selecting appropriate measurement tools is also important to assess the effects of donepezil on cognition, given that cognitive function includes many different domains in schizophrenia.Citation1,Citation2
Here we present a 12-week, randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov identifier NCT01490567) examining the effect of donepezil as an adjunct to atypical antipsychotic (risperidone or olanzapine) therapy on cognitive impairment in Chinese patients with chronic schizophrenia. Risperidone and olanzapine are commonly used new-generation antipsychotic medications in the People’s Republic of China. Unlike the previous research, participants in our study had chronic schizophrenia, a duration of illness of less than 10 years, excluded ex-smokers or current smokers, and included an extensive cognitive battery.
Materials and methods
Participants
All participants were recruited from the Second Xiangya Hospital of Central South University between June 2011 and November 2012. Consenting patients aged 18–40 years were eligible for the study if they met the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) criteria for schizophrenia determined by the Structured Clinical Interview for DSM-IV. Patients had to be clinically stable (Positive and Negative Syndrome Scale [PANSS]Citation16 total scores ≤60);Citation17 have a duration of illness of more than 2 years but less than 10 years; and be treated with risperidone or olanzapine at a stable dose for at least 4 weeks. We excluded patients with mental retardation or severe organic brain syndrome; those diagnosed with a serious or unstable medical condition; those who were pregnant or breastfeeding; those receiving concomitant anticholinergic drugs; ex-smokers or current smokers; patients treated with electroconvulsive therapy within the 6 months prior to screening; those who had made suicidal attempts or displayed ideation or violent behavior within the last 12 months; and those who had participated in other therapy programs.
Study design
The study was designed as a 12-week, double-blind, placebo-controlled, randomized trial. The study protocol was approved by the ethics committee of the Second Xiangya Hospital of Central South University, and was carried out in accordance with the Guideline for Good Clinical Practice and the Declaration of Helsinki. Every patient or their legal guardian provided written informed consent before enrollment.
After screening, patients were randomized to receive donepezil 5 mg/day or placebo in addition to their antipsychotic treatment. Doses of antipsychotic medications remained fixed throughout the study. Benzodiazepines for insomnia and propranolol for akathisia or tachycardia were permitted as needed during the study. Patients who received benzodiazepines needed to wait for 48 hours prior to cognitive testing.
Neuropsychological assessments
A nine-test neuropsychological battery was administered at baseline and at the end of the study. These nine tests were grouped into seven cognitive domains. Working memory was evaluated using the Wechsler Memory Scale Third Edition Spatial Span (WMS-III SST).Citation18 Verbal memory was tested using the Hopkins Verbal Learning Test-Revised.Citation19 Visual memory was assessed with the Brief Visuospatial Memory Test-Revised.Citation20 The tests for processing speed included the Trail-Making Test Part A (TMT-A),Citation21 Brief Assessment of Cognition in Schizophrenia-symbol coding,Citation22 and Category Fluency Test-animal naming.Citation23 Attention was evaluated with Continuous Performance Test-Identical Pairs (CPT-IP).Citation24 In addition, the Wisconsin Card Sorting TestCitation25 and Stroop Color and Word TestCitation26 were used to assess reasoning and problem-solving, and inhibition and interference control functions, respectively.
To calculate cognitive domain scores, all test measures were first converted to standardized z scores by setting the sample mean of each measure at baseline to zero and the standard deviation to 1. For domains with more than one test, summary scores were determined by calculating the mean of the z scores for the measures that comprised the domain, then converting the mean to a z score with a mean of zero and a standard deviation of one.Citation27
Clinical and safety assessments
The severity of psychopathology was assessed using the PANSS.Citation16 The Clinical Global Impressions (CGI) scale item for severity of illness was used to assess global changes.Citation28 Depressive symptoms were evaluated using the Calgary Depression Scale for Schizophrenia (CDSS).Citation29 The Simpson-Angus Rating ScaleCitation30 and the Abnormal Involuntary Movement ScaleCitation31 were used to assess extrapyramidal symptoms and dyskinetic movements, respectively. The PANSS, CDSS, CGI, Simpson-Angus Rating Scale, and Abnormal Involuntary Movement Scale ratings were administered monthly. Safety evaluations included physical examinations, electrocardiography, vital signs, and adverse events. A complete blood count and urinalysis were performed at baseline and at the end of the study. Intraclass correlation coefficients for these instruments ranged from 0.82 to 0.88. All raters were blinded to treatment assignment.
