Abstract
Background
The Mood Disorder Questionnaire (MDQ) has been translated to many languages and has been used in many countries as a screening instrument for bipolar disorder. The main objective of this study was to evaluate validity of the Thai version of the MDQ as a screening instrument for bipolar disorder in a psychiatric outpatient sample, and to determine its optimum question #1 item threshold value for bipolar disorder.
Methods
The English language Mood Disorder Questionnaire (MDQ) was translated into Thai. The process involved back-translation, cross-cultural adaptation, field testing of the prefinal version, as well as final adjustments. Two hundred and fifty major depressive disorder outpatients were further assessed by the Thai version of the MDQ and the Thai version of the Mini International Neuropsychiatric Interview (MINI). During the assessment, reliability and validity analyses, and receiver operating characteristic curve (ROC) analysis were performed.
Results
The Thai version of the MDQ screening had adequate internal consistency (Cronbach’s alpha =0.791, omega total =0.68, and omega hierarchical =0.69). The optimal question #1 item threshold value was at least five positive items, which yielded adequate sensitivity (76.5%), specificity (72.7%), positive predictive value (74.3%), and negative predictive value (75.0%). The ROC area under the curve (AUC) for this study was 0.82 (95% confidence interval: 0.70 to 0.90).
Conclusion
The Thai version of the MDQ had some useful psychometric properties for screening for bipolar disorder in a mood disorder clinic setting, with a recommended question #1 item threshold value of at least five positive items.
Introduction
Bipolar disorder is a common, serious, recurrent illness marked by episodes of depression and mood elevation, and entails serious psychosocial consequences (eg, suicide, incarceration, bankruptcy, divorce, employment termination, occupational disability, and diminished social function).Citation1 It is one of the leading causes of worldwide disability, especially in those aged 15–44 years.Citation2
Accurate diagnosis of individuals with bipolar disorder can be challenging because people with bipolar disorder commonly present in the more pervasive depressive phase and may not recall previous manic or mixed episodes, or may not be able to distinguish them from prior major depressive episodes, raising the risk that they may receive an inaccurate diagnosis of unipolar major depressive disorder.Citation3 For example, in one study, approximately 70% of patients with bipolar disorder were misdiagnosed,Citation4 with the most common incorrect initial diagnosis being unipolar major depressive disorder.Citation5,Citation6 Misdiagnosis can lead to inappropriate treatment and poor treatment outcome.Citation7 For example, standard antidepressants, which commonly provide adequate efficacy and tolerability in unipolar major depressive disorder, when administered to individuals with bipolar disorder, can have inadequate efficacy (leaving individuals depressed) and/or tolerability (causing emergence of manic symptoms).Citation8 Indeed, investigators have reported that as many as over one-third of bipolar disorder patients may endure as long as a decade of affective symptoms before appropriate diagnosis and treatment.Citation4,Citation9
Hirschfeld et al developed the Mood Disorder Questionnaire (MDQ), a brief self-report screening instrument for bipolar spectrum disorders; the MDQ detects past episodes of mania or hypomania via 13 yes/no items derived from both the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria and clinical experience.Citation10 In clinical settings, the MDQ has had good sensitivity and specificity (73% and 90% respectively), and it has been translated to multiple languages and validated in multiple countries.Citation11–Citation16 Although the sensitivity of the MDQ may be limited in community settings,Citation17 multiple studies have showed that the MDQ is a valid clinical screening instrument for bipolar disorder, even in international settings.Citation11–Citation16
However, the MDQ has not been translated into and validated in the Thai language. In the current study, the objective was to evaluate the validity of the Thai version of the MDQ as a screening instrument for bipolar disorder in major depressive disorder outpatients, and to determine its optimum question #1 item threshold value for bipolar disorder.
Methods
Study design
The study was conducted at an outpatient psychiatric clinic of Ramathibodi Hospital, Bangkok that primarily treats general psychiatric patients. The protocol was approved by the Ethics Committee on Human Experimentation of the Faculty of Medicine, Ramathibodi Hospital, Bangkok, Thailand. All subjects provided verbal and written informed consent prior to participation.
