Abstract
Background and purpose
Depression is a commonly occurring and persistent sequel of stroke affecting approximately 29% of patients. An immunological hypothesis has been put forward, and synthesis of kynurenine from tryptophan has been proposed to link inflammatory activity with neurotoxicity and neurotransmitter dysfunction. This study assessed the relationship between peripheral blood kynurenine and poststroke depressive symptoms.
Patients and methods
This was a multisite cross-sectional observational cohort study of patients with ischemic stroke. Depressive symptoms were assessed using the Center for Epidemiological Studies Depression (CES-D) scale and divided into high, medium, and low depressive symptom tertiles. Concentrations of kynurenine and tryptophan were assayed from fasting serum samples, and the kynurenine/tryptophan ratio was compared between tertiles. Serum cytokine concentrations were assayed in a subgroup of patients, and the ratio of proinflammatory (IL-6, IL-18, IFNγ, TNF, IL-1β) to anti-inflammatory (IL-10) cytokines compared.
NLM identifier
NCT00254020.
Results
In these patients (n=86, 52.3% male, mean age 71.7±14.2 years), there were no differences in kynurenine/tryptophan ratios between CES-D scale tertiles (F2,76=0.04, P=0.96) controlling for relevant covariates. For cytokines (n=53), serum IL-1β concentrations (F2,52=3.55, P=0.037) and serum ratios of IL-18/IL-10 (F2,52=3.30, P=0.046), IFNγ/IL-10 (F2,52=4.02, P=0.025), and IL-1β/IL-10 (F2,52=4.34, P=0.019) were elevated in the middle CES-D tertile. Post hoc analyses suggested that serum ratios of IL-18/IL-10 (ρ=0.28, P=0.04), and IL-1β/IL-10 (ρ=0.43, P=0.001), as well as IL-1β (ρ=0.29, P=0.04), were significantly associated with fatigue.
Conclusion
Peripheral kynurenine/tryptophan ratios were not associated with depressive symptoms in a poststroke population. However, in exploratory analyses a proinflammatory bias was identified specifically in patients with mild depressive symptoms and associated with poststroke fatigue, suggesting an avenue for future research.
Introduction
Poststroke depression (PSD) affects approximately a third of all stroke survivors.Citation1 The prevalence of PSD reaches a maximum at roughly 3–6 months, diminishes by 50% at 1 year, and may remain elevated for 2 years and beyond.Citation2,Citation3 As such, PSD can be chronic and nonremitting. Up to 35% of patients do not respond to currently available antidepressants, with high nonremission rates, and residual symptoms commonly persist.Citation4 A better understanding of the mechanisms associated with PSD is needed to direct the search for additional or adjunctive therapies.
A relationship between elevations in peripheral proinflammatory cytokines and depression has been suggested in many cross-sectional studies.Citation5 Many cytokines, particularly IFNγ, IL-6, IL-1β, and TNF, upregulate the indoleamine 2,3-dioxygenase enzyme in multiple central and peripheral cell types, which catalyzes the rate-limiting step in the synthesis of kynurenine (KYN) from tryptophan (TRP), thereby increasing their ratio (K/T) in blood.Citation6,Citation7 Changes in both the K/T ratio and concentrations of KYN and its metabolites have been demonstrated cross-sectionally in stroke.Citation8 These changes have also been associated with major depressive disorder (MDD)Citation9–Citation12 and depressive symptoms in patients with cardiovascular disease.Citation13 The further metabolism of KYN into excitotoxic metabolites, which are elevated centrally in MDD postmortem, has been suggested to contribute to neurodegeneration in ischemia and depressive disorders.Citation12,Citation14
A peripheral inflammatory response is observed post-stroke, due to a complex cascade of cytokine signals released from both innate and adaptive immune cells, including macrophages, neutrophils, and T-helper cells.Citation15,Citation16 Clinically, inflammatory status can be surveyed by considering relative concentrations of pro- and anti-inflammatory cytokines, eg, the anti-inflammatory cytokine IL-10 is produced by regulatory T cells, which drop precipitously poststroke.Citation16 IL-10 concentrations are often increased in tandem with proinflammatory cytokine concentrations in healthy individuals, but this relationship is lost in MDD,Citation17 where pro- versus anti-inflammatory cytokine imbalances have been found.Citation17–Citation20 Pro- to anti-inflammatory cytokine imbalances have also been associated with poorer outcomes poststroke, in agreement with the neuroprotective properties of regulatory T cells and IL-10 in animal ischemia models.Citation21–Citation25 Therefore, in the present study, the ratios of proinflammatory cytokines with IL-10 were explored in addition to cytokine concentrations.
