Abstract
There are studies showing that gene polymorphisms within the transforming growth factor-β (TGF-β) signaling constitute schizophrenia risk variants. However, the association between TGFB1 gene polymorphisms (+869T/C and +915G/C), TGF-β level with schizophrenia course, and its symptomatology together with cognitive functioning has not been investigated so far. We included 151 patients with schizophrenia and 279 healthy controls. Cognitive functioning was assessed using Rey Auditory Verbal Learning Test, Trail Making Test (TMT)-A and TMT-B, Verbal Fluency task, Stroop test, as well as selected subtests from the Wechsler Adults Intelligence Scale – Revised, Polish adaptation (WAIS-R-Pl): Digit Symbol Coding, Digit Span Forward and Backward, and Similarities. Additionally, serum TGF-β levels were measured in 88 schizophrenia patients and 88 healthy controls. Serum TGF-β level was significantly higher among patients with schizophrenia in comparison with healthy controls; however, the studied polymorphisms were not associated with TGF-β level in schizophrenia patients. Subjects carrying the +869T allele performed significantly worse in comparison with +869CC homozygotes on Stroop task, Verbal Fluency task and Digit Symbol Coding task. There was a significant difference in age of psychosis onset in female schizophrenia patients with respect to the TGFB1 +869T/C polymorphism. Additionally, adjustment for possible confounders revealed that there was a significant difference in cognitive performance on Digit Symbol Coding task with respect to the TGFB1 +869T/C polymorphism among female schizophrenia patients. Our results suggest that TGF-β signaling might be a valid link contributing to observed differences in age of onset and the level of cognitive decline between male and female schizophrenia patients.
Introduction
Cytokine alterations are increasingly recognized as part of schizophrenia pathophysiology.Citation1–Citation3 Some cytokines including interleukin (IL)-12, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) serve as trait markers of schizophrenia as they are increased in patients with first-episode psychosis and acute relapse, and during symptomatic stability. Other cytokines, including IL-1β, IL-6, and transforming growth factor-β (TGF-β) appear to be state markers, which are increased during acute relapses in comparison with periods of symptomatic stability.Citation4
TGF-β is a regulatory cytokine that influences proliferation, differentiation, and survival of lymphocytes, natural killer cells, dendritic cells, macrophages, mast cells, and granulocytes.Citation5 Interestingly, TGF-β has been found to regulate differentiation and survival of midbrain dopaminergic neurons.Citation6–Citation8 The recent genome-wide association studies data analysis revealed that TGF-β signaling is one of the top ranked pathways associated with schizophrenia.Citation9 In addition, overexpression of the ZNF804A gene, which is a susceptibility gene for schizophrenia supported by genome-wide association studies data, has been found to upregulate genes implicated in the TGF-β signaling.Citation10 There is also one postmortem study showing that the TGF-β signaling pathway genes are upregulated within hippocampal cells in schizophrenia patients.Citation11
The TGF-β gene (TGFB1) has biallelic single nucleotide polymorphisms (SNPs) at codon 10 (position +869) and at codon 25 (position +915), both causing amino acid substitutions and both known to influence alterations in TGF-β synthesis.Citation12,Citation13 Our investigation of these SNPs showed that the TGFB1 +869T/C polymorphism is associated with schizophrenia susceptibility.Citation14 The risk of schizophrenia was more than twofold higher in carriers of the T allele (TT and TC genotypes) in comparison with patients with the CC genotype. Interestingly, when we analysed sex differences, this association was significant in females, while in males it showed a trend towards statistical significance, suggesting the influence of sex differences on the TGF-β signaling.
Emerging evidence indicates that genetic factors influencing the TGF-β signaling may be associated with cognitive deficits. Loeys et alCitation15 identified heterozygous mutations in genes encoding TGF-β receptors (TGFBR1 and TGFBR2) that underlie the development of the syndrome of cardiovascular, craniofacial, neurocognitive, and skeletal disturbances. Furthermore, transgenic mice models overexpressing TGF-β are characterized by cognitive deficits mimicking those specific for Alzheimer’s disease.Citation16,Citation17 Finally, there is one study showing the association between the TGFB1 +869T/C polymorphism and the risk of Alzheimer’s disease.Citation18 However, there are no studies addressing the influence of the TGF-β signaling on cognitive functioning in schizophrenia.
The aim of this study was to investigate whether polymorphisms in the TGFB1 gene (+869T/C and +915G/C) or serum TGF-β level are associated with clinical manifestation, course of the disorder, and cognitive performance in schizophrenia.