Participants
The participants were native Thai-speaking adults (age ≥18 years) recruited between October 1, 2012 and January 31, 2014 from the psychiatric outpatient clinic at Ramathibodi Hospital; all had a clinical diagnosis of unipolar major depressive disorder, determined by a psychiatrist using the DSM IV, Text Revision (DSM-IV-TR) criteria. Participants with a psychiatric or physical disorder that prevented them from being interviewed or undermined their ability to provide accurate information, and those who declined participation in the study or refused to provide informed consent, were excluded.
Measures
The MDQ is a self-report measure for a lifetime history of mania or hypomania, consisting of 13 yes/no symptom questions based on the DSM-IV criteria for bipolar disorder. The symptom questions are followed by a single yes/no question about whether symptoms clustered during the same period of time. The final question evaluates the level of impairment resulting from the symptoms, with rating on a four-point scale (no problem, minor problem, moderate problem, or serious problem). After obtaining permission from the copyright holder, the MDQ was translated into Thai from the original English MDQ.Citation10 The MDQ was translated into Thai according to the guidelines for cross-cultural adaptation of self-report measures.Citation18 The process included two independent forward translations of the original English MDQ into Thai, consensus between translators on the forward translation, back-translation by a bilingual English teacher, and a review of the back-translation by the authors. Ten patients attending the outpatient department were invited to complete and to give comments on the prefinal version. Final modifications and adjustments were made accordingly.
The Mini International Neuropsychiatric Interview (MINI), Version 5, is a standardized clinical diagnostic interview schedule for DSM-IV Axis-I disorders.Citation19 It can be reliably administered by lay interviewers who have appropriate training. The Thai version of MINI (which was translated from the English version of the MINI, Version 5)Citation20 was used in this study as the “gold standard” diagnostic tool for identifying the presence of bipolar disorder.
Procedure
Subjects were recruited at their scheduled clinic visit, during which they completed the Thai version of the MDQ. Demographic data (eg, sex, date of birth, religion, marital status, education, and occupation) were recorded. After completing the questionnaires, participants were then assessed for bipolar disorder by two research assistants who were unaware of the participants’ MDQ results. The research assistants were trained to use the Thai version of MINI.Citation20 Interrater reliability of scoring was assessed. The agreement between raters was satisfactory (Kappa =0.91).
Statistical analysis
All statistical analysis was performed by using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA) and R and the Psy Package Version 3.1.0 (Northwestern University, Chicago, IL, USA). Descriptive and analytic statistics were compiled. Cronbach’s alpha coefficient and McDonald’s omegaCitation21 were used to assess the internal consistency of the scale. The receiver operating characteristic (ROC) curve was plotted to assess the screening performance of the questionnaire. Its accuracy was calculated in terms of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio for each possible cutoff, and the method of linear interpolation was used to calculate the sensitivity and specificity for each actually possible cutoff (number of positive answers). The optimal question #1 item threshold value was determined by maximizing the Youden’s index. For all analyses, a significant threshold of P<0.05 was used.
Results
Sample description
A total of 250 outpatients with a clinical diagnosis of unipolar major depressive disorder completed the Thai version of the Thai MDQ and were assessed using the Thai MINI. The mean age (standard deviation [SD]) of participants was 46.9 (13.8) years, and 79.2% of participants were female. Demographic data are provided in .
Table 1 Sample description (N=250)
Reliability and item analysis
The Cronbach’s alpha coefficient for the Thai version of the MDQ was 0.791. The McDonald’s omega total and omega hierarchical were 0.68 and 0.69 respectively. The frequency of endorsement of MDQ items ranged from 4.8% to 50.8%. The items that were endorsed most frequently were being “easily distracted” (50.8%), having “decreased need for sleep” (41.2%), and having “irritable mood” (36.8%). The items that were endorsed the least were having “increased interest in sex” (4.8%) and being “more social and outgoing” (6.4%). The corrected item-total correlations ranged from 0.24 to 0.57. All items, if deleted, would consistently decrease the total scale alpha ().