Statistical analysis
All analyses were conducted using the Statistical Package for Social Sciences version 17.0 software (SPSS Inc, Chicago, IL, USA). The alpha level for testing the statistical significance of effects was P=0.05 unless otherwise indicated. Data analysis was based on the intent-to-treat population, including all eligible patients with at least one follow-up assessment. For missing data, the last observation carried forward was used for analysis. Between-group comparisons of demographic and clinical characteristics at baseline were performed using the Student’s t-test for continuous variables and the χ2 test or Fisher’s Exact test for categorical variables. Between-group comparisons for cognitive scores at baseline were performed using analysis of covariance controlling for age and education.
The primary outcome measure was the change in cognitive scores from baseline to 12 weeks. Within-group comparisons of continuous variables between baseline and end point were examined using the paired-samples t-test. Between-group comparisons in cognitive function at end point were performed using analysis of covariance, controlling for baseline, age, and education. Bonferroni corrections were used to set the cut-off for statistical significance at 0.004 (0.05/14) and 0.008 (0.05/7) for multiple comparisons in 14 cognitive scores and in seven cognitive domain scores, respectively. Effect size (changes in cognitive domain from baseline to week 12) was calculated using Cohen’s formula.Citation32 In addition, the χ2 test or Fisher’s Exact test was used to compare between-group differences in adverse events.
Results
Baseline characteristics and follow-up
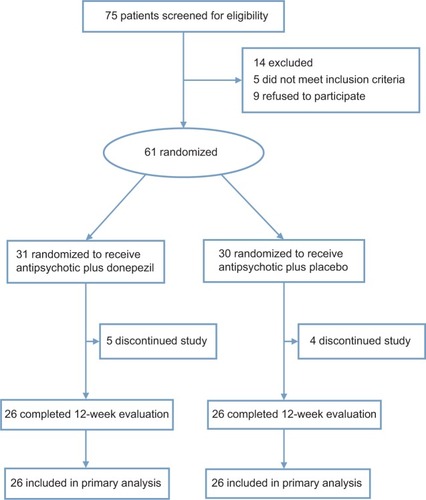
A total of 61 patients were enrolled in the study, and 52 completed cognitive function testing at baseline and at 12 weeks follow-up (). The demographic and clinical characteristics of the 61 patients are shown in . There were no significant differences between the two groups for age, sex, education, psychopathology scores, or duration of illness (all P≥0.083, ).
Table 1 Demographic and clinical characteristics of patients who received donepezil or placebo
Effects of treatment on cognitive function
When compared with baseline, significant improvements were found in the WMS-III SST, TMT-A, Category Fluency Test-animal naming, and Brief Visuospatial Memory Test-Revised total recall and delayed recall at end point in the donepezil group (all P≤0.018, ).
Table 2 Neuropsychological test scores of patients with schizophrenia who received donepezil or placebo
At week 12, cognitive function scores show significant differences in WMS-III SST, TMT-A, Category Fluency Test-animal naming, and Brief Visuospatial Memory Test-Revised total recall and delayed recall between the two groups (all P≤0.001, ).
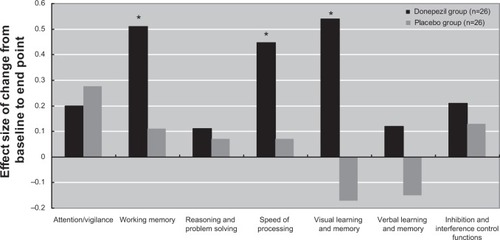
shows the effect size of changes in domain scores after 12 weeks of treatment with donepezil. Significant effects of donepezil were found in working memory, speed of processing, and visual learning and memory (all P≤0.008).