Material and methods
Subjects
We recruited 279 controls (61 females and 196 males of mean age 38.7±8.8 years), and 158 patients with schizophrenia (89 females and 69 males of mean age 38.0±11.9 years) including individuals recovered from acute relapse and stable outpatients.Citation14 All participants were Caucasians and came from the same geographic area – Lower Silesia. The study was approved by the Ethics Committee at Wroclaw Medical University and all subjects gave informed consent. The study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments. Participants were excluded on the basis of the following criteria: history of traumatic brain injury, neurologic disorders, severe physical health impairments, and comorbid substance use disorders with the exception of nicotine. Additionally, they had no current infections, allergies, nor present and past history of autoimmune disorders. Present, past, and family history of psychiatric illness was negative in all control subjects.
Genotyping of the TGFB1 polymorphisms (+869T/C and +915G/C) was performed in all participants according to the methodology described in our previous article,Citation14 while serum TGF-β level was assessed in the randomly selected group of 88 schizophrenia subjects and 88 healthy controls matched for sex, age and genotype distribution.
Assessment of psychopathology and cognitive functions
A diagnosis of schizophrenia was based on Diagnostic and Statistical Manual of Mental Disorders IV criteria and established by the same two senior board psychiatrists. Lifetime psychopathology and course of schizophrenia were assessed using the Operational Criteria for Psychotic Illness (OPCRIT) checklist.Citation19 In turn, psychopathology on the day of assessment was evaluated using the Positive and Negative Syndrome Scale (PANSS),Citation20 the Scale for Assessment of Positive Symptoms (SAPS),Citation21 and the Scale for Assessment of Negative Symptoms (SANS).Citation22 Assessment of cognitive performance was performed in schizophrenia patients using the following tests: Rey Auditory Verbal Learning Test,Citation23 Trail Making Test (TMT-A, TMT-B),Citation24 verbal fluency tests (F, A, and S letters,Citation25 and supermarket),Citation26 Stroop test,Citation27 as well as selected Wechsler Adults Intelligence Scale – Revised, Polish adaptation (WAIS-R-Pl) subtests (Digit Symbol Coding Test, Digit Span Forward and Backward, and Similarities).Citation28
Genotyping
Genomic DNA was obtained from peripheral blood leukocytes (from whole frozen blood) using the QIAamp DNA Blood Mini Kit (Qiagen NV, Venlo, the Netherlands). The TGFB1 +869T/C (rs1800470, c.29C>T) and the TGFB1 +915G/C (rs1800471, c.74G>C) polymorphisms were examined by the polymerase chain reaction (PCP) with sequence specific primers technique (single specific primer-PCR) using the PCYTGEN kit (One Lambda, Canoga Park, CA, USA). The PCR products were visualized on 2% agarose gel.
TGF-β measurement
Serum samples were stored in aliquots at −80°C. Serum concentrations were measured blinded for case and control status. Serum hs-CRP was measured with use of the C-reactive protein extended range method on the Dimension® clinical chemistry system (Siemens Healthcare Diagnostics Inc., Newark, NJ, USA). Serum levels of TGF-β were measured using a commercially available Human TGF-β Immunoassay (Quantikine® ELISA; R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer’s instructions.
Statistics
The differences in serum levels of TGF-β between subjects with different TGFB1 +869T/C and +915G/C genotypes, as well as different allele carriers in both schizophrenia patients and healthy controls, were compared using Kruskal–Wallis test and Mann–Whitney U-test. The analysis of covariance was performed to control for age and sex. Demographic and clinical data with respect to the TGFB1 +869T/C polymorphism among patients with schizophrenia were compared using Kruskal–Wallis test (body mass index [BMI], age, pack-year smoking index, chlorpromazine equivalent, years of education, disease duration, number of previous episodes) and χ2 test (sex, education, family history of schizophrenia, course of the disorder). Kaplan–Meier survival analysis was used to assess the difference in the age of psychosis onset between subjects with different TGFB1 +869T/C and +915G/C genotypes. Differences between two survival curves were tested with log-rank test. Cognitive performance and psychopathological manifestation with respect to the TGFB1 +869T/C and +915G/C were compared using Kruskal–Wallis test and Mann–Whitney U-test. Correlations between clinical variables and cognitive functioning with serum TGF-β level were assessed using Spearman’s rank correlations (Spearman’s rho). The association between the TGFB1 polymorphisms and TGF-β serum level with cognitive functioning was performed using linear regression analysis adjusting for age, education level, illness duration, total PANSS score, BMI, smoking, and chlorpromazine equivalent. Differences were considered as statistically significant if the two-tailed P-value was less than 0.05. All analyses were performed using the Statistical Package for Social Sciences version 20.
Results
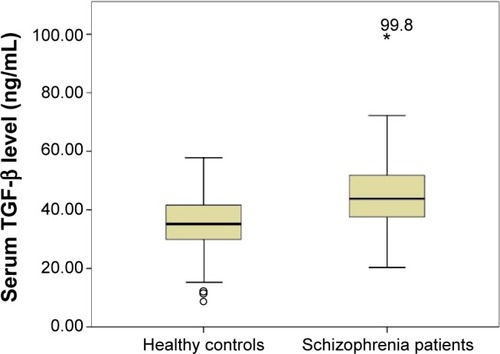
Serum TGF-β levels were significantly higher in schizophrenia patients in comparison with healthy controls (median ± interquartile range: 43.56±13.36 ng/mL and 35.15±11.83 ng/mL, respectively, P<0.0001) (). This difference was still statistically significant after covarying for age and sex of the participants (F=25.28, P<0.0001).