Table 2 Question #1 item-level values and item-total correlation for the Thai version of the MDQ (N=250)
Validity analysis
In 250 participants who were diagnosed with major depressive disorder, a MINI diagnosis of bipolar disorder was given to 60 participants (24.0%) (19 [7.6%] bipolar I disorder¸ and 41 [16.4%] bipolar II disorder).
As applied in the validity study of the original (English) version of the MDQ, when the response to the second question was positive and the third question affirmed moderate to severe severity, the item threshold values for the first question, which includes 13 subitems, were determined by ROC analysis. Area under the curve (AUC) was 0.82 (95% confidence interval [CI]: 0.70–0.90, P<0.0001) ().
Figure 1 Sensitivity and specificity of the MDQ for bipolar disorder at different cutoffs.
Abbreviation: MDQ, Mood Disorder Questionnaire.
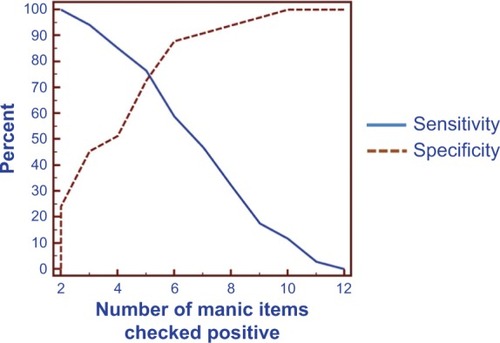
The sensitivity, specificity, PPV, NPV, and likelihood ratio for the Thai version of the MDQ using different question #1 positive item thresholds are provided in . For the Thai version of the MDQ, the sensitivity and specificity at the question #1 item threshold value of at least four positive items were 85.3%, and 51.5%, respectively. Using a question #1 item threshold value of at least five positive items, sensitivity and specificity were 76.5% and 72.7%, respectively. Using a question #1 item threshold value of at least six positive items, sensitivity and specificity were 58.8% and 87.9%, respectively.
Table 3 Sensitivity, specificity, PPV, NPV, and LR of the Thai version of the MDQ for diagnosis of bipolar disorder
By maximizing the Youden’s index, a score of five or more positive items was chosen as the optimal question #1 item threshold value for bipolar disorder as it provided a good balance, with not only adequate sensitivity but also, adequate specificity. By using this five or more question #1 item threshold, more than seven out of ten people with a bipolar disorder would be expected to be correctly identified by the Thai version of the MDQ, whereas more than seven out of ten of those who did not have a bipolar disorder would be expected to be successfully screened out.
Discussion
The internal consistency of the Thai version of the MDQ in this study (Cronbach’s alpha coefficient =0.79) was marginally lower than in the studies of the original English MDQ from the United States (alpha coefficient =0.84–0.90).Citation10,Citation17 However, the reliability of the Thai MDQ was within the acceptable range as Cronbach’s alpha coefficient was greater than 0.70.Citation22 The participants in this study had high rates of endorsement of distractibility, decreased need for sleep, and irritable mood, which were consistent with results from previous studies.Citation10,Citation17
When the Thai version of the MDQ was examined as a continuous measure, its validity was supported by an AUC value of 0.82 (95% CI: 0.70–0.90), which being greater than 0.80, suggested adequate accuracy. The sensitivity and specificity using a question #1 item threshold value of at least five positive items were also adequate, being 76.5% and 72.7%, respectively. Moreover, using a question #1 item threshold value of at least five positive items also yielded an adequate PPV and NPV, of 74.3% and 75.0%, respectively. Results from other studies of the MDQ in clinical settings have included PPV ranging from to 18.4%–96% (depending on cutoff).Citation12,Citation23,Citation24 In our study, the optimal question #1 item threshold value of at least five positive items was comparable with that reported in studies from ChinaCitation13,Citation25 but was more liberal than the question #1 item threshold value of at least seven positive items reported in studies from the United States,Citation10 France,Citation26 Turkey,Citation24 and Hong Kong.Citation23 It has been proposed that the difference in optimal cutoff value from these studies might partly be due to cultural differences.Citation25
