Abstract
Background
The current study’s aim is to evaluate the possible interaction effects of khat chewing on treatment of paranoid schizophrenic patients.
Patients and methods
In the study group, 42 male subjects suffered from paranoid schizophrenia and were classified according to their khat chewing habits into two subgroups: either khat-chewer subgroup (SKc; n=21; r=11, h=10) or non-khat-chewer subgroup (SNKc; n=21, r=11, h=10). Each subgroup was further subdivided according to type of treatment into r (risperidone) and h (haloperidol). Healthy male subjects (37) were subdivided into healthy khat-chewer as positive controls (HKc, n=17) and healthy non-khat-chewer as negative controls (HNKc, n=20). Plasma dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid, 5-hydroxytryptamine (serotonin), 5-hydroxyindoleacetic acid, epinephrine, and norepinephrine were estimated.
Results
ANOVA and post hoc analysis showed that dopamine was illustrating significant elevation in all khat chewing groups. DOPAC was illustrating significant decrease in all khat chewing groups with an interesting outcome showing significant increase in DOPAC in SNKcr group due to risperidone effect. Homovanillic acid, serotonin, hydroxyindoleacetic acid, and norepinephrine were illustrating significant elevations in all khat chewing groups. Epinephrine was illustrating significant elevation in all chewers than non-chewers groups. Unexpected significant decrease in epinephrine in the SNKcr group indicated that risperidone drug is decreasing epinephrine through indirect mechanism involving calcium.
Conclusion
Khat chewing in schizophrenic patients is contraindicated because it aggravates the disease symptoms, attenuates all used treatment medications, and deteriorates all biochemical markers of the patients.
Introduction
Schizophrenia is a severe neuropsychiatric mental disorder caused by inherent dysfunction of the brain, occurs in about 1% of the population, and is characterized by hallucinations, delusions, and thinking or speech disturbances. Schizophrenia presents positive symptoms such as auditory hallucination, mood abnormality, lack of insight into the illness delusions, and initial vagueness in speech, whereas the negative symptoms present emotional apathy, lack of drive, and social withdrawal.Citation1
Functional and structural disconnectivities are among the most reproducible neurophysiological abnormalities associated with schizophrenia and refer to macroscopic neuroanatomical dynamics and are mainly measured by diffusion-tensor imaging, tractography, volumetric magnetic resonance imaging, and magnetization-transfer imaging.Citation2
Neuroinflammatory mechanisms implicated in schizophrenia include glial (astroglial loss and activation, microglial activation, and priming), immunologic (cytokines, chemokines, and prostaglandins), and oxidative (reactive oxygen and nitrogen species) aberrations. These mechanisms are thought to result in glutamatergic (hypofunction) and dopaminergic (limbic hyperfunction, frontal hypofunction) dysregulations.Citation3
Schizophrenia can be genetic, although in many cases of identical twins, only one sibling developed schizophrenia. Another postulation is the dopamine (DA) receptor’s abnormality, mainly D2 receptors. Other factors could implicate in schizophrenia such as toxins, family environment, migration, and socioeconomic factors.Citation1
Khat leaves (Catha edulis Forsk) has a stimulant effect and comes from West Asia and countries bordering the Red Sea in east coast Africa. Khat is traditionally consumed by keeping the slowly chewed leaves in the side of the cheek, releasing the Khat active constituents, and swallowing with saliva.Citation4,Citation5
High consumption of Khat resulted in a state resembling paranoid schizophrenia or maniac behavior. A short-lived schizophrenic from psychotic illness and mania due to excessive khat chewing was documented presenting psychotic paranoid delusions and manic-like psychosis.Citation6–Citation10
Khat contains cathinone and cathine, which have sympathomimetic effects mediated through cathinone-induced catecholamine secretion by releasing catecholamines from presynaptic storage sites, and inhibit catecholamines reuptake and also monoamine oxidase enzyme (MAO). These amines include DA and serotonin.Citation11 Khat may have also MAO inhibiting action.Citation12
DA or β-(3,4-dihydroxyphenyl)ethylamine is an endogenous neurotransmitter catecholamine transmitting signals between neurons that are separated by a synaptic cleft.Citation13 Serotonin (5-hydroxytryptamine [5-HT]) is an indolamine monoamine neurotransmitter in the central nervous system (CNS), mainly in the basal ganglia and limbic system.Citation14,Citation15