All results for patients and controls were in Hardy–Weinberg equilibrium (P>0.05). The association of the TGFB1 polymorphisms with serum TGF-β level are presented in and . With respect to the TGFB1 +869T/C polymorphism, healthy controls with CT and CC genotypes were characterized by higher levels of serum TGF-β in comparison with subjects with TT genotype (P=0.005) (). There was no significant association between the TGFB1 +869T/C polymorphism and TGF-β level in schizophrenia patients. With respect to the TGFB1 +915G/C polymorphism, there was no significant association with serum TGF-β either in schizophrenia patients or in healthy controls (P>0.05) ().
Table 1 The comparison of TGF-β levels between schizophrenia patients and healthy controls with respect to distinct genotypes and alleles’ carriers of the TGFB1 +869T/C polymorphism
Table 2 The comparison of TGF-β levels between schizophrenia patients and healthy controls with respect to distinct genotypes and alleles’ carriers of the TGFB1 +915G/C polymorphism
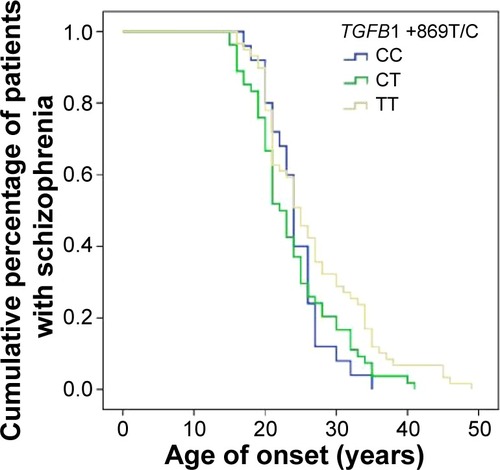
There was no association between the TGFB1 +869T/C nor +915G/C polymorphisms with domains evaluated by the OPCRIT checklist such as: disturbances related to speech and form of thought; affect and associated features; appearance and behavior; as well as abnormal beliefs and abnormal perceptions (data not shown). However, there was a significant difference in age of psychosis onset between schizophrenia patients with distinct TGFB1 +869T/C polymorphism genotypes (log-rank test χ2=6.54, P=0.038) (). When the patients were divided with respect to sex, there was still a significant association in females (log-rank test χ2=9.27, df=2, P=0.01) but not in males (log-rank test χ2=0.57, df=2, P=0.75). With respect to the TGFB1 +915G/C polymorphism, there were no significant associations with age of psychosis onset either in female or in male subjects (data not shown).
Figure 2 Kaplan–Meier survival plot of genotype-specific age of onset distribution curves with respect to the TGFB1 +869T/C polymorphism.
General characteristics with respect to the TGFB1 + 869T/C polymorphism are provided in . There was no significant association between the TGFB1 +869T/C polymorphism and demographic variables, basic clinical characteristics (number of previous episodes, illness duration, family history of schizophrenia, and chronic course of the disorder), or possible confounding factors (chlorpromazine equivalent, BMI, and pack-year index for cigarette smoking). Similarly, the TGFB1 +915G/C polymorphism was not associated with any of these variables (data not shown). There was no statistically significant difference between demographic and clinical variables among male and female patients with respect to the TGFB1 +869T/C and +915G/C polymorphisms (data not shown).
Table 3 General demographic and clinical characteristics of schizophrenia patients with respect to the TGFB1 +869T/C polymorphism
All of the patients had been medicated on the day of the assessment. The mean duration of treatment was 12.24±12.24 years. The majority of patients were treated with second-generation antipsychotic drugs (14.58% olanzapine, 27.08% risperidone, 12.50% quetiapine, 21.87% clozapine, 11.45% ziprasidone, 2.08% aripiprazole, 2.08% sertindole), while the rest of the patients were treated with first-generation antipsychotics (2.08% chlorpromazine, 1.04% perazine, 1.04% zuclopenthixol, 4.16% haloperidol). The mean value of daily chlorpromazine equivalent dose was 571.84±401.98 mg/day.Citation29
Correlations between TGF-β level and clinical variables are shown in . TGF-β serum level was not associated with illness duration, number of previous episodes, number of years of completed education, age, pack-year smoking index, BMI, or chlorpromazine equivalent dosage. Serum TGF-β was not associated either with schizophrenia psychopathology or cognitive performance (). There was no difference between male and female patients with respect to age (P=0.09), number of previous episodes (P=0.56), BMI (P=0.25), pack-year index of cigarette smoking (P=0.18), chlorpromazine equivalent dosage (P=0.72), education level (P=0.17), number of years of completed education (P=0.11), PANSS total score (P=0.10), or any of the cognitive tests (P>0.05). However, there was a difference in the age of onset (females 26.6±7.8 years, males 23.12±4.44 years, P=0.016), which is one of the most commonly described aspects of sex dimorphism in schizophrenia. There was no significant difference in TGF-β level between female and male patients (median ± interquartile range: 44.15±13.70 ng/mL, 42.85±14.10 ng/mL, respectively, P=0.47). Additionally, the TGFB1 +869T/C polymorphism did not influence TGF-β level either in female (P=0.78) or in male patients (P=0.19). Similarly, the TGFB1 +915G/C polymorphism did not influence TGF-β level either in female (P=0.65) or in male patients (P=0.14).