Our study has several noteworthy limitations. First, participants were patients already being treated in a university hospital; therefore, the participants may not be representative of the patients usually treated in non-university community settings and were certainly not representative of heterogeneous groups of individuals with and without depression in the community, in general. Second, although the Thai version of the MINI performed well in a validity study,Citation19 it is still possible that it overestimated or underestimated the rate of manic/hypomanic symptoms in our study. Third, our somewhat liberal question #1 item threshold value of at least five versus seven (used in some countries) positive items could have overestimated the prevalence of clinically significant manic/hypomanic symptoms. Fourth, the test-retest reliability of the Thai version of the MDQ was not assessed. Generally, this type of reliability is used for measuring the stability of a scale over time, and it is usually assessed after a short period of time. Fifth, there were substantial limitations on the MDQ’s ability to accurately detect “bipolarity”, including the risks of misdetection of borderline personality disorderCitation27 and attention-deficit/hyperactivity disorderCitation28 as bipolar disorder. Finally, the ability to detect bipolarity in this study was only assessed in patients who were already professionally diagnosed as having had major depressive episodes; in this regard, our sample had a larger percentage of female subjects and a later onset age compared with most cohorts of patients with bipolar disorder.
Conclusion
In summary, the Thai version of the MDQ had some useful psychometric properties for screening for bipolar disorder in a mood disorder clinic setting, with a recommended question one item threshold value of at least five positive items. Thus, the Thai MDQ may be a useful instrument to identify a risk for bipolar disorder in Thai clinical settings. However, if the patient screens positive for possible bipolar disorder with the MDQ, the physician should proceed with full clinical evaluation for bipolar disorder.Citation29 Further population-based research is needed to assess whether the Thai version of the MDQ would be useful in other settings, particularly as the original English MDQ had limited sensitivity in a (nonclinical) community setting.
Author contributions
All the Thai authors contributed to the design of the study and wrote the protocol. Pichai Ittasakul and Terence A Ketter managed the literature searches and analyses. Pattarabhorn Wisajun and Sudawan Jullagate were responsible for data collection. Pichai Ittasakul and Punjaporn Waleeprakhon wrote the draft manuscript. Terence A Ketter revised the draft manuscript. Thus, all authors contributed to development of the manuscript, revised it critically for important intellectual content, and gave their approval of this version to be published.
Acknowledgments
This study was supported by grants from the Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
Disclosure
The authors report no conflicts of interest in this work.
References
- GoodwinFKJamisonKRManic-Depressive Illness: Bipolar Disorders and Recurrent Depression2nd edNew York, NYOxford University Press2007
- World Health OrganizationThe World Health Report 2001 Mental Health: New Understanding, New HopeGenevaWorld Health Organization2001
- KetterTAHandbook of Diagnosis and Treatment of Bipolar DisordersArlington, VAAmerican Psychiatric Publishing, Inc2010
- HirschfeldRMLewisLVornikLAPerceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorderJ Clin Psychiatry200364216117412633125
- GhaemiSNBoimanEEGoodwinFKDiagnosing bipolar disorder and the effect of antidepressants: a naturalistic studyJ Clin Psychiatry20006110804808 quiz 80911078046
- GhaemiSNSachsGSChiouAMPandurangiAKGoodwinKIs bipolar disorder still underdiagnosed? Are antidepressants overutilized?J Affect Disord1999521–313514410357026