Effects of treatment on clinical efficacy and safety outcomes
Compared with baseline, significant improvements were found in PANSS scores and CDSS total scores at end point (all t≥2.302, all P≤0.030). There were no significant differences in PANSS total score or CDSS total score between two groups at end point (all F≤3.314, all P≥0.075). No significant differences were found in rates of adverse events between the two groups (P≥0.492, ).
Table 3 Safety measures of patients with schizophrenia who received donepezil or placebo
Discussion
This placebo-controlled, double-blind study investigated the efficacy and tolerability of donepezil as adjunctive therapy for cognitive impairment in Chinese patients with chronic schizophrenia. The study indicates that donepezil was safe and well tolerated, and effective as an add-on treatment to improve cognitive function in this patient population.
Our data show that at the 12-week end point, the donepezil group had significant improvements in the WMS-III SST, Brief Visuospatial Memory Test total recall and delayed recall, TMT-A, and Category Fluency Test-animal naming. Effect size analysis showed that the donepezil group experienced moderate improvement in working memory, speed of processing, and visual learning and memory. The difference between the largest effect size for the cognitive domain score (0.54) and the smaller effect size (≤0.27) is ≥0.27, or about one third of a standard deviation (1). If we assume that the smaller effect sizes reflect only practice effects, then the difference (≥0.27) could represent an actual treatment effect, albeit a “small” effect with low clinical significance.Citation32
A number of previous studies have investigated the cognitive effects of acetylcholinesterase inhibitors in schizophrenia.Citation5,Citation9–Citation12 Some trials suggested that donepezil and other acetylcholinesterase inhibitors may have potential benefits for cognitive impairment in schizophrenia,Citation5,Citation12 but a large multicenter study by Keefe et al failed to demonstrate any cognitive effects of donepezil in patients with schizophrenia or schizoaffective disorder.Citation11 Most patients were smokers or ex-smokers, and the mean duration of schizophrenia was long (18 years) in their study. A previous study suggested that cognitive impairment is progressive during the course of the illness;Citation33 therefore, some of the study participants may not have responded well to donepezil in the study by Keefe et al. The evidence for an effect of cigarette smoking on cognitive deficits in schizophrenia is mixed. Several studies have showed that nicotine improves the cognitive deficits,Citation34,Citation35 but others have found little or no association between smoking and cognitive deficits in schizophrenia.Citation36 Cigarette smoking can desensitize nicotine receptors in patients with schizophrenia, who do not show the normal upregulation following chronic nicotine use.Citation37 This might have prevented donepezil reaching its full potential with regard to cognitive enhancement in patients with schizophrenia who smoked, in the study by Keefe et al.Citation11
The cholinergic system has been implicated in the regulation of attention, memory, processing speed, and sensory gating,Citation38 all of which are impaired in schizophrenia. Autoradiography studies have shown alterations in the density and expression of acetylcholine receptors in the brains of patients with schizophrenia.Citation39,Citation40 Post mortem studies have also demonstrated changes in muscarinic and nicotinic receptor availability or expression.Citation41,Citation42 The activity of choline acetyltransferase, a biomarker of cholinergic neuronal function, in the parietal cortex of patients with schizophrenia was shown to be negatively correlated with the severity of cognitive impairment.Citation43 These studies suggest that schizophrenia is associated with multiple abnormalities of the cholinergic system. Thus, cholinesterase inhibitors may be useful in the treatment of cognitive impairment in patients with schizophrenia.
Donepezil had limited effects on psychotic symptoms. There was no difference in effects on symptoms of psychopathology between the two groups in our study. Donepezil was also well tolerated. The incidence of adverse events was similarly low in both groups.