While psychosocial, genetic, and physical disability correlates of PSD have been identified, relationships between inflammatory markers and PSD have not been consistently observed.Citation26–Citation28 Although some studies have described relationships between cytokines and fatigue or sickness-like behavior,Citation26,Citation28,Citation29 rather than severe PSD per se, few studies have taken into account that different contributing factors may underlie severe and mild PSD strata. The present study undertook analyses without assuming linear severity relationships. Therefore, the present study examined relationships between the K/T ratio and symptoms of depression in patients poststroke and explored relationships between ratios of pro-to anti-inflammatory cytokines with depressive symptoms. The study also examined possible relationships between cytokines and specific symptoms to account for qualitative differences between patients in depressive symptoms post-stroke. We hypothesized that the K/T ratio (primary) and concentrations of other inflammatory markers would differ between subjects with mild, moderate, and severe depressive symptoms in a poststroke population.
Patients and methods
Participants
This cross-sectional observational study recruited participants admitted to acute care regional stroke centers in Toronto, ON, Canada. Consecutive patients meeting National Institute of Neurological and Communicative Disorders and StrokeCitation30 and World Health Organization MONICA (multinational MONItoring of trends and determinants in CArdiovascular disease)Citation31 criteria for stroke were invited to participate in this study. Patients were recruited from Sunnybrook Health Sciences Centre (Toronto, ON, Canada) and three additional regional stroke centers (Toronto Rehabilitation Institute, St John’s Rehabilitation Centre, and Baycrest Hospital). All patients were referred to the primary study site at Sunnybrook for uniform assessment by the same study personnel. The study was approved by local research ethics boards from each institution, and all participants provided written informed consent. All study procedures were followed in accordance with institutional research ethics boards.
Acute ischemic infarcts were verified from computed tomography or magnetic resonance imaging reports performed at admission for all patients. Inclusion criteria also required participants to speak and understand English. Exclusion criteria were: prestroke diagnosis of dementia or significant cognitive impairment (Mini-Mental State Examination [MMSE] score ≤24), primary hemorrhagic stroke, decreased level of consciousness, severe aphasia or dysarthria, significant acute medical or neurological illness other than stroke, and the presence of a premorbid axis I psychiatric disorder other than depression. Histories of major depression or stroke were permitted and controlled for in statistical analyses as needed.
Assessments
All neuropsychiatric testing was done within 24 hours of the blood draw. Severity of depressive symptoms was assessed using the Center for Epidemiological Studies Depression (CES-D) scale.Citation32 The CES-D scale is a self-report instrument that was used in the National Institutes of Neurological Communicative Disorders Stroke Data Bank,Citation33 and previously validated in stroke patients using a structured psychiatric interview. The CES-D scale has been shown to have high concurrent validity with other depression measures in stroke patients (both observer-reported and self-reported),Citation34 and has been used specifically as a measure of PSD.Citation34–Citation37 CES-D scale scores have also been dichotomized: a score ≥16 was highly predictive of clinical depression (sensitivity 86%, specificity 90%, positive predictive value 80%),Citation38 and a score of 16 or greater has been considered a valid and reliable indicator of PSD.Citation39 The CES-D scale was administered to the participant in the presence of trained research personnel, with assistance if necessary. The MMSECitation40 was selected as a cognitive screening instrument to exclude significant cognitive dysfunction (MMSE score ≤24), because it is brief, widely used, and validated in acute care settings.Citation41 Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS),Citation42 either completed by clinicians upon patient admission or extracted from the medical chart using a standardized method.Citation43 For patients with an available clinical computed tomography scan, stroke lesions were traced and lesion locations and volumes recorded. Patient demographics, risk factors, comorbidities, and concurrent medications were obtained from hospital charts and by patient interview.
Blood sampling and serum analyses
Fasting blood was collected via venipuncture in SST™ Vacutainer® (BD, Franklin Lakes, NJ, USA) tubes at 7.30 am ±30 minutes on the morning after the clinical assessments were conducted. Blood samples were centrifuged at 1,000 g for 10 minutes at 4°C, and serum was separated and stored at −80°C until it was assayed (Sunnybrook Health Sciences Centre). TRP and KYN concentrations were determined by high-performance liquid chromatography (HPLC), as described previously.Citation44,Citation45 TRP was measured by isocratic reverse-phase HPLC without derivatization or fluorescence detection.
