Abstract
Accumulating evidence suggests that neuroinflammation affecting microglia plays an important role in the etiology of schizophrenia, and appropriate control of microglial activation may be a promising therapeutic strategy for schizophrenia. Minocycline, a second-generation tetracycline that inhibits microglial activation, has been shown to have a neuroprotective effect in various models of neurodegenerative disease, including anti-inflammatory, antioxidant, and antiapoptotic properties, and an ability to modulate glutamate-induced excitotoxicity. Given that these mechanisms overlap with neuropathologic pathways, minocycline may have a potential role in the adjuvant treatment of schizophrenia, and improve its negative symptoms. Here, we review the relevant studies of minocycline, ranging from preclinical research to human clinical trials.
Introduction
Schizophrenia is a chronic and often debilitating illness affecting approximately 1% of the world population.Citation1 It is a complex disorder characterized by profound disturbance of perception, cognition, emotion, and social function. The onset of schizophrenia is typically in late adolescence or early adulthood, and includes distinctive symptoms, commonly referred to as positive, negative, and cognitive. To date, effective treatments for schizophrenia have been limited to medications with antidopaminergic activity, which alleviate symptoms by augmenting dysfunctional neurotransmitter systems.Citation2 While antipsychotics are most effective for positive symptoms, negative and cognitive symptoms are less well addressed.Citation3 Treatment of patients with minimal or no response to adequate doses of antipsychotics represents an enormous challenge for clinicians. These antipsychotic-resistant patients constitute up to 25%–30% of all patients suffering from schizophrenia.Citation2,Citation4,Citation5 One of the main reasons for this is our lack of understanding of the etiology of schizophrenia.
Although the exact mechanism of schizophrenia remains to be elucidated, several hypotheses have been proposed, including disruption of neurotransmitter systems,Citation6–Citation10 genetic factors,Citation11 and neurodevelopmental and neurotoxic mechanisms.Citation12,Citation13 In recent years, however, growing evidence has supported the idea that neuroinflammation, in particular that focused on the microglia, plays an important role in the etiology of schizophrenia, so appropriate control of microglial activation may be a promising strategy in the treatment of the disease.Citation1,Citation14–Citation16 Minocycline, a second-generation tetracycline, has a distinct neuroprotective profile independent of its antibacterial activity.Citation17 Minocycline is almost completely absorbed when taken orally and shows excellent penetration of brain tissue. These properties, as well as its beneficial effect in animal models of neurologic disorders, has led to investigation of its potential use in the treatment of schizophrenia.Citation18,Citation19 Recent reports have demonstrated a possible antipsychotic effect for minocycline, which is a potent inhibitor of microglial activation. In these studies, use of minocycline as an adjuvant to antipsychotics was reported to be beneficial in patients with schizophrenia.Citation20–Citation23 Here we review the existing literature focusing on the preclinical and clinical potential of minocycline in the treatment of schizophrenia and propose directions for future research.
Overview of minocycline: how to confer neuroprotection?
Microglial hypothesis for schizophrenia
Although dopaminergic neurotransmission is involved in the pathophysiology of schizophrenia, the exact mechanism leading to dopaminergic dysfunction remains unclear. Accumulating evidence indicates the significance of neuroinflammation involving microglia in schizophrenia.Citation16,Citation24 Maternal inflammation during critical stages of gestation has been shown to form the basis of the link between prenatal infection and schizophrenia.Citation25,Citation26 Longitudinal studies in animal models further indicate that infection-induced developmental neuroinflammation may be pathologically relevant beyond the antenatal and neonatal periods, and may contribute to progression of disease, associated with gradual development of full-blown schizophrenia.Citation27 Arion et al carried out a DNA microarray study that showed increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia.Citation28 Narayan et al profiled genome-wide expression patterns in the prefrontal cortex in subjects with schizophrenia at different stages of the illness. Their study demonstrated that the molecular basis for schizophrenia changes between the early to chronic stages, providing evidence that the nature of schizophrenia changes with disease progression, with the long-term illness possibly being associated with inflammation, stimulus response, and immune function.Citation29
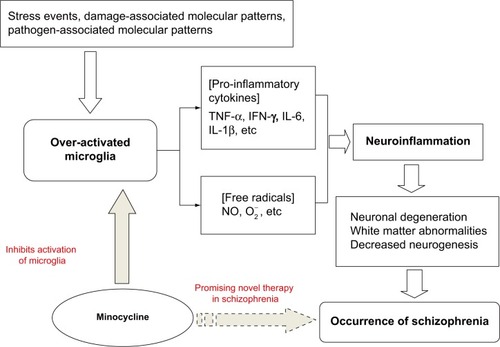
Microglial cells are the primary reservoirs of proinflammatory cytokines, such as interleukin-6, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ), and act as the main antigen-presenting cells in the central nervous system (CNS).Citation30 Microglia are important for the cross-talk between the immune system and glutamatergic neurotransmission.Citation31 The cells gradually become present in the brain and participate in various aspects of brain development, including cell death, axonal remodeling, synaptogenesis, and synaptic pruning.Citation32–Citation36 Prolonged microglial hyperactivity may lead to neuronal apoptosis and brain damage, which are commonly seen in neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease.Citation37,Citation38 A neurodegenerative and neurodevelopmental process is indicated in the course of schizophrenia and may be associated with microglial activation.Citation39,Citation40 Animal studies, autopsy studies, and