Table 4 Correlations between TGF-β level and clinical variables in patients with schizophrenia
Table 5 Psychopathological manifestation and cognitive performance with respect to the TGFB1 +869T/C polymorphism
There was no significant difference in psychopathological manifestation assessed using PANSS, SAPS, and SANS with respect to the TGFB1 +869T/C and +915G/C polymorphisms (P>0.05). There was no significant association between TGFB1 +915G/C polymorphism and cognitive functioning in schizophrenia (data not shown); however, subjects carrying the +869T allele performed worse in comparison with +869CC homozygotes on Stroop test (P=0.02), Verbal Fluency task (F words) (P=0.03), and on Digit Symbol Coding task (P=0.06).
After adjustment for possible confounding factors, such as age, education level, illness duration, total PANSS score, BMI, smoking, chlorpromazine equivalent, and TGF-β serum level, there was a statistically significant difference in cognitive performance on Digit Symbol Coding task with respect to the TGFB1 +869T/C polymorphism among female schizophrenia patients (β=−0.57, t=−2.78, P=0.02).
Discussion
There are numerous studies showing the influence of the immune system deregulation on the risk of schizophrenia and its clinical manifestation.Citation30,Citation31 However, the association between the TGFB1 gene polymorphisms and TGF-β level with schizophrenia course and symptomatology, together with cognitive functioning, has not been investigated so far.
In our study, we showed that TGF-β level was significantly higher in patients with schizophrenia than in healthy control subjects, even after covarying for age and sex. The majority of previous studies on medicated and drug-naïve first-episode patients have also shown higher TGF-β levels in schizophrenia subjects in comparison to healthy controls,Citation32–Citation34 with the exception of one study that failed to show this association.Citation35
Studies, which have attempted to establish the relationship between the TGFB1 gene polymorphisms and TGF-β level, have yielded ambiguous results.Citation36,Citation37 In the present study, we found that the TGFB1 +869T/C and +915G/C polymorphisms are not associated with TGF-β level in schizophrenia patients. However, we revealed that the TGFB1 +869T/C but not the TGFB1 +915G/C polymorphism is associated with TGF-β level in healthy controls. The explanation of a differential contribution of the TGFB1 +869T/C polymorphism in schizophrenia patients and healthy controls would originate from a complex regulation of gene expression within the TGF-β signaling pathway. In the recent study by Umeda-Yano et alCitation10 it was found that overexpression of the ZNF804A gene may lead to altered expression of genes encoding proteins acting on the TGF-β signaling pathway. Notably, large data sets indicate that the SNP (rs1344706) in the ZNF804A gene is one of the top genetic variants associated with schizophrenia.Citation38
In our study, we showed no association between TGF-β levels and clinical variables, including current (PANSS, SANS, SAPS) or lifetime (OPCRIT) psychopathological manifestation of schizophrenia. Other researchers have also found no correlations with current clinical status as assessed by the Brief Psychiatric Rating ScaleCitation30 or PANSS.Citation39 However, the analysis of counter-regulatory cytokines ratio, such as IFN-γ/TGF-β ratio, has shown a weak negative correlation with negative symptoms and general psychopathology subscales of PANSS,Citation31 as well as the total PANSS score.Citation31,Citation32 Additionally, a weak negative correlation has been found between the IL-17/TGF-β ratio and the negative and general psychopathology PANSS subscales.Citation32
We found no significant association between the TGFB1 gene polymorphisms and schizophrenia current and life-time symptomatology, or variables associated with course of the disorder. However, we found that the TGFB1 +869T/C polymorphism may predict age of psychosis onset in female patients. Indeed, females with the +869TT and +869CT genotypes had significantly later age of psychosis onset in comparison with females with the +869CC genotype. These findings are in agreement with the results that were previously published by our group.Citation14 We found that the risk of schizophrenia is more than twofold higher in the +869T allele carriers in comparison with those with the +869CC genotype. When we conducted a separate analysis of male and female subjects, we found that this association was significant in females, while in males it reached a trend toward significant difference. Several lines of evidence support strong heritability of age at onset in schizophrenia.Citation40–Citation42 Moreover, there is a plethora of studies linking SNPs to age of psychosis onset. Although the majority of these studies show that the risk allele for schizophrenia is simultaneously associated with earlier age of psychosis onset, there is a considerable number of studies linking the risk polymorphic variant to later age of psychosis onset.Citation43–Citation45
Cognitive impairment serves as the core component of schizophrenia symptomatology that accounts for poor functional outcome.Citation46,Citation47 Although the efficacy of current pharmacological strategies with respect to psychotic symptoms seems to be sufficient, their impact on cognition leaves much to be desired.Citation48 Therefore, our understanding of biological mechanisms underlying cognitive dysfunction in schizophrenia patients appears to be a major challenge for current psychiatric research. However, immune alterations are sparsely addressed in studies focused on cognitive impairment in schizophrenia patients. There is evidence that C-reactive protein (CRP) level negatively correlates with cognitive functioning.Citation49,Citation50 It has also been found that this relationship is augmented by Herpes Simplex Virus-1Citation51,Citation52 and cytomegalovirus seropositivity.Citation52 Additionally, the leukemia inhibitory factor gene polymorphism has been associated with deterioration in working memory function in patients with schizophrenia.Citation53 With respect to cytokine serum levels in schizophrenia, cognitive impairment has been associated with higher IL-6Citation50 and IL-18Citation54 as well as lower IL-2Citation55 levels.