For KYN, an equal volume of 3% perchloric acid was used for protein precipitation. After centrifugation, the concentration of L-KYN in the supernatant was measured by HPLC with ultraviolet detection at 258 nm. The mobile phase consisted of 9% acetonitrile in 0.05 M potassium phosphate monobasic, pumped through a reverse-phase 5 μm octadecyl column of 250×4.6 mm (Symmetry®; Waters, Milford, MA, USA). For those participants who provided enough sample, analyses of serum cytokines IL-6, IL-10, IFNγ, TNF, IL-1β and IL-18 were performed using a multiplex suspension bead-array immunoassay (Luminex, Austin, TX, USA). Cytokine concentrations were calculated from the median fluorescence intensities using the Luminex analyzer based on standard curve data obtained using the serially diluted cytokine standards provided in kits (BioSource, Burlington, ON, USA). Assay sensitivities were 0.2 pg/mL for IL-10, 1.0 pg/mL for IFNγ, 0.09 pg/mL for TNF, 0.057 pg/mL for IL-1β, 0.1 pg/mL for IL-6, and 12.5 pg/mL for IL-18. Biochemical assays were performed blinded to all clinical information.
Statistical analysis
For initial descriptive analyses, relationships between CES-D scale total tertiles and serum concentration of KYN, TRP, K/T ratio, lesion volumes, and clinical and demographic covariates were compared. To test the primary hypothesis, a univariate analysis of covariance (ANCOVA) model was performed to assess differences in the K/T ratio between low, intermediate, and highest CES-D scale tertiles, where all important covariates from initial descriptive analyses were included. NIHSS score, age, sex, and history of depression were chosen as covariates a priori, since these were identified previously as independent predictors of PSDCitation2,Citation46 and antidepressant use due to associations with depressive symptoms and immune markers.Citation19,Citation20,Citation47 Age in particular has been found to be positively correlated with the K/T ratio,Citation48 pro- and anti-inflammatory cytokines, and depression scores.Citation2 CES-D scale tertiles were chosen to best model the hypothesis that elevated K/T might manifest as mild depressive symptoms.
Since serum cytokine concentrations below the limits of detectability indicate low serum concentrations, values returned below detectable limits were imputed at the lower limit of detectability for that analyte: 0.2 pmol/L for IL-10, 1.00 pmol/L for IFNγ, 0.09 pmol/L for TNF, and 0.06 pmol/L for IL-1β. Serum assays and stroke-lesion volumes were log-transformed to maintain a normal distribution. For calculating proinflammatory cytokine to IL-10 ratios, they were calculated with raw values and log-transformed afterwards. Associations between immune imbalance and PSD were investigated using individual cytokine concentrations and the ratios of pro- to anti-inflammatory cytokines as exploratory outcomes in all patients for whom these data were available. ANCOVA models were performed to determine which serum cytokines or cytokine ratios were significantly elevated across CES-D scale tertiles. Tukey’s post hoc test was used to identify difference between specific tertiles.
To explore which depressive symptoms were associated with the K/T ratio, or serum cytokines or their ratios, bivariate Spearman’s correlations were conducted between each CES-D scale item and the K/T ratio, as well as the significant serum cytokine ratios from previous analyses. Possible heterogeneity due to histories of depression or antidepressant use were also explored in post hoc analyses. All statistical analyses were performed using SPSS software (version 20; IBM, Armonk, NY, USA). Error bars in all figures represent standard deviation.
Sample size
A sample size of at least 84, divided into three groups, provides a power of 80% to detect the minimum effect size that was observed previously in a cardiovascular populationCitation13 with a two-sided α-value of 0.05.
Results
Patient characteristics
A total of 382 post-ischemic stroke patients were screened for the study, of whom 138 patients were carefully selected who met inclusion criteria and did not meet any criterion for exclusion. A total of 86 patients (mean age 71.7±14.2 years, 47.7% female) who agreed to participate and for whom sufficient serum samples were obtained were included in this analysis. Demographics and clinical characteristics of each tertile are presented in . CES-D scale scores ranged from 0 to 48, with a mean ± standard deviation CES-D scale score of 13.5±11.5. Based on 33rd and 66th percentile CES-D scale scores, CES-D scale tertile ranges were 0–6, 7–16, and 17–48, respectively. Of all demographic and clinical characteristics, only smoking (current) and hypertension differed between CES-D scale tertiles. Concurrent medications did not differ significantly between CES-D scale tertiles. As continuous variables, only NIHSS score (r=0.25, P=0.02) and age (r=0.23, P=0.04) were associated with CES-D scale scores.