positron emission tomography (PET) studies have demonstrated that the neuropathology of schizophrenia is associated with microglial activation.Citation41–Citation49 Bayer et al found that there were more activated microglia in the frontal cortex and hippocampus in patients with schizophrenia post mortem than in controls.Citation45 Their findings were strengthened further by positive results from other autopsy studies in patients with schizophrenia.Citation43,Citation44,Citation46,Citation47 The evidence from autopsy studies suggests that schizophrenia is associated with an increased number of activated microglia cells. However, autopsy studies cannot reflect dynamic changes in microglial activation over time. PET, a noninvasive brain imaging technique, provides the opportunity to study the presence of microglial activation in vivo. Van Berckel et al found overactive microglia in patients with schizophrenia of recent onset.Citation48 Doorduin et al also reported finding more activated microglia in the hippocampus of patients with schizophrenia than in healthy volunteers, and suggested that focal neuroinflammation may play an important role in schizophrenia.Citation49 As such, there has been growing evidence of a “microglial hypothesis of schizophrenia” () whereby stressful events, damage-associated molecular patterns, and pathogen-associated molecular patterns activate microglia in the CNS via immunologic or inflammatory activators. Proinflammatory cytokines and free radicals released by overactivated microglia can cause neuronal degeneration, abnormalities in white matter, and decreased neurogenesis. The damage done to the neuron by overactivated microglia may thus lead eventually to the development of schizophrenia.Citation1,Citation16 If microglial pathology proves to be an important causative factor in schizophrenia, the processes involving modulators of microglial activation may represent a novel therapeutic strategy.Citation50,Citation51
Figure 1 Microglia hypothesis of schizophrenia.
Abbreviations: IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; NO, nitric oxide; O2−, superoxide.
Targeting microglia
Inflammation of the CNS is detrimental to neurogenesis in the adult hippocampus.Citation52,Citation53 The negative effects of inflammation on differentiation and survival of neuronal cells are due to microglia-derived proinflammatory cytokines.Citation52,Citation54 Interleukin-1β and TNF-α have been reported to inhibit neurogenesis in vivo.Citation55,Citation56 However, neurogenesis can be restored by anti-inflammatory compounds such as minocycline, indomethacin, and cyclo-oxygenase-2 inhibitors (eg, celecoxib) which inhibit microglial activation.Citation52,Citation53,Citation57,Citation58 Other reports have shown that minocycline is able to inhibit microglial activation via anti-inflammatory and antioxidant properties which are independent of its antimicrobial action.Citation59–Citation67 Yrjanheikki et al showed that minocycline is able to provide neuroprotection against global ischemia in gerbils and focal brain ischemia in rats.Citation59,Citation60 This neuroprotection was associated with reduced activation of microglia and inhibition of interleukin-1β-converting enzyme, suggesting that minocycline may function by reducing the cytotoxic effects of the microglia. Minocycline can also reduce the proliferation and activation of resting microglia.Citation61 Tikka et al investigated whether minocycline was able to reduce excitotoxicity in a primary neuronal culture, and found that glutamate-induced microglial activation occurred via the p38 mitogen-activated protein kinase pathway and that minocycline inhibited activation of this pathway in microglia, providing neuroprotection against excitotoxicity.Citation62 Their findings were strengthened further by positive results in other studies using animal models of ischemic injury to the brain and neurodegenerative diseases.Citation63–Citation65 In addition, it was found that interleukin-1β levels following traumatic brain injury can be reduced in mice by a high dose of minocycline, and that increased TNF-α and nitric oxide levels in rats treated with 3-nitropropioic acid (an inhibitor of succinate dehydrogenase, an electron transport enzyme) can be attenuated by treatment with minocycline.Citation66,Citation67 Minocycline has also been reported to reduce superoxide production by granular neurons in the rat following treatment with N-methyl-D-aspartate, a glutamate receptor agonist.Citation68 Overall, the above studies suggest that minocycline targets overactive microglia and removes proinflammatory cytokines and oxygen radicals.Citation59–Citation67
Attenuation of apoptosis
Increasing evidence indicates that the neurodegenerative course of schizophrenia involves an increased susceptibility to apoptotic death, and activation of the apoptotic process results in rapid neuronal death.Citation69–Citation73 The mechanisms of cell death in neurodegenerative diseases include oxidative stress, excitotoxicity, mitochondrial dysfunction, and inflammation.Citation74 Proinflammatory cytokines, including TNF-α, have been well characterized as mediators of oxidative stress and induce apoptosis in human cortical neurons as well as oligodendrocytes.Citation75,Citation76 Apoptosis is indirectly suppressed by minocycline by inhibition of proinflammatory cytokines and nitric oxide.Citation77
Moreover, minocycline has been shown to be an inhibitor of poly-(ADP-ribose), polymerase-1, and matrix metalloproteinases.Citation78,Citation79 The antiapoptotic profile of minocycline has been attributed to upregulation of the anti-apoptotic protein Bcl-2, reduced expression of caspases, and inhibition of release of proapoptotic proteins from the mitochondria.Citation80–Citation84 Animal studies have shown that minocycline is able to increase expression of antiapoptotic proteins and decrease expression of these proteins after treatment with transforming growth factor beta-2. Hydrogen peroxide can also be reversed by minocycline, which leads to inhibition of apoptosis.Citation85,Citation86 Minocycline may directly activate the kinase G pathway by activation of kinase G1, leading to increased expression of Bcl-2.Citation19,Citation87 Minocycline is able to block the mitochondrial permeability transition pore (a large-conductance mega channel in the inner mitochondrial membrane), inhibiting release of proapoptotic proteins from the mitochondria.Citation88–Citation91