Our study is the first to address cognitive correlates of TGF-β level and the TGFB1 gene polymorphisms in schizophrenia. We found no association between TGF-β level and cognition; however, our findings suggest that the TGFB1 +869T/C polymorphism is associated with cognitive functioning in schizophrenia. In our study, the +869T allele carriers performed worse on tests measuring processing speed (Digit Symbol Coding task, Stroop congruent task) and verbal fluency (F words). Interestingly, after adjustment for potential confounders, such as age, education level, illness duration, total PANSS score, BMI, smoking, chlorpromazine equivalent, and TGF-β serum level, the +869T allele carriers scored significantly lower only on Digit Symbol Coding task in comparison with the +869CC homozygotes, among female patients. The meta-analysis of neuropsychological measures in schizophrenia revealed that Digit Symbol Coding task shows significantly larger effect in differentiating schizophrenia and healthy subjects in comparison with other cognitive measures including verbal memory, executive functioning, or working memory tasks.Citation56 Digit Symbol Coding task is considered to be a cognitive endophenotype – a measurable biomarker that is correlated with an illness due to shared underlying genetic influences. It has been shown to have high heritability and stable trait-like qualities, and is associated with prognosis, as well as functional outcome among patients with schizophrenia.Citation56
Interestingly, studies investigating the polymorphism in the ZNF804A gene (rs1344706), known to upregulate genes implicated in the TGF-β signaling,Citation10 have shown its correlation with several domains of cognitive performance including episodic and working memory, executive functions, verbal learning, and recall.Citation57–Citation61 Most interestingly, the ZNF804A gene polymorphism (rs1344706) has been found to predict poorer executive control of attention in schizophrenia, overlapping partially with the results of our study.Citation60 Additionally, there is one study that established a susceptibility link between the TGFB1 +869T/C polymorphism and Alzheimer’s disease,Citation18 suggesting that cognitive decline both in schizophrenia and dementia may be partially influenced by the same set of genes associated with the immune system.Citation62,Citation63
Our results, showing the association of the TGFB1 +869T/C polymorphism with age of onset and cognitive performance in female patients, point to the list of sex differences in schizophrenia (for review seeCitation64,Citation65). Indeed, female patients with schizophrenia tend to have later age of psychosis onset;Citation66 more affectiveCitation67 and less negative symptoms;Citation68 as well as less severe course of the disorder.Citation69 The presence of sex differences in schizophrenia has been attributed to neuroprotective and antidopaminergic properties of estradiol.Citation65,Citation69,Citation70 To support hormonal underpinnings, it has been found that there is a fluctuation in the severity of psychopathological manifestation across the menstruation cycle, with deterioration of symptomatology during low-estrogen phases.Citation71 Furthermore, chronic psychosis is prone to improvement during pregnancy.Citation72 Finally, low-estrogen lifespan phases including perimenstrual phase of the menstrual cycle, postmenopause, and the period after abortion are characterized by higher susceptibility to psychosis.Citation71 Interestingly, it has been found that females with first-episode psychosis are characterized by significantly higher TGF-β levels.Citation33 There is also a crosstalk between TGF-β and estradiol. It has been found that estradiol increases the production of TGF-β in cortical and hypothalamic astrocytes.Citation73,Citation74 On the other hand, TGF-β may stimulate the production of estradiol via promotion of the basal secretion of follicle-stimulating hormone.Citation75 However, these interactions are yet to be investigated in schizophrenia patients.