Khat psychostimulant activity is mediated through release of DA and 5-HT.Citation16 The metabolism of serotonin is primarily done by MAO. The principle metabolite of serotonin is hydroxyindoleacetic acid (HIAA).Citation17
Intermittent oral administration of C. edulis extract (200 mg/kg) induces DOPAC significant reduction with increment of urinary catecholamine (HVA) indicating amine oxidase activity inhibition.Citation4,Citation18,Citation19
Khat effect is due to DA release rather than 5-HT release.Citation16,Citation20 Cathinone (beta-keto analog of amphetamine) is the main psychoactive alkaloid of fresh khat leaves and has indirect sympathomimetic mechanism through modulating the release of catecholamines, mainly DA from storage sites in the synaptic terminal, and indirectly affects electrolyte balance by increasing copper and calcium and decreasing zinc plasma concentrations. Cathinone (−) isomer is approximately three times more potent than (+) isomer at DA terminals in the CNS.Citation12,Citation20–Citation23
It was reported that cathinone may act on noradrenaline transporters.Citation24 Schizophrenia is treated by neuroleptic drugs, which have an antipsychotic effect; haloperidol drug binds preferentially to D2 and Alpha 1 receptors at low dose (ED50 =0.13 mg/kg and 0.42 mg/kg, respectively) and 5-HT2 receptors at a higher dose (ED50 =2.6 mg/kg) with pronounced DA antagonism. Haloperidol, a non-selective DA antagonist, was found to reduce cathinone-induced biting and licking movements.Citation25 Risperidone is a second-generation antipsychotic with more pronounced serotonin antagonism than DA antagonism.Citation26
This study was aimed to evaluate the interference of Khat chewing with antipsychotic medications by assaying DA, serotonin, epinephrine, norepinephrine, and their related metabolites.
Patients and methods
Ethical consideration
Written consent was taken from all participants as a routine admission protocol. As the hospital is a mental and psychiatric health hospital (Al-Amal Hospital) in Sana’a, Yemen, it is a charge-free governmental hospital, and all patients were informed that their diagnosis and biological samples taking during diagnosis will be recorded and could be used anonymously for education and research purposes.
The research protocol followed all the ethical regulations stated by the scientific research ethical committee in the university. Protocol, consent format, recorded information, and sampling procedures were approved by the committee. The university and the hospital ethical regulations prohibit disclosing names and personal information of any patients without legislative procedure or applying any invasive protocol was not needed for the diagnosis such as tissue biopsy.
All our blood samples were collected during regular and routine diagnosis procedure of patients without using any invasive protocols. The authors are confirmed that they did not administer khat in purpose for the patients. Khat groups were classified by asking the patients during patient history recording as khat is a common traditional medicine and being used routinely in a widespread manner in Yemen.
Study design
The current study was carried out as a case–control study. In all, 79 Yemeni male subjects were recruited (aged 39.64±0.44 years, body mass index averaged 8±1.2 kg/m2, and were nonsmokers), out of which 42 subjects suffered from late-onset paranoid schizophrenia. They were examined and treated during temporary remission in the psychiatric department and were classified according to their khat chewing habits into two main subgroups: either khat-chewer subgroup (SKc; n=21, r=11, h=10), patients chewed khat during treatment (when they were discharged for holidays), or non-khat-chewer subgroup (SNKc; n=21, r=11, h=10). Each subgroup further subdivided according to type of treatment into r (risperidone) and h (haloperidol). The daily doses of risperidone and haloperidol were 5 mg and 10 mg, respectively.
Healthy subjects who chewed khat and were not suffering from paranoid schizophrenia were taken as positive controls (HKc, n=17). Healthy subjects who did not chew khat and were not suffering from paranoid schizophrenia were taken as negative controls (HNKc, n=20).
All subjects (in groups HKc or SKc) were demonstrating khat chewing habits, determined as daily abuse with duration of 4–8 hours, and khat chewing habit was highly comparable within the patient groups in quantity (average 100–120 g).
Patients’ inclusion criteria considered age, sex, onset of disease, previous readmission to hospital, past history of mental illness, family history of mental illness, and date of admission of each patient, while the exclusion criteria excluded dementia or mental retardation diagnosis, any intermittent therapy (on/off), and any patient exposed to severe drug side effects, shifted from one drug to another, or administered any concomitant psychotropic medications.