Minocycline has also been shown to reduce glutamate excitotoxicity in the glutamatergic pathways.Citation92–Citation99 It has been reported that minocycline-treated rat cortical neurons show increased cell viability following glutamate administration, which indicates that minocycline is able to reduce glutamate-induced neurotoxicity.Citation92,Citation93 GluR1 receptors, which are critical for the cognitive processes that are impaired in schizophrenia, has been shown to be involved in the pathobiology of schizophrenia.Citation94,Citation95 Minocycline affects a subtype of glutamate receptors known as GluR1 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors in neurons in vitro and in the CNS in vivo. Increased membrane localization of the GluR1 AMPA receptor improves glutamatergic activity and modulates neuroplasticity.Citation96 Overall, these findings suggest that minocycline has an antiapoptotic profile and is involved in several pathways known to be disturbed in schizophrenia.
Minocycline in schizophrenia: preclinical studies
Minocycline has been reported to reduce inflammation and provide neuroprotection in a variety of experimental models, including ischemic stroke, brain and spinal cord injury, multiple sclerosis, and Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease.Citation17 Du et al have reported on the neuroprotective effects of minocycline in animal models of stroke/ischemic injury and Huntington’s disease, and shown that minocycline can prevent nigrostriatal dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease.Citation97 Minocycline has been shown to play an important role in the treatment of disorders involving neuronal damage.Citation17,Citation97 However, in a rat model of intracerebral hemorrhage, Wasserman et al found that minocycline did not reduce neuronal loss outside the hematoma or striatal tissue, despite reducing the number of neutrophils and activated microglia. These authors suggested that minocycline did not appear to target the mechanisms responsible for cell death in this model of intracerebral hemorrhage.Citation98 However, the complex behavioral tests needed to assess functional recovery in this model of intracerebral hemorrhage were not used, and it was not known whether rescuing the band of damaged neurons at the edge of the hematoma would have lasting benefits.
The neurotherapeutic potential of minocycline in the treatment of mental disorders has also been explored.Citation19,Citation99–Citation101 It has been reported that minocycline is able to ameliorate behavioral changes as well as neurotoxicity at dopaminergic terminals after administration of methamphetamine, a compound that causes long-term cognitive deficits and psychiatric signs such as hallucination and delusions.Citation99 Hashimoto et al did a PET study in conscious monkeys, and found that minocycline attenuated the reduction of dopamine transporters in monkeys treated with methamphetamine. They suggested that minocycline protects against methamphetamine-induced neurotoxicity in the monkey brain.Citation102 Levkovitz et alCitation100 compared the effects of minocycline and haloperidol in an animal model of schizophrenia (N-methyl-D-aspartate antagonist, dizocilpine maleate, MK801). After 3 days of treatment with minocycline 35 mg/kg/day, the rats were injected with MK801 and assessed using behavioral tests. The findings showed that MK801 caused cognitive visuospatial memory deficits and changes in sensorimotor gating, similar to those evident in schizophrenia. Minocycline reversed the cognitive effects of MK801, and the effect was similar to that of haloperidol. Levkovitz et al suggested that minocycline may be able to protect against the disturbed cognitive processes seen in the MK801 animal model of schizophrenia.Citation100 Zhang et al also indicated that minocycline attenuates the behavioral changes (such as acute hyperlocomotion and prepulse inhibition deficits) occurring in mice after administration of dizocilpine, and suggested that minocycline could be a potential therapeutic compound for schizophrenia.Citation103 Monte et al reported the prevention and reversal of ketamine-induced schizophrenia-related behavior by minocycline.Citation101 Moreover, minocycline has been shown to have beneficial effects on cognition in animal models of schizophrenia. In a study by Fujita et al saline 10 mL/kg/day or phencyclidine 10 mg/kg/day were subcutaneously administered in mice for 10 days. Three days after the final dose of saline or phencyclidine, vehicle (10 mL/kg/day, physiologic saline) or minocycline (4.0 or 40 mg/kg/day) was administered intraperitoneally for 14 consecutive days. One day after the final injection, a novel object recognition test was performed, and it was found that phencyclidine-induced cognitive deficits in mice were significantly improved by subsequent subchronic administration of minocycline (40 mg/kg), suggesting minocycline could be a potential therapeutic compound for cognitive deficits in schizophrenic patients.Citation104 Improvement by minocycline of impaired recognition memory in methamphetamine-treated mice was also reported by Mizoguchi et al.Citation105
In summary, these findings indicate that minocycline may be useful in the treatment in schizophrenia. However, regardless of how encouraging the preclinical studies have been, clinical trials are required to verify these effects of minocycline.