Our results should be interpreted with caution due to limitations that require further discussion. Firstly, it should be noted that our sample size was limited; however, it is one of the largest studies analyzing serum cytokine levels with respect to cognitive functioning in schizophrenia.Citation50,Citation54,Citation55,Citation76 Secondly, all our patients were medicated, while the association of TGF-β level with cognitive functioning in unmedicated first-episode schizophrenia patients would be much more reliable. However, it should be noted that we found no association between TGF-β level and daily chlorpromazine equivalent dose, and previous studies have shown higher TGF-β levels in both drug-naïve and medicated schizophrenia patients in comparison to controls.Citation32 Additionally, our study assessed cognitive functions only at one point while, in the future, longitudinal design might bring insight into how changes in antipsychotic treatment are translated into changes in TGF-β level and cognitive performance with time, especially in first-episode patients. Moreover, in our study, we did not assess menstrual cycle stage in female participants. This should be included in the future studies’ protocols, as this information may shed more light on the reciprocal interaction between estrogen and TGF-β levels in the brain, possibly playing a role in the sex dimorphism of schizophrenia. Finally, future studies should look into the TGFB1 +869T/C polymorphism together with TGF-β level on all indices associated with metabolic syndrome, including hypertension and glucose intolerance, especially due to their high prevalence in schizophrenia and their influence on cognition in this group of patients.
Acknowledgments
We are deeply grateful to all patients and healthy controls participating in this study. We thank Anna Partyka for the genotyping. This work was supported by research grant “IL-2, IL-6, IFN-gamma and TGF-beta gene polymorphism in patients with schizophrenia” awarded by Ministry of Science and Higher Education, Grant Number N N402 465237.
Disclosure
The authors report no conflicts of interest in this work.
References
- PotvinSStipESepehryAAGendronABahRKouassiEInflammatory cytokine alterations in schizophrenia: a systematic quantitative reviewBiol Psychiatry200863880180818005941
- KirkpatrickBMillerBJInflammation and schizophreniaSchizophr Bull20133961174117924072812
- ZakharyanRBoyajyanAInflammatory cytokine network in schizophreniaWorld J Biol Psychiatry201415317418724041158
- MillerBJBuckleyPSeaboltWMellorAKirkpatrickBMeta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effectsBiol Psychiatry201170766367121641581
- LiMOWanYYSanjabiSRobertsonAKFlavellRATransforming growth factor-beta regulation of immune responsesAnnu Rev Immunol2006249914616551245
- CaiJSchleidtSPelta-HellerJHutchingsDCannarsaGIacovittiLBMP and TGF-β pathway mediators are critical upstream regulators of Wnt signaling during midbrain dopamine differentiation in human pluripotent stem cellsDev Biol20133761627323352789
- KrieglsteinKSuter-CrazzolaraCFischerWHUnsickerKTGF-beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP + toxicityEMBO J19951447367427882977
- PoulsenKTArmaniniMPKleinRDHynesMAPhillipsHSRosenthalATGF beta 2 and TGF beta 3 are potent survival factors for midbrain dopaminergic neuronsNeuron1994135124512527946360
- JiaPWangLMeltzerHYZhaoZCommon variants conferring risk of schizophrenia: a pathway analysis of GWAS dataSchizophr Res20101221–3384220659789
- Umeda-YanoSHashimotoRYamamoriHThe regulation of gene expression involved in TGF-β signaling by ZNF804A, a risk gene for schizophreniaSchizophr Res20131461–327327823434502
- BenesFMLimBMatzilevichDWalshJPSubburajuSMinnsMRegulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolarsProc Natl Acad Sci U S A200710424101641016917553960
- AwadMREl-GamelAHasletonPTurnerDMSinnottPJHutchinsonIVGenotypic variation in the transforming growth factor-beta1 gene: association with transforming growth factor-beta1 production, fibrotic lung disease, and graft fibrosis after lung transplantationTransplantation1998668101410209808485
- YamadaYMiyauchiAGotoJAssociation of a polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to osteoporosis in postmenopausal Japanese womenJ Bone Miner Res19981310156915769783545
- FrydeckaDMisiakBBeszlejJAGenetic variants in transforming growth factor-β gene (TGFB1) affect susceptibility to schizophreniaMol Biol Rep201340105607561424065520
- LoeysBLChenJNeptuneERA syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2Nat Genet200537327528115731757
- OngaliBNicolakakisNLecruxCTransgenic mice overexpressing APP and transforming growth factor-beta1 feature cognitive and vascular hallmarks of Alzheimer’s diseaseThe Am J Pathol2010177630713080
- NicolakakisNAboulkassimTAliagaATongXKRosa-NetoPHamelEIntact memory in TGF-β1 transgenic mice featuring chronic cerebrovascular deficit: recovery with pioglitazoneJ Cereb Blood Flow Metab201131120021120571524
- ArosioBBergamaschiniLGalimbertiL+10 T/C polymorphisms in the gene of transforming growth factor-beta1 are associated with neurodegeneration and its clinical evolutionMech Ageing Dev20071281055355717889927
- McGuffinPFarmerAHarveyIA polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT systemArch Gen Psychiatry19914887647701883262
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- AndreasenNCMethods for assessing positive and negative symptomsMod Probl Pharmacopsychiatry19902473882336066
- AndreasenNCNegative symptoms in schizophrenia. Definition and reliabilityArch Gen Psychiatry19823977847887165477
- ReyAL’examen psychologique dans les cas d’encéphalopathie traumatique. Les problèmes [The psychological examination in cases of traumatic encephalopathy. Problems]Archives de Psychologie194128215228 French.