There are several potential limitations to our study. First, the dose of 5 mg/day is lower than that used in previous similar research.Citation44 Donepezil is administered once daily in a dose range of 5–10 mg for Alzheimer’s disease, but in some Asian countries, the recommended dose is lower.Citation8 Second, our patients were treated with donepezil for 12 weeks, which might be too short to demonstrate the effect of donepezil on cognitive function. Donepezil significantly improves cognitive function in Alzheimer’s dementia for up to 6 months.Citation8 Higher doses or a longer treatment duration may have yielded greater cognitive improvement. Third, the sample size was small. Future randomized controlled investigations with a larger sample size are warranted. Finally, our study excluded smokers, so the generalizability of the findings might be limited. However, the rate of cigarette smoking in Chinese patients with schizophrenia is 13.9%, so is lower than the rates reported in most of the previous studies.Citation45
In summary, the results of this double-blind, placebo-controlled study suggest a potential cognitive benefit from adjunctive use of donepezil in Chinese patients with schizophrenia.
Acknowledgments
We are very grateful to Xiaoduo Fan from UMass Memorial Medical Center, University of Massachusetts Medical School, Worcester, MA, USA, for assistance in revising our manuscript. This research was supported by grants from the National Natural Science Foundation of China (30900485, 81270019) and the National R&D Special Fund for the Health Profession (201002003).
Disclosure
The authors report that they have no direct conflict of interest or grant support that is directly related to the content of the study.
References
- GreenMFKernRSBraffDJMintzJNeurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”?Schizophr Bull200026111913610755673
- GoldbergTEGoldmanRSBurdickKECognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect?Arch Gen Psychiatry200764101115112217909123
- KeefeRSHarveyPDCognitive impairment in schizophreniaHandb Exp Pharmacol2012213113723027411
- PreskornSHGawrylMDgetluckNPalfreymanMBauerLOHiltDCNormalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophreniaJ Psychiatr Pract2014201122424419307
- RibeizSRBassittDPArraisJAAvilaRSteffensDCBottinoCMCholinesterase inhibitors as adjunctive therapy in patients with schizophrenia and schizoaffective disorder: a review and meta-analysis of the literatureCNS Drugs201024430331720297855
- AmentaFTayebatiSKPathways of acetylcholine synthesis, transport and release as targets for treatment of adult-onset cognitive dysfunctionCurr Med Chem200815548849818289004
- PohankaMAlpha-7 Nicotinic acetylcholine receptor is a target in pharmacology and toxicologyInt J Mol Sci20121322219223822408449
- Di SantoSGPrinelliFAdorniFCaltagironeCMusiccoMA meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer’s diseaseJ Alzheimers Dis201335234936123411693
- BuchananRWConleyRRDickinsonDGalantamine for the treatment of cognitive impairments in people with schizophreniaAm J Psychiatry20081651828917986678
- LindenmayerJPKhanAGalantamine augmentation of long-acting injectable risperidone for cognitive impairments in chronic schizophreniaSchizophr Res20111252–326727720850275
- KeefeRSMalhotraAKMeltzerHYEfficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trialNeuropsychopharmacology20083361217122817625502
- EricksonSKSchwarzkopfSBPalumboDBadgley-FleemanJSmirnowAMLightGAEfficacy and tolerability of low-dose donepezil in schizophreniaClin Neuropharmacol200528417918416062097
- TriccoACSoobiahCBerlinerSEfficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysisCMAJ2013185161393140124043661
- KumariV1PostmaPNicotine use in schizophrenia: the self medication hypothesesNeurosci Biobehav Rev20052961021103415964073
- ThakurathiNVincenziBHendersonDCAssessing the prospect of donepezil in improving cognitive impairment in patients with schizophreniaExpert Opin Investig Drugs2013222259265