Minocycline in schizophrenia: clinical studies
Miyaoka et al reported two cases of minocycline being used as adjunctive treatment. In these cases, minocycline produced a remarkable improvement in individuals with acute schizophrenia who had predominantly catatonic symptoms.Citation106 These observations are supported by a case report of a patient with schizophrenia given minocycline 200 mg/day for 8 weeks in addition to a stable dose of olanzapine 20 mg/day.Citation107 The combination of minocycline and antipsychotic treatment significantly reduced positive symptoms, with no negative symptoms seen in the baseline assessment. Significantly decreased hyperperfusion in the posterior cingulate gyrus along with a simultaneous improvement of positive symptoms was observed after treatment with minocycline. Based on these case reports, Miyaoka et al conducted an open-label study in 22 patients with schizophrenia, in whom minocycline was administered for 4 weeks as an adjunct to antipsychotic medication. Minocycline was initiated as 100 mg orally twice daily for the first week, and 150 mg orally three times daily from weeks 2 through 4. The patients showed statistically significant and robust clinical improvements on the Positive and Negative Syndrome Scale, and these improvements were maintained at the follow-up evaluation 4 weeks after the end of treatment with minocycline, with few adverse events.Citation108 However, this was an open-label study without a control group, and the effects of the adjunctive medication cannot be ruled out as an explanation for the observed improvement.
Interestingly, some studies indicate that minocycline may have specific effects on negative symptoms and impaired cognitive function in patients with schizophrenia, which contribute to their poor social and occupational functioning. Case reports indicate the effective role of add on minocycline in treating negative symptoms in schizophrenia.Citation109,Citation110 Recently, Levkovitz et al carried out a double-blind, randomized, placebo-controlled study demonstrating that add on treatment with minocycline 200 mg/day for 6 months had a beneficial effect on negative symptoms and general outcomes in patients with early-phase schizophrenia. A similar pattern was seen for cognitive functioning, mainly of the executive type, ie, working memory, cognitive shifting, and cognitive planning.Citation111 Seventy patients with early-phase schizophrenia were recruited for the study; however, of 54 patients randomly allocated in a 2:1 ratio to the minocycline group, only 13 completed the 6-month trial. This high dropout rate limits the reliability of the results of this study. Another randomized, double-blind, placebo-controlled clinical trial also suggested that addition of minocycline (200 mg/day for one year) to treatment as usual in patients with early psychosis could reduce negative symptoms without a detectable effect on cognition.Citation112 The investigators recruited 144 patients with psychosis (including schizophrenia, schizoaffective disorder, psychosis not otherwise specified, or schizophreniform disorder) within 5 years of first onset in Brazil and Pakistan, and 94 completed the trial. Although the dropout rates were not high when compared with other trials, the validity and reliability of cognitive measures in two different racial groups and cultures could have increased the variability of the results. In addition, neither the type nor dose of antipsychotic medication was controlled, which may have further increased the variability and obscured the effects of the medication. Sofuoglu et al showed that minocycline attenuated dextroamphetamine-induced subjective reward effects and improved reaction times on a Go No-Go task in healthy volunteers.Citation113
Although case reports should to be interpreted with caution and large-scale studies are needed to determine the effectiveness of minocycline and related compounds in clinical use, the results of the above studies suggest that minocycline may be a safe and effective adjunct to antipsychotic medication for the treatment of schizophrenia.
Conclusion
Although the etiology of schizophrenia remains unclear, there has been growing evidence suggesting that neuroinflammation, characterized by overactive microglia, plays an important role in the neuropathology of schizophrenia. The microglial hypothesis of schizophrenia may shed new light on a therapeutic strategy for the disease.Citation1,Citation16 According to this hypothesis and the results of previous related studies, we suggest that overactivation of microglia can lead to neuronal degeneration and abnormal development in the brain, and that this neuropathologic process may be more likely to result in negative symptoms and cognitive impairment in schizophrenia.Citation1,Citation16,Citation21–Citation23,Citation48,Citation49 Neuroinflammation could constitute the basic framework for further study in the neuropathology of negative schizophrenia.