Table 1 Clinical demographic characteristics by depression tertiles (n=86)
K/T ratio and depressive symptoms
KYN, TRP, and K/T ratio assay results are reported in . The K/T ratio did not differ between CES-D scale tertiles (F2,76=0.04, P=0.96; see ) in a model controlling for NIHSS score (F1,76=1.12, P=0.28), age (F1,76=4.84, P=0.03), sex (F1,76=0.15, P=0.70), history of depression (F1,76=3.19, P=0.08), antidepressant use (F1,76=0.22, P=0.64), hypertension (F1,76=1.39, P=0.24), and smoking (F1,76=4.54, P=0.04). Neither KYN (F2,76=0.054, P=0.96) nor TRP (F2,76=0.44, P=0.64) concentrations individually differed between CES-D scale tertiles; however, TRP was significantly lower in patients with a history of depression (F2,76=5.43, P=0.022) and in older subjects (F1,76=6.48, P=0.013).
Figure 1 Mean serum kynurenine/tryptophan (K/T) ratios in low (0–6), medium (7–16), and high (17–47) Center for Epidemiological Studies Depression (CES-D) scale tertiles (n=86).
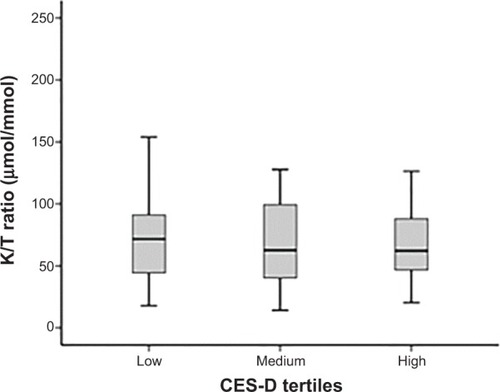
Table 2 Immunological assay resultsTable Footnote*
Cytokine concentrations and ratios
Cytokines were assayed in the 53 patients who provided sufficient serum for analysis, and results are reported in . This subset of patients did not differ from patients who did not provide sufficient serum with respect to age, sex, depression history, hypertension, or other comorbidities (all P>0.05). Of these immunological markers, all KYN, TRP, K/T ratio, IL-6, and IL-18 levels were within detectable range. Imputations were made for 21% of IL-1β, 27% of TNF, 60% of IL-10, and 5.1% of IFNγ samples. Comparison of serum concentrations of IL-6, IL-10, IL-18, IFNγ, TNF, and IL-1β, and their ratios with IL-10 across CES-D scale tertiles are shown in . Of the individual cytokines, only IL-1β concentrations differed significantly between CES-D scale tertiles. Ratios of IL-18/IL-10, IFNγ/IL-10 and IL-1β/IL-10 were also significantly different between CES-D scale tertiles. Post hoc tests revealed that serum IL-18/IL-10 (P=0.046), IFNγ/IL-10 (P=0.025), and IL-1β/IL-10 (P=0.019) () were elevated in the middle CES-D scale tertile compared to the first CES-D scale tertile.
Figure 2 Mean serum cytokine/IL-10 ratios in low (0–6), medium (7–16), and high (17–47) Center for Epidemiological Studies Depression (CES-D) scale tertiles; n=53 for each panel.
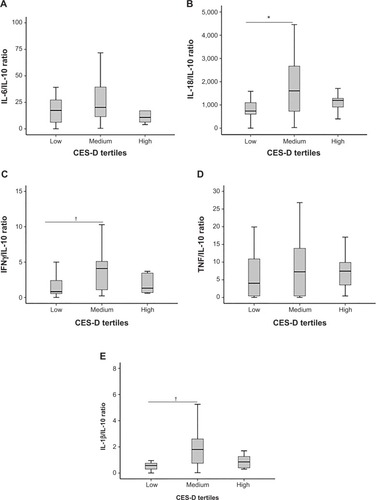
Table 3 Differences between cytokines and cytokine ratios by CES-D tertiles (n=53)
Post hoc analyses
Post hoc analyses of specific depressive symptoms suggested that serum ratios of IL-18/IL-10 (ρ=0.28, P=0.04) and IL-1β/IL-10 (ρ=0.43, P=0.001), as well as IL-1β (ρ=0.29, P=0.04) were significantly associated with CES-D scale item 7 (“everything was an effort”). However, this association was not found with K/T ratios (ρ=0.044, P=0.69). The IFNγ/IL-10 ratio was associated with CES-D scale items 11 (“restless sleeping”, ρ=0.35, P=0.01) and 17 (“crying spells”, ρ=0.29, P=0.04). The IL-18/IL-10 ratio was also associated with CES-D scale item 17 (ρ=0.29, P=0.04). IL-1β was associated with CES-D scale item 15 (“people were unfriendly”, ρ=0.30, P=0.03).