- KaySRFiszbeinAOplerLAThe Positive and Negative Syndrome Scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- GuoXZhaiJLiuZEffect of antipsychotic medication alone vs combined with psychosocial intervention on outcomes of early-stage schizophrenia: a randomized, 1-year studyArch Gen Psychiatry201067989590420819983
- WechslerDWMS-R: Wechsler Memory Scale-Revised: ManualSan Antonio, TX, USAPsychological Corporation1987
- BrandtJBenedictRHBHopkins Verbal Learning Test-Revised: Professional ManualLutz, FL, USAPsychological Assessment Resources2001
- BenedictRBrief Visuospatial Memory Test-Revised: Professional ManualOdessa, FL, USAPsychological Assessment Resources1997
- ReitanRMWolfsonDThe Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation2nd edSouth Tucson, AZ, USANeuropsychology Press1997
- KeefeRSBrief Assessment of Cognition in Schizophrenia (BACS) Manual-A. Version 21Durham, NC, USADuke University Medical Center1999
- SpreenQStraussEA Compendium of Neuropsychological Tests: Administration, Norms, and Commentary2nd edNew York, NY, USAOxford University Press1998
- CornblattBARischNJFarisGFriedmanDErlenmeyer-KimlingLThe Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal familiesPsychiatry Res19882622232383237915
- HeatonRKWisconsin Card Sorting Test Manual: Revised and ExpandedLutz, FL, USAPsychological Assessment Resources1993
- GoldenCJStroop Color and Word Test: A Manual for Clinical Experimental UsesWood Dale, IL, USAStoelting Co1978
- KeefeRSSweeneyJAGuHEffects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparisonAm J Psychiatry200716471061107117606658
- GuyWECDEU Assessment Manual for PsychopharmacologyRockville, MD, USAUS Department of Health, Education, and Welfare Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration1976
- AddingtonDAddingtonJMaticka-TyndaleEAssessing depression in schizophrenia: the Calgary Depression ScaleBr J Psychiatry Suppl19932239448110442
- SimpsonGMAngusJWA rating scale for extrapyramidal side effectsActa Psychiatr Scand Suppl197021211194917967
- LaneRDGlazerWMHansenTEBermanWHKramerSIAssessment of tardive dyskinesia using the Abnormal Involuntary Movement ScaleJ Nerv Ment Dis198517363533573998720
- CohenJA power primerPsychol Bull1992112115515919565683
- BilderRMGoldmanRSRobinsonDNeuropsychology of first episode schizophrenia: initial characterization and clinical correlatesAm J Psychiatry2000157454955910739413
- KrishnadasRJauharSTelferSShivashankarSMcCreadieRGNicotine dependence and illness severity in schizophreniaBr J Psychiatry2012201430631222878134
- WintererGWhy do patients with schizophrenia smoke?Curr Opin Psychiatry201023211211920051860
- BarnesMLawfordBRBurtonSCSmoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use?Aust N Z J Psychiatry2006406–757558016756583
- KumariVPostmaPNicotine use in schizophrenia: the self medication hypothesesNeurosci Biobehav Rev20052961021103415964073
- FureyMLPietriniPHaxbyJVCholinergic enhancement and increased selectivity of perceptual processing during working memoryScience200029055002315231911125148
- CrookJMTomaskovic-CrookECopolovDLDeanBDecreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formationBiol Psychiatry200048538138810978721
- BreeseCRLeeMJAdamsCEAbnormal regulation of high affinity nicotinic receptors in subjects with schizophreniaNeuropsychopharmacology200023435136410989262
- FreedmanRHallMAdlerLELeonardSEvidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophreniaBiol Psychiatry199538122337548469
- GuanZZZhangXBlennowKNordbergADecreased protein level of nicotinic receptor alpha-7 subunit in the frontal cortex from schizophrenic brainNeuroreport19991081779178210501574
- PowchikPDavidsonMHaroutunianVPostmortem studies in schizophreniaSchizophr Bull19982423253419718627
- TsunoNDonepezil in the treatment of patients with Alzheimer’s diseaseExpert Rev Neurother20099559159819402770
- WangCYXiangYTWengYZCigarette smoking in patients with schizophrenia in China: prospective, multicentre studyAust N Z J Psychiatry201044545646220397788