- ReitanRMThe relation of the trail making test to organic brain damageJ Consult Psychol195519539339413263471
- BentonALDifferential behavioural effects in frontal lobe diseaseNeuropsychologia196865360
- KesslerJDenzlerPMarkowitschHJDemenztest [Test for dementia]WeinheimBeltz Test GmbH1988 German
- JensenARScoring the Stroop testActa Psychol (Amst)19652453984085841721
- BrzezińskiJHornowskaESkala Inteligencji Wechslera dla Dorosłych. Wersja Zrewidowana. Polska Adaptacja WAIS-R (PL) [Scale of the Wechsler Adult Intelligence. The revised version. Polish adaptation WAIS-R (PL)]WarszawaWydawnictwo Naukowe PWN1996 Polish
- WoodsSWChlorpromazine equivalent doses for the newer atypical antipsychoticsJ Clin Psychiatry200364666366712823080
- NaKSJungHYKimYKThe role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophreniaProg Neuropsychopharmacol Biol Psychiatry20144827728623123365
- JonesALMowryBJPenderMPGreerJMImmune dysregulation and self-reactivity in schizophrenia: do some cases of schizophrenia have an autoimmune basis?Immunol Cell Biol200583191715661036
- KimYKMyintAMLeeBHTh1, Th2 and Th3 cytokine alteration in schizophreniaProg Neuropsychopharmacol Biol Psychiatry20042871129113415610925
- BorovcaninMJovanovicIRadosavljevicGElevated serum level of type-2 cytokine and low IL-17 in first episode psychosis and schizophrenia in relapseJ Psychiatr Res201246111421142622974591
- BorovcaninMJovanovicIRadosavljevicGAntipsychotics can modulate the cytokine profile in schizophrenia: attenuation of the type-2 inflammatory responseSchizophr Res2013147110310923602340
- LinCCChangCMChangPYHuangTLIncreased interleukin-6 level in Taiwanese schizophrenic patientsChang Gung Med J201134437538121880192
- HutchinsonIVTurnerDMSankaranDAwadMRSinnottPJInfluence of cytokine genotypes on allograft rejectionTransplant Proc19983038628639595127
- HanIBRopperAEJeonYJAssociation of transforming growth factor-beta1 gene polymorphism with genetic susceptibility to ossification of the posterior longitudinal ligament in Korean patientsGenet Mol Res20131244807481623479171
- O’DonovanMCCraddockNNortonNIdentification of loci associated with schizophrenia by genome-wide association and follow-upNat Genet20084091053105518677311
- KimYKMyintAMVerkerkRScharpeSSteinbuschHLeonardBCytokine changes and tryptophan metabolites in medication-naive and medication-free schizophrenic patientsNeuropsychobiology200959212312919390223
- HareEGlahnDCDassoriAHeritability of age of onset of psychosis in schizophreniaAm J Med Genet B Neuropsychiatr Genet2010153B129830219350535
- VassosEShamPCCaiGCorrelation and familial aggregation of dimensions of psychosis in affected sibling pairs from ChinaBr J Psychiatry2008193430531018827292
- HustedJAGreenwoodCMBassettASHeritability of schizophrenia and major affective disorder as a function of age, in the presence of strong cohort effectsEur Arch Psychiatry Clin Neurosci2006256422222916331352
- VoiseyJSwagellCDHughesIPLawfordBRYoungRMMorrisCPA novel DRD2 single-nucleotide polymorphism associated with schizophrenia predicts age of onset: HapMap tag-single-nucleotide polymorphism analysisGenet Test Mol Biomarkers2012162778121861710
- HanninenKKatilaHSaarelaMInterleukin-1 beta gene polymorphism and its interactions with neuregulin-1 gene polymorphism are associated with schizophreniaEur Arch Psychiatry Clin Neurosci20082581101517901998
- SacchettiEBocchio-ChiavettoLValsecchiPG308A tumor necrosis factor alpha functional polymorphism and schizophrenia risk: meta-analysis plus association studyBrain Behav Immun200721445045717234379
- KeefeRSHarveyPDCognitive impairment in schizophreniaHandb Exp Pharmacol2012213113723027411
- BarchDMCeaserACognition in schizophrenia: core psychological and neural mechanismsTrends Cogn Sci2012161273422169777
- IbrahimHMTammingaCATreating impaired cognition in schizophreniaCurr Pharm Biotechnol20121381587159422283754
- DickersonFStallingsCOrigoniAC-reactive protein is elevated in schizophreniaSchizophr Res2013143119820223218564