As an inhibitor of microglial activation, minocycline has anti-inflammatory, antioxidant, and antiapoptotic properties, and can modulate glutamate-induced excitotoxicity. The therapeutic potential of minocycline has been demonstrated in studies using animal models of schizophrenia.Citation19,Citation99–Citation105 In addition to the evidence presented here, recent clinical trials have demonstrated that minocycline can be used as an add on therapy in schizophrenia, especially for amelioration of negative symptoms.Citation106–Citation112
Because of the limited and preliminary nature of the studies mentioned above, more detailed studies are required to determine whether minocycline is effective as an adjunctive treatment for symptoms in patients with schizophrenia who derive limited benefit from standard antipsychotic treatment. Moreover, considering the complicated etiology and heterogeneity of schizophrenia, it will be necessary to categorize the disease phenotype in further studies, including patient status and the severity of negative symptoms, which possibly need to be used as inclusion/exclusion criteria or stratifying variables. Finally, given the mechanism of action of minocycline and the data on the role of overactivated microglia in schizophrenia, future clinical studies should include inflammatory markers in blood or cerebrospinal fluid, and functional imaging such as PET to verify the hypothesis that subgroups of schizophrenic patients with neuroinflammation are the most appropriate candidates for augmentation therapy with minocycline.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81071093).
Disclosure
The authors report no conflicts of interest in this work.
References
- MonjiAKatoTKanbaSCytokines and schizophrenia: microglia hypothesis of schizophreniaPsychiatry Clin Neurosci200963325726519579286
- KaneJMCorrellCUPast and present progress in the pharmacologic treatment of schizophreniaJ Clin Psychiatry20107191115112420923620
- LeuchtSArbterDEngelRRKisslingWDavisJMHow effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trialsMol Psychiatry200914442944718180760
- EssaliAAl HajHNLiCRathboneJClozapine versus typical neuroleptic medication for schizophreniaCochrane Database Syst Rev20091CD00005919160174
- AsenjoLCKomossaKRummel-KlugeCClozapine versus other atypical antipsychotics for schizophreniaCochrane Database Syst Rev201011CD00663321069690
- GilmourGDixSFelliniLNMDA receptors, cognition and schizophrenia – testing the validity of the NMDA receptor hypofunction hypothesisNeuropharmacology20126231401141221420987
- TerryAVJrRole of the central cholinergic system in the therapeutics of schizophreniaCurr Neuropharmacol20086328629219506725
- SanderKKottkeTStarkHHistamine H3 receptor antagonists go to clinicsBiol Pharm Bull200831122163218119043195
- MeltzerHYSerotonergic mechanisms as targets for existing and novel antipsychoticsHandb Exp Pharmacol20122128712423129329
- GinovartNKapurSRole of dopamine D(2) receptors for antipsychotic activityHandb Exp Pharmacol2012212275223129327
- MulleJGSchizophrenia genetics: progress, at lastCurr Opin Genet Dev201222323824422424801
- GuptaSKulharaPWhat is schizophrenia: a neurodevelopmental or neurodegenerative disorder or a combination of both? A critical analysisIndian J Psychiatry2010521212720174514
- RapoportJLGieddJNGogtayNNeurodevelopmental model of schizophrenia: update 2012Mol Psychiatry201217121228123822488257
- DeanBUnderstanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studiesInt J Neuropsychopharmacol2011147997101221156092
- MeyerUAnti-inflammatory signaling in schizophreniaBrain Behav Immun20112581507151821664451
- MonjiAKatoTAMizoguchiYNeuroinflammation in schizophrenia especially focused on the role of microgliaProg Neuropsychopharmacol Biol Psychiatry201342511512122192886
- YongVWWellsJGiulianiFCashaSPowerCMetzLMThe promise of minocycline in neurologyLancet Neurol200431274475115556807
- KimHSSuhYHMinocycline and neurodegenerative diseasesBehav Brain Res2009196216817918977395
- DeanOMData-FrancoJGiorlandoFBerkMMinocycline: therapeutic potential in psychiatryCNS Drugs201226539140122486246
- KellerWRKumLMWehringHJKoolaMMBuchananRWKellyDLA review of anti-inflammatory agents for symptoms of schizophreniaJ Psychopharmacol201327433734223151612
- LevkovitzYLeviUBrawYCohenHMinocycline, a second-generation tetracycline, as a neuroprotective agent in an animal model of schizophreniaBrain Res2007115415416217488642
- LevkovitzYMendlovichSRiwkesSA double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophreniaJ Clin Psychiatry201071213814919895780