- GoldbergJFErnstCLFeatures associated with the delayed initiation of mood stabilizers at illness onset in bipolar disorderJ Clin Psychiatry2002631198599112444811
- PacchiarottiIBondDJBaldessariniRJThe International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disordersAm J Psychiatry2013170111249126224030475
- LishJDDime-MeenanSWhybrowPCPriceRAHirschfeldRMThe National Depressive and Manic-depressive Association (DMDA) survey of bipolar membersJ Affect Disord19943142812947989643
- HirschfeldRMWilliamsJBSpitzerRLDevelopment and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder QuestionnaireAm J Psychiatry2000157111873187511058490
- ZaratieguiRLorenzoLSVázquezGHValidation of the Argentine version of the Mood Disorder QuestionnaireVertex20112297165171 Spanish22091450
- de Sousa GurgelWRebouçasDBNegreiros de MatosKJCarneiroAHGomes de Matos e SouzaFGrupo de Estudos em Transtornos Afetivos Affective Disorders Study GroupBrazilian Portuguese validation of Mood Disorder QuestionnaireCompr Psychiatry201253330831221632039
- YangHCYuanCMLiuTBValidity of the Chinese version Mood Disorder Questionnaire (MDQ) and the optimal cutoff screening bipolar disordersPsychiatry Res2011189344645021402414
- Sanchez-MorenoJVillagranJMGutierrezJREDHIPO (Hypomania Detection Study) GroupAdaptation and validation of the Spanish version of the Mood Disorder Questionnaire for the detection of bipolar disorderBipolar Disord200810340041218402628
- TwissJJonesSAndersonIValidation of the Mood Disorder Questionnaire for screening for bipolar disorder in a UK sampleJ Affect Disord20081101–218018418261804
- LinCJShiahISChuHReliability and validity of the Chinese Version of the Mood Disorder QuestionnaireArch Psychiatr Nurs2011251536221251602
- HirschfeldRMHolzerCCalabreseJRValidity of the mood disorder questionnaire: a general population studyAm J Psychiatry2003160117818012505821
- BeatonDEBombardierCGuilleminFFerrazMBGuidelines for the process of cross-cultural adaptation of self-report measuresSpine (Phila Pa 1976)200025243186319111124735
- SheehanDVLecrubierYSheehanKHThe Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10J Clin Psychiatry199859Suppl 20S22S33 quiz S34–S57
- KittirattanapaiboonPKhamwongpinMThe validity of the Mini International Neuropsychiatric Interview (M.I.N.I.)-Thai versionJournal of Mental Health of Thailand2005133126136
- RevelleWZinbargRECoefficients alpha, beta, omega and the glb: comments on SijtsmaPsychometrika2009741145154
- StreinerDLNormanGRScaling responsesHealth Measurement Scales: A Practical Guide to Their Development and Use2nd edOxfordOxford University Press19952853
- ChungKFTsoKCCheungEWongMValidation of the Chinese version of the Mood Disorder Questionnaire in a psychiatric population in Hong KongPsychiatry Clin Neurosci200862446447118778445
- KonukNKiranSTamamLKaraahmetEAydinHAtikLValidation of the Turkish version of the mood disorder questionnaire for screening bipolar disordersTurk Psikiyatri Derg2007182147154 Turkish17566880
- GanZHanZLiKValidation of the Chinese version of the “Mood Disorder Questionnaire” for screening bipolar disorder among patients with a current depressive episodeBMC Psychiatry201212822293033
- Weber RougetBGervasoniNDubuisVGex-FabryMBondolfiGAubryJMScreening for bipolar disorders using a French version of the Mood Disorder Questionnaire (MDQ)J Affect Disord200588110310816054228
- ZimmermanMGalioneJNRuggeroCJScreening for bipolar disorder and finding borderline personality disorderJ Clin Psychiatry20107191212121720361913
- ZimmermanMGalioneJNChelminskiIYoungDDalrympleKPsychiatric diagnoses in patients who screen positive on the Mood Disorder Questionnaire: Implications for using the scale as a case-finding instrument for bipolar disorderPsychiatry Res2011185344444920656360
- HirschfeldRMThe Mood Disorder Questionnaire: A simple, patient-rated screening instrument for bipolar disorderPrim Care Companion J Clin Psychiatry20024191115014728