Sociodemographic and clinical characteristics
On admission, all patients underwent a routine clinical interview with specialist psychiatrist to register psychotic symptoms using the rating instrument that assesses even different symptoms of schizophrenia instrument; positive and negative syndrome scale (PANSS)Citation27 comprises three components: positive (P), negative (N), and cognitive or general psychopathology (G). Positive syndrome is composed of symptoms such as delusions, hallucinations, and disorganized thinking. Negative syndrome is characterized by deficits in cognitive, affective, and social functions, including blunting affect and passive withdrawal. General psychopathology is composed of many deficits in cognition such as disorientation, poor attention, lack of insight, and active social avoidance. Positive and negative subscales each contain seven items (P1–P7, N1–N7); General psychopathology subscale contains 16 items with the major emphasis on cognition (G1–G16). Diagnosis at discharge was given according to the ICD-10 classification of mental and behavioral disorders.Citation28
Sample collection
Venous blood samples were collected 2 hours after lunch in the HNKc group, 1 hour after treatment in the SNKc group, 1 hour after treatment and starting of continued khat chewing in SKc, and 1 hour after starting of continued khat chewing in HKc (they chewed the khat outside the hospital before coming to outpatient psychiatric department and then underwent blood drawing) using vacutainer. Blood samples were collected on EDTA tubes and immediately centrifuged at 3,500× g for 10 minutes, and separated plasma was divided into six separated aliquots in Eppendorf (micro centrifuge) tubes and stored at −70°C for later analysis of DA (Cat No CEA851Ge), homovanillic acid (HVA) (Cat No CED717Ge), 5-hydroxytryptamine (serotonin) (Cat No CEA808Ge), 5-HIAA (Cat No CEB005Ge), epinephrine (Cat No CEA858Ge), and norepinephrine (Cat No CEA907Ge) using ELISA kits from Cloud-Clone Corp Company (Houston, TX, USA) and following the manufacturer instructions, while 3,4-dihydroxyphenylacetic acid (DOPAC) was measured using microdialysis-HPLC as described by Dethy et al.Citation29
Data analysis
Data were collected and analyzed using SPSS version 15 (SPSS Inc., Chicago, IL, USA). One-way ANOVA was used to assess the difference between frequencies (the associations between khat chewing and paranoid schizophrenia). Tukey’s post hoc test was used to test the difference between subgroup means, and F value test tested the multivariate effect of investigated subjects using multivariate Pillai’s trace, Wilks’ lambda, Hotelling trace, and Roy’s largest root tests. These tests are based on the linearly independent pairwise comparisons among the estimated marginal means. Pearson’s chi-squared test was used to test the significant differences between PANSS scores from study baseline and after 6 weeks and 12 weeks duration; Phi and Cramer’s V were used as symmetric measures to evaluate the chi-square correlation strength. Observed difference was considered to be significant at P<0.05.
Results
All analyzed data in the ANOVA table () and multivariate tests table () are showing highly significant correlations between groups and within groups.
Table 1 One-way ANOVA analysis showing correlation between groups and within groups
Table 2 Multivariate tests
Post hoc tests for all tested groups (multiple comparisons, Tukey’s HSD) in – show () significant elevation in DA in all khat chewing groups, while SKch and SKcr groups are showing significant elevation in DA level than HKc group, exhibiting the significant positive effect of khat on DA increment in both SKch and SKcr groups. DOPAC () is illustrating significant decrease in all khat chewing groups with an interesting outcome, the unexpected significant increase in SNKcr group. HVA is illustrating significant elevation in all khat chewing groups; SKch group is illustrating significant increase in HVA than SKcr group ().
Figure 1 Effects of khat chewing and paranoid schizophrenia on plasma level of dopamine (A) and its related metabolites, DOPAC (B) and HVA (C).
Abbreviations: H, healthy; N, non; K, khat; c, chewing; S, schizophrenic; h, haloperidol; r, risperidone; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; SE, standard error; SEM, standard error of the mean.