In antidepressant free-patients (n=80), K/T ratios did not differ between CES-D scale tertiles (F2,79=1.49, P=0.19). In those with cytokine assays (n=47), ratios of IL-1β/IL-10 (F2,46=4.87, P=0.01), IFNγ/IL-10 (F2,46=3.83, P=0.03) and IL-1β concentrations (F2,46=4.41, P=0.02) remained different between tertiles, and there remained a trend for IL-18/IL-10 (F2,46=3.01, P=0.06).
In patients without a premorbid history of depression (n=80), K/T ratios did not differ between CES-D scale tertiles (F2,79=0.41, P=0.67). In those with cytokine assays (n=51), ratios of IFNγ/IL-10 (F2,50=3.87, P=0.03), IL-18/IL-10 (F2,50=3.35, P=0.04), IL-1β/IL-10 (F2,50=4.26, P=0.02), and IL-1β concentrations (F2,50=4.01, P=0.03) remained different between tertiles.
To facilitate comparison of our results with other studies, we repeated the main analyses using an established clinically important CES-D scale cutoff of ≥16. K/T ratios did not differ between groups (F1=2.05, P=0.15) in a model adjusted as per the main analysis.
Discussion
In this study, no relationship was identified between the K/T ratio and CES-D scale scores. This finding was maintained after excluding those with antidepressant use or a history of depression. In the context of MDD, both positiveCitation10–Citation12 and negative findings with KYN metabolism have been reported.Citation49–Citation51 As replicated in this study, a recent meta-analysis found disability to be among the most significant predictors of PSD,Citation2 suggesting a minor role for immunological contributions. However, the present findings should be interpreted in light of other known properties of the KYN pathway and additional observations. For instance, whereas the production of quino-linic acid and its intermediates by KYN monooxygenase in microglia may induce oxidative stress and excitotoxicity,Citation14 KYN catabolism into kynurenic acid by KYN aminotransferase II in astrocytes may be neuroprotective.Citation52 Therefore, the production of both neurotoxic and neuroprotective metabolites when in balance may have no net effect on depressive symptoms.Citation10,Citation53 Immunoregulatory effects of KYN may curb neuroinflammation,Citation54 resulting in the neuroprotective properties observed in ischemia models,Citation55 which might obscure deleterious roles of KYN metabolites.Citation54,Citation55 Therefore, while the present study establishes that the peripheral K/T ratio is not a PSD biomarker, it does not preclude possible roles of KYN or its metabolites in the brain.
In the present study, higher K/T ratios and lower TRP concentrations were associated with older age. TRP concentrations were lower in subjects with a history of depression, although KYN concentrations were not different. Therefore, the present study replicates the relationship between low TRP and depressive diathesis seen in other populations, but does not provide evidence to support a role for KYN synthesis in this relationship. Mean K/T ratios in the present study (78 μmol/mmol) and another stroke studyCitation8 were higher than those previously reported in MDD patients (25 μmol/mmol)Citation10 or in coronary artery disease patients (40 μmol/mmol), where depressive symptoms measured by the CES-D scale were associated with the K/T ratio.Citation13 Marked elevations in the K/T ratio poststroke may be above the levels associated with depressive symptoms and instead indicative of other poststroke sequelae.Citation8,Citation56,Citation57
Relationships between cytokines (ie, IL-1β) and cytokine ratios (IL-18/IL-10, IL-1β/IL-10, and IFNγ/IL-10) with depressive symptoms were identified in agreement with someCitation24,Citation26,Citation27,Citation29 but not all previous studies.Citation22,Citation24,Citation25 These exploratory results suggest that the risk of mild PSD symptoms might be better characterized by inflammatory imbalance; IL-1β and the immunologic ratios studied tended to be highest in the intermediate depressive symptoms tertile. These inverted U-shaped curves would be consistent with animal data, in which inflammatory activity produces “sickness behaviors” that sometimes transition into a more severe syndrome reminiscent of human depression.Citation58
The disagreement between studies that dichotomized patients into depressed or nondepressed categories may be partly explained by the heterogeneity in the presentation of depressive episodes poststroke; the present post hoc analyses agree with previous studies,Citation28,Citation29,Citation59 suggesting that somatic symptoms (ie, fatigue and sleep disturbance) may be associated with immune dysregulation, in addition to some core affective symptoms (ie, sadness, crying, and negative social bias). Clinically, cytokines have been associated with post-stroke fatigue but not depression per se.Citation28,Citation29 Our exploratory finding that cytokine ratios (IL-18/IL-10 and IL-1β/IL-10) as well as IL-1β were most consistently associated with CES-D scale item 7 (“everything was an effort”) rather than mood items are consistent with this. This item, either with or without the inertia item (“could not get going”), has been operationalized to measure fatigue.Citation60,Citation61 A recent study examined fatigue more specifically in patients poststroke, finding relationships with TRP availability and a KYN metabolite, but also finding no relationship with PSD.Citation62 Taken together, the results suggest a potential relationship between inflammatory cascades and fatigue and milder depressive symptoms, which may be qualitatively different from MDD.