- ChaudhryIBHallakJHusainNMinocycline benefits negative symptoms in early schizophrenia: a randomized double-blind placebo-controlled clinical trial in patients on standard treatmentJ Psychopharmacol20122691185119322526685
- MullerNMyintAMSchwarzMJInflammation in schizophreniaAdv Protein Chem Struct Biol201288496822814706
- AshdownHDumontYNgMPooleSBoksaPLuheshiGNThe role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophreniaMol Psychiatry2006111475516189509
- ShenQLiZQSunYThe role of pro-inflammatory factors in mediating the effects on the fetus of prenatal undernutrition: implications for schizophreniaSchizophr Res2008991–3485518065207
- MeyerUDevelopmental neuroinflammation and schizophreniaProg Neuropsychopharmacol Biol Psychiatry201342203422122877
- ArionDUngerTLewisDALevittPMirnicsKMolecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophreniaBiol Psychiatry200762771172117568569
- NarayanSTangBHeadSRMolecular profiles of schizophrenia in the CNS at different stages of illnessBrain Res2008123923524818778695
- BessisABechadeCBernardDRoumierAMicroglial control of neuronal death and synaptic propertiesGlia200755323323817106878
- SteinerJBogertsBSarnyaiZBridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrityWorld J Biol Psychiatry201213748249221707463
- AlliotFGodinIPessacBMicroglia derive from progenitors, originating from the yolk sac, and which proliferate in the brainBrain Res Dev Brain Res19991172145152
- Pont-LezicaLBechadeCBelarif-CantautYPascualOBessisAPhysiological roles of microglia during developmentJ Neurochem2011119590190821951310
- SchlegelmilchTHenkeKPeriFMicroglia in the developing brain: from immunity to behaviourCurr Opin Neurobiol201121151020817438
- TremblayMEMajewskaAKA role for microglia in synaptic plasticity?Commun Integr Biol20114222022221655446
- TremblayMEStevensBSierraAWakeHBessisANimmerjahnAThe role of microglia in the healthy brainJ Neurosci20113145160641606922072657
- NurunBNTanakaTKaminoKToll-like receptor 3 mediated hyperphosphorylation of tau in human SH-SY5Y neuroblastoma cellsPsychiatry Clin Neurosci200660Suppl 1S27S33
- BlockMLHongJSMicroglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanismProg Neurobiol2005762779816081203
- LiebermanJAIs schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspectiveBiol Psychiatry199946672973910494440
- Perez-NeriIRamirez-BermudezJMontesSRiosCPossible mechanisms of neurodegeneration in schizophreniaNeurochem Res200631101279129417006758
- NakkiRKoistinahoJSharpFRSagarSMCerebellar toxicity of phencyclidineJ Neurosci1995153 Pt 2209721087891155
- NakkiRNickolenkoJChangJSagarSMSharpFRHaloperidol prevents ketamine- and phencyclidine-induced HSP70 protein expression but not microglial activationExp Neurol199613722342418635538
- SteinerJMawrinCZiegelerADistribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralizationActa Neuropathol2006112330531616783554
- SteinerJBielauHBrischRImmunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicideJ Psychiatr Res200842215115717174336
- BayerTABusleiRHavasLFalkaiPEvidence for activation of microglia in patients with psychiatric illnessesNeurosci Lett1999271212612810477118
- RadewiczKGareyLJGentlemanSMReynoldsRIncrease in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenicsJ Neuropathol Exp Neurol200059213715010749103
- Wierzba-BobrowiczTLewandowskaELechowiczWStepieńTPasennikEQuantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenicsFolia Neuropathol2005432818916012909
- Van BerckelBNBossongMGBoellaardRMicroglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography studyBiol Psychiatry200864982082218534557
- DoorduinJde VriesEFWillemsenATde GrootJCDierckxRAKleinHCNeuroinflammation in schizophrenia-related psychosis: a PET studyJ Nucl Med200950111801180719837763
- TakahashiNSakuraiTRoles of glial cells in schizophrenia: possible targets for therapeutic approachesNeurobiol Dis201353496023146995
- FrickLRWilliamsKPittengerCMicroglial dysregulation in psychiatric diseaseClin Dev Immunol2013201360865423690824
- MonjeMLTodaHPalmerTDInflammatory blockade restores adult hippocampal neurogenesisScience200330256511760176514615545
- EkdahlCTClaasenJHBondeSKokaiaZLindvallOInflammation is detrimental for neurogenesis in adult brainProc Natl Acad Sci U S A200310023136321363714581618
- CacciEClaasenJHKokaiaZMicroglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitroJ Neurosci Res200580678979715884015
- IosifREEkdahlCTAhleniusHTumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesisJ Neurosci200626389703971216988041
- KanekoNKudoKMabuchiTSuppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrusNeuropsychopharmacology200631122619262616823390
- MullerNKrauseDDehningSCelecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatmentSchizophr Res20101211–311812420570110
- MullerNRiedelMScheppachCBeneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophreniaAm J Psychiatry200215961029103412042193
- YrjänheikkiJKeinänenRPellikkaMHökfeltTKoistinahoJTetracyclines inhibit microglial activation and are neuroprotective in global brain ischemiaProc Natl Acad Sci U S A1998952615769157749861045
- YrjänheikkiJTikkaTKeinänenRGoldsteinsGChanPHKoistinahoJA tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic windowProc Natl Acad Sci U S A19999623134961350010557349
- DommeguessMAPlaisantFVermeyCGressensPEarly microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotectionNeuroscience2003121361962814568022
- TikkaTFiebichBLGoldsteinsGKeinanenRKoistinahoJMinocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microgliaJ Neurosci20012182580258811306611
- ArvinKLHanBHDuYLinSZPaulSMHoltzmanDMMinocycline markedly protects the neonatal brain against hypoxic-ischemic injuryAnn Neurol2002521546112112047
- ChenMOnaVOLiMMinocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington diseaseNat Med20006779780110888929
- WuDCJackson-LewisVVilaMBlockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson diseaseJ Neurosci20022251763177111880505
- HomsiSFedericoFCrociNMinocycline effects on cerebral edema: relations with inflammatory and oxidative stress markers following traumatic brain injury in miceBrain Res2009129112213219631631
- AhujaMBishnoiMChopraKProtective effect of minocycline, a semi-synthetic second-generation tetracycline against 3-nitropropionic acid (3-NP)-induced neurotoxicityToxicology20082442–311112218164115
- Garcia-MartinezEMSanz-BlascoSKarachitosAMitochondria and calcium flux as targets of neuroprotection caused by minocycline in cerebellar granule cellsBiochem Pharmacol201079223925019682437
- DavisKLStewartDGFriedmanJIWhite matter changes in schizophrenia: evidence for myelin-related dysfunctionArch Gen Psychiatry200360544345612742865
- KumraSAshtariMCervellioneKLWhite matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging studyJ Am Acad Child Adolesc Psychiatry200544993494116113622
- SalisburyDFKurokiNKasaiKShentonMEMcCarleyRWProgressive and interrelated functional and structural evidence of post-onset brain reduction in schizophreniaArch Gen Psychiatry200764552152917485604
- Hulshoff PolHEKahnRSWhat happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophreniaSchizophr Bull200834235436618283048
- GlantzLAGilmoreJHLiebermanJAJarskogLFApoptotic mechanisms and the synaptic pathology of schizophreniaSchizophr Res2006811476316226876
- OlanowCWJennerPBrooksDDopamine agonists and neuroprotection in Parkinson’s diseaseAnn Neurol1998443 Suppl 1S167S1749749590
- MedinaSMartinezMHernanzAAntioxidants inhibit the human cortical neuron apoptosis induced by hydrogen peroxide, tumor necrosis factor alpha, dopamine and beta-amyloid peptide 1-42Free Radic Res200236111179118412592670
- BuntinxMMoreelsMVandenabeeleFCytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosisJ Neurosci Res200476683484515160395
- ThomasMLeWDMinocycline: neuroprotective mechanisms in Parkinson’s diseaseCurr Pharm Des200410667968614965330
- AlanoCCKauppinenTMVallsAVSwansonRAMinocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrationsProc Natl Acad Sci U S A2006103259685969016769901
- BrundulaVRewcastleNBMetzLMBernardCCYongVWTargeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosisBrain.2002125Pt 61297130812023318
- WangJWeiQWangCYHillWDHessDCDongZMinocycline up-regulates Bcl-2 and protects against cell death in mitochondriaJ Biol Chem200427919199481995415004018
- CastanaresMVeraYErkkilaKMinocycline up-regulates BCL-2 levels in mitochondria and attenuates male germ cell apoptosisBiochem Biophys Res Commun2005337266366916202388
- ScarabelliTMStephanouAPasiniEMinocycline inhibits caspase activation and reactivation, increases the ratio of XIAP to SMAC/DIABLO, and reduces the mitochondrial leakage of cytochrome C and smac/DIABLOJ Am Coll Cardiol200443586587414998631
- ChenMOnaVOLiMMinocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington diseaseNat Med20006779780110888929
- TengYDChoiHOnarioRCMinocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injuryProc Natl Acad Sci U S A200410193071307614981254
- YangDLiuXZhangRIncreased apoptosis and different regulation of pro-apoptosis protein bax and antiapoptosis protein bcl-2 in the olfactory bulb of a rat model of depressionNeurosci Lett20115041182221903151
- KerntMNeubauerASEiblKHMinocycline is cytoprotective in human trabecular meshwork cells and optic nerve head astrocytes by increasing expression of XIAP, survivin, and Bcl-2Clin Ophthalmol2010459160420668721