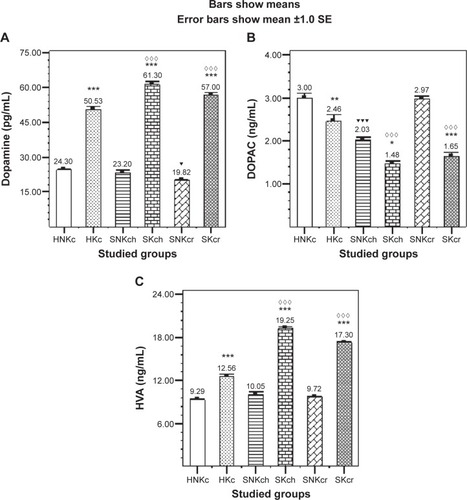
Table 3 Effects of khat chewing and paranoid schizophrenia on plasma levels of dopamine and its related metabolites (DOPAC and HVA), in comparison with healthy non-khat-chewer subjects
Table 4 Effects of khat chewing and paranoid schizophrenia on plasma levels of serotonin (5-HT) and its metabolite (HIAA), in comparison with healthy non-khat-chewer subjects
Table 5 Effects of khat chewing and paranoid schizophrenia on plasma levels of norepinephrine and epinephrine, in comparison with healthy non-khat-chewer subjects
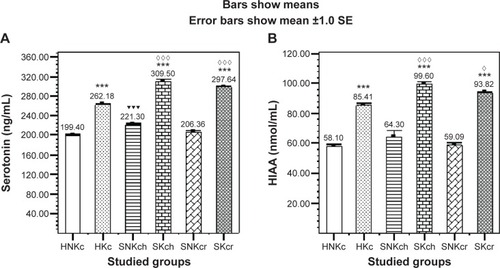
Serotonin () is illustrating significant elevation in all khat chewing groups with significant increase in both SKch and SKcr groups, and HIAA () is illustrating significant elevation in all khat chewing groups with significant increase in SKch and SKcr groups.
Figure 2 Effects of khat chewing and paranoid schizophrenia on plasma level of serotonin (A) and its related metabolite HIAA (B).
Abbreviations: H, healthy; N, non; K, khat; c, chewing; S, schizophrenic; h, haloperidol; r, risperidone; HIAA, hydroxyindoleacetic acid; SE, standard error; SEM, standard error of the mean.
Norepinephrine () is illustrating significant elevation in all khat chewing groups with significant increase in both SKch and SKcr groups. Epinephrine () is illustrating significant elevation in all chewers than non-chewers. Another interesting finding is encountered in the unexpected significant decrease in epinephrine in the SNKcr group, indicating that risperidone drug is possibly decreasing plasma epinephrine. shows the improvement/deterioration in symptoms according to khat chewing within 12 weeks duration. shows the sociodemographic and clinical characteristics.
Figure 3 Effects of Khat chewing and paranoid schizophrenia on plasma level of norepinephrine (A), and epinephrine (B).
Abbreviations: H, healthy; N, non; K, khat; c, chewing; S, schizophrenic; h, haloperidol; r, risperidone; SE, standard error; SEM, standard error of the mean.
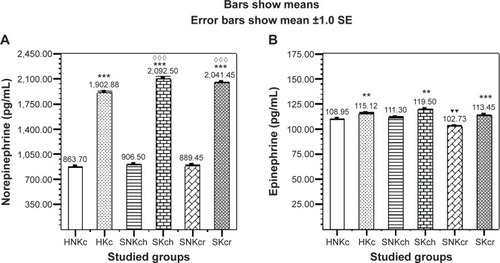
Table 6 Positive, negative syndrome, and general psychopathology scales of patients with paranoid schizophrenia receiving haloperidol or risperidone across 12-week study period
Table 7 Sociodemographic and clinical characteristics
Discussion
In vitro studies reported that cathinone may act on noradrenaline transporters and serotonin receptors and induce motor activities and was found to have four times higher affinity than racemic amphetamine for serotonin receptors in isolated rat fundus.Citation24,Citation30,Citation31
Repeated doses of amphetamines 5 mg/kg twice daily for successive 5 days in rats induce behavioral sensitization phenomenon due to DA release.Citation32,Citation33
In addition, administration of khat or cathinone to rats after unilateral lesion of substantia nigra with 6-hydroxydopamine induced ipsilateral rotation, in a similar fashion to amphetamine, suggesting that they have indirect DA-releasing actions on the CNS.Citation34
Several preclinical studies on rats treated with cathinone (3.2 mg/kg) showed increase in DA efflux measured by microdialysis (55%), and the behavioral effect of cathinone in animals is attributed to increasing the level of DA release or inhibiting its reuptake,Citation12,Citation35,Citation36 and this might explain the khat-inducing behavioral sensitization phenomenon. Locomotor sensitization and deficits in prepulse inhibition induced by psychostimulants are two paradigms that have been widely studied as animal behavioral models of amphetamine psychosis. Repeated oral administration of a standardized C. edulis extract (containing a dose of 1 mg cathinone per kilogram body weight) or (−) cathinone (1.5 mg/kg) to rats induced a strong locomotor sensitization and led to a gradual deficit in prepulse inhibition.Citation4
Chronic administration of either the whole khat extract or cathinone in rats results in a significant depletion of DA in several brain areas, particularly on the nigrostriatal DA terminal projections,Citation4 and exhibits the same neurotoxic effect of chronic amphetamine administration on the dopaminergic innervations of caudate, inducing their degeneration.Citation37