As a possible limitation, inflammatory markers were not assessed in the acute phase of stroke and examined prospectively, which may have revealed different relationships; however, previous reports suggest that elevations in KYN and its metabolites persist for weeks to years following stroke or traumatic brain injury,Citation8,Citation63 and the present findings suggest that elevations in other inflammatory markers are relevant to depressive symptoms in the subacute phase. These positive findings suggest that it would have been possible to observe a relationship if the K/T ratio was related to PSD. Although the CES-D scale is not a diagnostic instrument, the aim of this study was to characterize the relationship between the K/T ratio and depressive symptoms, for which the CES-D scale has been validated. Moreover, exploratory findings support the hypothesis that there may be nonlinear immunological relationships with depressive symptoms, suggesting the utility of nondichotomous assessment. While KYN and some KYN metabolites can cross the blood–brain barrier,Citation64 peripheral blood concentrations may not represent central concentrations and KYN metabolites were not assayed; however, peripheral KYN has been correlated with cerebrospinal fluid KYN and quinolinic acid concentrationsCitation65 and with post-stroke sequelae,Citation8,Citation57 justifying its exploration as a biomarker for PSD. Finally, the main finding of the present study does not support the primary hypothesis, and the positive findings reported were based on exploratory and post hoc analyses in a subgroup of patients without correction for multiple comparisons. Although the relationship between cytokines and fatigue was determined based on a CES-D scale item, the validity of this item has been established,Citation60,Citation61 and the results concur with those of other studies that used more nuanced fatigue assessments.Citation28,Citation29 Although the exploratory results do not provide rigorous evidence, they are confluent with extant literature and provide an impetus for appropriately powered replicative and/or prospective studies. Finally, the associations observed in this study do not imply causation.
Conclusion
K/T ratios were not associated with depressive symptoms in the acute phase following an ischemic infarct, although associations between depressive symptoms and disability and vascular risk factors were confirmed, and relationships between serum TRP concentrations and histories of depression and age were found. Exploratory analyses suggested that inflammatory activity was associated with mild–moderate but not with severe depressive symptoms. Prospective studies might clarify relationships between immune imbalance and the evolution of depressive symptoms poststroke.
Acknowledgments
This research was supported by grants from the Heart and Stroke Foundation of Canada (T6383 and NA5857). Dr Swardfager was supported by fellowships from the Canadian Partnership for Stroke Recovery and the Toronto Rehabilitation Institute.
Disclosure
The authors report no conflicts of interest in this work.