- FrydeckaDMisiakBPawlak-AdamskaEInterleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestationEur Arch Psychiatry Clin Neurosci Epub2014912
- DickersonFStallingsCOrigoniAVaughanCKhushalaniSYolkenRAdditive effects of elevated C-reactive protein and exposure to Herpes Simplex Virus type 1 on cognitive impairment in individuals with schizophreniaSchizophr Res20121341838822048011
- ShirtsBHPrasadKMPogue-GeileMFDickersonFYolkenRHNimgaonkarVLAntibodies to cytomegalovirus and Herpes Simplex Virus 1 associated with cognitive function in schizophreniaSchizophr Res20081062–326827418801645
- OkahisaYUjikeHKunugiHLeukemia inhibitory factor gene is associated with schizophrenia and working memory functionProg Neuropsychopharmacol Biol Psychiatry201034117217619879916
- ZhangXYTangWXiuMHInterleukin 18 and cognitive impairment in first episode and drug naïve schizophrenia versus healthy controlsBrain Behav Immun20133210511123499732
- AsevedoERizzoLBGadelhaAPeripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophreniaPhysiol Behav201412919419824576679
- DickinsonDRamseyMEGoldJMOverlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophreniaArch Gen Psychiatry200764553254217485605
- Van Den BosscheMJDocxLMorrensMLess cognitive and neurological deficits in schizophrenia patients carrying risk variant in ZNF804ANeuropsychobiology201266315816622948380
- WaltersJTCorvinAOwenMJPsychosis susceptibility gene ZNF804A and cognitive performance in schizophreniaArch Gen Psychiatry201067769270020603450
- NicodemusKKElvevågBFoltzPWRosensteinMDiaz-AsperCWeinbergerDRCategory fluency, latent semantic analysis and schizophrenia: a candidate gene approachCortex20145518219124447899
- BalogZKissIKériSZNF804A may be associated with executive control of attentionGenes Brain Behav201110222322721040459
- ChenMXuZZhaiJEvidence of IQ-modulated association between ZNF804A gene polymorphism and cognitive function in schizophrenia patientsNeuropsychopharmacology20123771572157822373944
- ErikssonUKPedersenNLReynoldsCAAssociations of gene sequence variation and serum levels of C-reactive protein and interleukin-6 with Alzheimer’s disease and dementiaJ Alzheimers Dis201123236136921116047
- MillerBJCulpepperNRapaportMHC-reactive protein levels in schizophrenia: a review and meta-analysisClin Schizophr Relat Psychoses20147422323023428789
- AbelKMDrakeRGoldsteinJMSex differences in schizophreniaInt Rev Psychiatry201022541742821047156
- MarkhamJASex steroids and schizophreniaRev Endocr Metab Disord201213318720721603930
- AlemanAKahnRSSeltenJPSex differences in the risk of schizophrenia: evidence from meta-analysisArch Gen Psychiatry200360656557112796219
- KosterALajerMLindhardtARosenbaumBGender differences in first episode psychosisSoc Psychiatry Psychiatr Epidemiol2008431294094618574541
- ThorupAPetersenLJeppesenPGender differences in young adults with first-episode schizophrenia spectrum disorders at baseline in the Danish OPUS studyJ Nerv Ment Dis2007195539640517502805
- SeemanMVLangMThe role of estrogens in schizophrenia gender differencesSchizophr Bull19901621851942197713
- DluzenDENeuroprotective effects of estrogen upon the nigrostriatal dopaminergic systemJ Neurocytol2000295–638739911424955
- WuYCHillRAGogosAvan den BuuseMSex differences and the role of estrogen in animal models of schizophrenia: interaction with BDNFNeuroscience2013239678323085218
- McNeilTFKaijLMalmquist-LarssonAWomen with nonorganic psychosis: factors associated with pregnancy’s effect on mental healthActa Psychiatr Scand19847032092196496145
- DhandapaniKMWadeFMMaheshVBBrannDWAstrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathwaysEndocrinology200514662749275915746252
- BuchananCDMaheshVBBrannDWEstrogen-astrocyte-luteinizing hormone-releasing hormone signaling: a role for transforming growth factor-beta(1)Biol Reprod20006261710172110819775
- YingSYInhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormoneEndocr Rev1988922672933136011
- LvMHTanYLYanSXDecreased serum TNF-alpha levels in chronic schizophrenia patients on long-term antipsychotics: correlation with psychopathology and cognitionPsychopharmacology (Berl) Epub2014624