Cathinone has to penetrate to intraneuronal sites in order to evoke release, and the uptake inhibitors prevent this penetration; therefore, DA release inhibitors or pretreatment with the relatively selective dopaminergic neurotoxin 6-hydroxydopamine significantly attenuates cathinone-induced effects,Citation25,Citation32 while Zelger et alCitation38 have demonstrated that pretreatment with reserpine (monoamine store depleting agent) or methyl-p-tyrosine (a catecholamine synthesis inhibitor) abolished locomotor and increased stereotyped behavior induced by cathinone. On the other hand, pretreatment with haloperidol, a non-selective DA antagonist, was found to reduce biting and licking movements caused by cathinone.
In vivo microdialysis in rats after acute intraperitoneal administration of cathinone, in a similar fashion to amphetamine, increased levels of DA but decreased levels of DA metabolite DOPAC in a dose-dependent manner,Citation19 and similar to amphetamine, cathinone led to depletion of serotonin and its corresponding metabolites in both anterior and posterior striatum, which suggests that aggression in this paradigm is enhanced presumably by decreasing the level of serotonin and its metabolites.Citation34
In human, khat induces hypomanic illness with grandiose delusions and paranoid or schizophrenic psychosis with persecutory delusions associated with mainly auditory hallucinations, fear, and anxiety, resembling amphetamine psychosis,Citation21,Citation39 and induced psychotic states have been described in over 20 case reports,Citation40 while recent community-based studies showed that khat use is associated with severe psychiatric problems.Citation41
There are a number of reports of psychiatric disorders secondary to khat chewing with features of manic-like psychosisCitation6 and paranoid psychosis.Citation7,Citation8
Accordingly, two cases of homicide and combined homicide and suicide have been reported following consumption of khat.Citation7,Citation39
In addition to cathinone, other khat cathine alkaloid or d-norpseudoephedrine has been identified as an additional psychoactive ingredient with psychostimulant properties.Citation42
Khat withdrawal syndrome includes dysphoria, depression, irritability, anxiety, poor concentration, hypersomnia, fatigue, paranoia, akathisia, and drug craving.Citation43,Citation44
Several investigators claim that khat use is not necessarily linked to psychological morbidity; any association that is found may reflect an interaction with other environmental factors.Citation10
This study’s results come in agreement with all the previous studies, as schizophrenic patients khat chewing groups (SKch and SKcr) for treated patients showed significant increase in DA, DA metabolite HVA, serotonin, serotonin metabolites HIAA, epinephrine, and norepinephrine than schizophrenic patients non-khat chewing groups (SNKch and SNKcr) and control healthy groups (HNKc and HKc). This outcome was explained by khat induction release effect on both DA and serotonin through khat cathinone and cathine alkaloids.
This study revealed two interesting outcomes. The first one was the significant increase in DOPAC in the SNKcr group and was expected to show the same decrement pattern like other non-khat-chewer group, SNKch, and this outcome could be explained as risperidone treatment induced a decrement in plasma DA levels and increment in plasma DOPAC in the schizophrenic patients.Citation45 Khat chewing in the SKcr group had compensated the induction effect of risperidone, and the DOPAC plasma level was declined. This might deeply explain the mechanism of action for risperidone through affecting DA biosynthesis and metabolism.
The second interesting outcome was the marked significant decrease in epinephrine in the SNKcr group than other groups, particularly SNKch and HNKc groups, and this could be explained as serotonin concentration dependent can increase Ca2+ and its effect is blocked by a broad-spectrum 5-HT antagonist (metergoline). Calcium triggers the exocytosis of chromaffin granules, releasing epinephrine into the bloodstream.Citation46–Citation48 Risperidone is a 5-HT antagonist; therefore, it blocks Ca2+ elevation by serotonin and consequently blocks release of epinephrine into the bloodstream.