References
- HackettMLYapaCParagVAndersonCSFrequency of depression after stroke: a systematic review of observational studiesStroke2005361330134015879342
- AyerbeLAyisSWolfeCDRuddAGNatural history, predictors and outcomes of depression after stroke: systematic review and meta-analysisBr J Psychiatry2013202142123284148
- WhyteEMMulsantBHVanderbiltJDodgeHHGanguliMDepression after stroke: a prospective epidemiological studyJ Am Geriatr Soc20045277477815086660
- ChenYPatelNCGuoJJZhanSAntidepressant prophylaxis for post-stroke depression: a meta-analysisInt Clin Psychopharmacol20072215916617414742
- DowlatiYHerrmannNSwardfagerWA meta-analysis of cytokines in major depressionBiol Psychiatry20106744645720015486
- FujigakiSSaitoKSekikawaKLipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanismEur J Immunol2001312313231811477543
- PembertonLAKerrSJSmytheGBrewBJQuinolinic acid production by macrophages stimulated with IFN-gamma TNF-alpha and IFN-alphaJ Interferon Cytokine Res1997175895959355959
- DarlingtonLGMackayGMForrestCMStoyNGeorgeCStoneTWAltered kynurenine metabolism correlates with infarct volume in strokeEur J Neurosci2007262211222117892481
- GabbayVLiebesLKatzYThe kynurenine pathway in adolescent depression: preliminary findings from a proton MR spectroscopy studyProg Neuropsychopharmacol Biol Psychiatry201034374419778568
- MyintAMKimYKVerkerkRScharpeSSteinbuschHLeonardBKynurenine pathway in major depression: evidence of impaired neu-roprotectionJ Affect Disord20079814315116952400
- RiedelWJKlaassenTSchmittJATryptophan, mood, and cognitive functionBrain Behav Immun20021658158912401472
- SteinerJWalterMGosTSevere depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission?J Neuroinflammation201189421831269
- SwardfagerWHerrmannNDowlatiYIndoleamine 2,3-dioxygenase activation and depressive symptoms in patients with coronary artery diseasePsychoneuroendocrinology2009341560156619540675
- SchwarczRWhetsellWOJrManganoRMQuinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brainScience19832193163186849138
- LambertsenKLBiberKFinsenBInflammatory cytokines in experimental and human strokeJ Cereb Blood Flow Metab2012321677169822739623
- SwardfagerWWinerDAHerrmannNWinerSLanctôtKLInterleukin-17 in post-stroke neurodegenerationNeurosci Biobehav Rev20133743644423370232
- DhabharFSBurkeHMEpelESLow serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depressionJ Psychiatr Res20094396296919552919
- KenisGMaesMEffects of antidepressants on the production of cytokinesInt J Neuropsychopharmacol2002540141212466038
- KuberaMLinAHKenisGBosmansEvan BockstaeleDMaesMAnti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratioJ Clin Psychop-harmacol200121199206
- MaesMSongCLinAHNegative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretionNeuropsychopharmacology19992037037910088138
- Basic KesVSimundicAMNikolacNTopicEDemarinVPro-inflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcomeClin Biochem2008411330133418801351
- KimJMStewartRKimSWAssociations of cytokine gene polymorphisms with post-stroke depressionWorld J Biol Psychiatry20121357958721793642
- LieszASuri-PayerEVeltkampCRegulatory T cells are key cerebroprotective immunomodulators in acute experimental strokeNat Med20091519219919169263
- SuJAChouSYTsaiCSHungTHCytokine changes in the pathophysiology of poststroke depressionGen Hosp Psychiatry201234353922055333
- YangLZhangZSunDXuZZhangXLiLThe serum interleukin-18 is a potential marker for development of post-stroke depressionNeurol Res20103234034620482998
- BossùPSalaniFCacciariCDisease outcome alexithymia and depression are differently associated with serum IL-18 levels in acute strokeCurr Neurovasc Res2009616317019534720
- JiménezISobrinoTRodríguez-YáñezMHigh serum levels of leptin are associated with post-stroke depressionPsychol Med2009391201120919356259
- OrmstadHAassHCAmthorKLund-SørensenNSandvikLSerum levels of cytokines, glucose, and hemoglobin as possible predictors of poststroke depression, and association with poststroke fatigueInt J Neurosci201212268269022812657
- OrmstadHAassHCAmthorKFLund-SørensenNSandvikLSerum cytokine and glucose levels as predictors of poststroke fatigue in acute ischemic stroke patientsJ Neurol201125867067621365457
- FoulkesMAWolfPAPriceTRMohrJPHierDBThe Stroke Data Bank: design methods and baseline characteristicsStroke1988195475543363586
- [No authors listed]The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaborationJ Clin Epidemiol1988411051143335877
- RadloffLSThe CES-D scale: a self-report depression scaled for research in the general populationAppl Psychol Meas19771385401
- KunitzSCGrossCRHeymanAThe pilot Stroke Data Bank: definition, design, and dataStroke1984157407466464070