- TangCMHwangCSChenSDYangDINeuroprotective mechanisms of minocycline against sphingomyelinase/ceramide toxicity: roles of Bcl-2 and thioredoxinFree Radic Biol Med201150671072121184825
- ZhuSStavrovskayaIGDrozdaMMinocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in miceNature20024176884747811986668
- WangXZhuSDrozdaMMinocycline inhibits caspase- independent and dependent mitochondrial cell death pathways in models of Huntington’s diseaseProc Natl Acad Sci U S A200310018104831048712930891
- FuksBTalagaPHuartCIn vitro properties of 5-(benzylsulfonyl)-4-bromo-2- methyl- 3(2H)-pyridazinone: a novel permeability transition pore inhibitorEur J Pharmacol20055191–2243016099453
- LeungAWHalestrapAPRecent progress in elucidating the molecular mechanism of the mitochondrial permeability transition poreBiochim Biophys Acta200817777–894695218407825
- MorimotoNShimazawaMYamashimaTNagaiHHaraHMinocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damageBrain Res20051044181515862784
- KrausRLPasiecznyRLariosa-WillinghamKTurnerMSJiangATraugerJWAntioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activityJ Neurochem200594381982716033424
- WiedholzLMOwensWAHortonREMice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviorsMol Psychiatry200813663164017684498
- WoolleyMLWatersKAGartlonJEEvaluation of the pro-cognitive effects of the AMPA receptor positive modulator, 5-(1-piperidinylcarbonyl)-2,1,3-benzoxadiazole (CX691), in the ratPsychopharmacology (Berl)20092021–334335418795266
- WangJQAroraAYangLPhosphorylation of AMPA receptors: mechanisms and synaptic plasticityMol Neurobiol200532323724916385140
- DuYMaZLinSMinocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s diseaseProc Natl Acad Sci U S A20019825146691467411724929
- WassermanJKSchlichterLCNeuron death and inflammation in a rat model of intracerebral hemorrhage: effects of delayed minocycline treatmentBrain Res20071136120821817223087
- ZhangLKitaichiKFujimotoYProtective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamineProg Neuropsychopharmacol Biol Psychiatry20063081381139316839653
- LevkovitzYLeviUBrawYCohenHMinocycline, a second-generation tetracycline, as a neuroprotective agent in an animal model of schizophreniaBrain Res2007115415416217488642
- MonteASde SouzaGCMcIntyreRSPrevention and reversal of ketamine-induced schizophrenia related behavior by minocycline in mice: possible involvement of antioxidant and nitrergic pathwaysJ Psychopharmacol201327111032104324045882
- HashimotoKTsukadaHNishiyamaSFukumotoDKakiuchiTIyoMProtective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a positron emission tomography study in conscious monkeysBiol Psychiatry200761557758116712806
- ZhangLShirayamaYIyoMHashimotoKMinocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpineNeuropsychopharmacology20073292004201017228338
- FujitaYIshimaTKunitachiSPhencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antibiotic drug minocyclineProg Neuropsychopharmacol Biol Psychiatry200832233633917884273
- MizoguchiHTakumaKFukakusaAImprovement by minocycline of methamphetamine-induced impairment of recognition memory in micePsychopharmacology (Berl)2008196223324117909751
- MiyaokaTYasukawaRYasudaHHayashidaMInagakiTHoriguchiJPossible antipsychotic effects of minocycline in patients with schizophreniaProg Neuropsychopharmacol Biol Psychiatry200731130430717030375
- ChavesCde MarqueCRWichert-AnaLFunctional neuroimaging of minocycline’s effect in a patient with schizophreniaProg Neuropsychopharmacol Biol Psychiatry201034355055220138948
- MiyaokaTYasukawaRYasudaHHayashidaMInagakiTHoriguchiJMinocycline as adjunctive therapy for schizophrenia: an open-label studyClin Neuropharmacol200831528729218836347
- KellyDLVyasGRichardsonCMAdjunct minocycline to clozapine treated patients with persistent schizophrenia symptomsSchizophr Res20111331–325725821872445
- JhamnaniKShivakumarVKalmadySRaoNPVenkatasubramanianGSuccessful use of add-on minocycline for treatment of persistent negative symptoms in schizophreniaJ Neuropsychiatry Clin Neurosci2013251E06E0723487204
- LevkovitzYMendlovichSRiwkesSA double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophreniaJ Clin Psychiatry201071213814919895780
- ChaudhryIBHallakJHusainNMinocycline benefits negative symptoms in early schizophrenia: a randomized double-blind placebo-controlled clinical trial in patients on standard treatmentJ Psychopharmacol20122691185119322526685
- SofuogluMMooneyMKostenTWatersAHashimotoKMinocycline attenuates subjective rewarding effects of dextroamphetamine in humansPsychopharmacology (Berl)20112131616820838775