- AgrellBDehlinOComparison of six depression rating scales in geriatric stroke patientsStroke198920119011942772980
- MorrisPLRobinsonRGde CarvalhoMLLesion characteristics and depressed mood in the stroke data bank studyJ Neuropsychiatry Clin Neurosci199681531599081550
- RamasubbuRFlintABrownGAwadGKennedySA neuroendo-crine study of serotonin function in depressed stroke patients compared to non depressed stroke patients and healthy controlsJ Affect Disord19995212113310357025
- RamasubbuRRobinsonRGFlintAJKosierTPriceTRFunctional impairment associated with acute poststroke depression: the Stroke Data Bank StudyJ Neuropsychiatry Clin Neurosci19981026339547463
- ParikhRMEdenDTPriceTRRobinsonRGThe sensitivity and specificity of the Center for Epidemiologic Studies Depression Scale in screening for post-stroke depressionInt J Psychiatry Med1988181691813170080
- ShinarDGrossCRPriceTRBankoMBolducPLRobinsonRGScreening for depression in stroke patients: the reliability and validity of the Center for Epidemiologic Studies Depression ScaleStroke1986172412453961834
- FolsteinMFFolsteinSEMcHughPR“Mini-mental state” A practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res1975121891981202204
- TombaughTNMcIntyreNJThe mini-mental state examination: a comprehensive reviewJ Am Geriatr Soc1992409229351512391
- BrottTMarlerJROlingerCPMeasurements of acute cerebral infarction: lesion size by computed tomographyStroke1989208718752749847
- WilliamsLSYilmazEYLopez-YunezAMRetrospective assessment of initial stroke severity with the NIH Stroke ScaleStroke20003185886210753988
- AndersonGMPurdyWCLiquid chromatographic-fluorometric system for the determination of indoles in physiological samplesAnal Chem197951283286420401
- ForrestCMMackayGMStoyNTryptophan loading induces oxidative stressFree Radic Res2004381167117115621693
- HackettMLAndersonCSPredictors of depression after stroke: a systematic review of observational studiesStroke2005362296230116179565
- KuberaMKenisGBosmansEScharpeSMaesMEffects of serotonin and serotonergic agonists and antagonists on the production of interferon-gamma and interleukin-10Neuropsychopharmacology200023899810869889
- FrickBSchroecksnadelKNeurauterGLeblhuberFFuchsDIncreasing production of homocysteine and neopterin and degradation of tryptophan with older ageClin Biochem20043768468715302611
- FrazerAPandeyGNMendelsJMetabolism of tryptophan in depressive diseaseArch Gen Psychiatry1973295285354748313
- MøllerSEKirkLHonoréPTryptophan tolerance and metabolism in endogenous depressionPsychopharmacology (Berl)19827679836805013
- WoodKHarwoodJCoppenAThe effect of antidepressant drugs on plasma kynurenine in depressed patientsPsychopharmacology (Berl)197859263266104330
- NozakiKBealMFNeuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal ratsJ Cereb Blood Flow Metab1992124004071569135
- SchwarczRPellicciariRManipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunitiesJ Pharmacol Exp Ther200230311012235226
- MezrichJDFechnerJHZhangXJohnsonBPBurlinghamWJBradfieldCAAn interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cellsJ Immunol20101853190319820720200
- GiglerGSzenasiGSimoANeuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbilsEur J Pharmacol200756411612217407777
- BrounsRVerkerkRAertsTThe role of tryptophan catabolism along the kynurenine pathway in acute ischemic strokeNeurochem Res2010351315132220490917
- GoldABHerrmannNSwardfagerWThe relationship between indoleamine 2,3-dioxygenase activity and post-stroke cognitive impairmentJ Neuroinflammation201181721324164
- DantzerRO’ConnorJCFreundGGJohnsonRWKelleyKWFrom inflammation to sickness and depression: when the immune system subjugates the brainNat Rev Neurosci20089465618073775
- MaesMGaleckiPVerkerkRRiefWSomatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activityNeuro Endocrinol Lett20113226427321712776
- De VriesJVan der SteegAFRoukemaJAPsychometric properties of the Fatigue Assessment Scale (FAS)Int J Clin Health Psychol201010125139
- VestergaardSNayfieldSGPatelKVFatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disabilityJ Gerontol A Biol Sci Med Sci200964768219176328
- OrmstadHVerkerkRAmthorKFSandvikLActivation of the kynurenine pathway in the acute phase of stroke and its role in fatigue and depression following strokeJ Mol Neurosci Epub2014325
- MackayGMForrestCMStoyNTryptophan metabolism and oxidative stress in patients with chronic brain injuryEur J Neurol200613304216420391
- FukuiSSchwarczRRapoportSITakadaYSmithQRBlood–brain barrier transport of kynurenines: implications for brain synthesis and metabolismJ Neurochem199156200720171827495
- RaisonCLDantzerRKelleyKWCSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depressionMol Psychiatry20101539340319918244