According to PANSS scores assessed at study startup, after 6 weeks, and then after 12 weeks duration (), khat chewing prohibited the improvements in SKcr PANSS positive and PANSS negative and significantly deteriorated the PANSS general scale.
This study showed two limitations: we could not confirm the khat effect on SKch PANSS positive because the SNKch group showed non-significant mild improvement and the other limitation is the discontinuation of khat administration during hospitalization periods.
In conclusion, khat chewing in schizophrenic patients is contraindicated because it aggravates the disease symptoms, attenuates all used treatment medications, and deteriorates all biochemical markers of the patients.
Author contributions
Both authors were involved and contributed to the proposal design, conception, and design of the manuscript; analyzed, collected, assembled, and interpreted the data; provided the study material, intellectual content, and graphics design; and were involved in manuscript writing and the final approval of the manuscript.
Acknowledgments
We would like to express our gratitude to medical staff of Al-Amal Hospital for their cooperation during the study.
Disclosure
We declare no conflict of interests with any other party. This study was completely covered financially by the authors, and no grants or funds by any type either governmental or industrial were received.
References
- DavisKKahnRSKoGDavidsonMDopamine in schizophrenia: a review and reconceptualizationAm J Psychiatry1991148147414861681750
- SouhelNDanielMPNeuroinflammation and white matter pathology in schizophrenia: systematic reviewSchizophr Res2015161110211224948485
- NajjarSPearlmanDMAlperKNajjarADevinskyONeuroinflammation and psychiatric illnessJ Neuroinflammation2013104323547920
- BanjawMSchmidtWBehavioural sensitization following repeated intermittent oral administration of Catha edulis in ratsBehav Brain Res200515618118915582104
- BalintEFalkayGBalintGKhat-a controversial plantMiddle Eur J Med2009121604614
- GoughSPCooksonIBKhat induced schizophreniform psychosis in UK (letter)Lancet1984i455
- AlemAShibreTKhat induced psychosis and its medico-legal implication: a case reportEthiop Med J1997351371399577014
- NielenRJvan der HeijdenFMTuinerSVerhoevenWMKhat and mushrooms associated with psychosisWorld J Biol Psychiatry20045495315048636
- Al HaboriMThe potential adverse effects of habitual use of Catha edulis (khat)Expert Opin Drug Saf200541145115416255671
- OdenwaldMNeunerFSchauerMKhat use as risk factor for psychotic disorders: a cross-sectional and case–control study in SomaliaBMC Med2005351515707502
- SchechterMMeehanSMConditioned place preference produced by the psychostimulant cathinoneEur J Pharmacol19932321351388096187
- Al-HebshiNSkaugNKhat (Catha edulis) – an updated reviewAddict Biol20051029930716318950
- DaniJAZhouFMSelective dopamine filters of glutamate striatal afferentsNeuron20044252252415157413
- BaumgartenHGGrozdanovicZPsychopharmacology of central serotonergic systemsPharmacopsychiatry1995273798614704
- KandelERSchwartzJHJessellTMPrinciple of Neuronal Science4th edNew YorkMcGraw-Hill/Appleton & Lange Companies, Inc2000
- CalcagnettiDJSchechterMDPlace preference for the psychostimulant-cathinone is blocked by pretreatment with a dopamine release inhibitorProg Neuropsychopharmacol Biol Psychiatry1993176376498103235
- MeltzerHYLiZKanedaYIchikawaJSerotonin receptors: their key role in drugs to treat schizophreniaProg Neuropsychopharmacol Biol Psychiatry2003271159117214642974
- NenciniPAhmedAMAmiconiGElmiASTolerance develops to sympathetic effects of khat in humansPharmacology1984281501546718481
- PehekEASchechterMDYamamotoBKEffects of cathinone and amphetamine on the neurochemistry of dopamine in vivoNeuropharmacology199029117111762293059
- KalixPThe pharmacology of khatGen Pharmacol1984151791876376274
- KalixPThe pharmacology of psychoactive alkaloids from Ephedra and CathaJ Ethnopharmacol1991322012081881158
- FeyissaAMKellyJPA review of the neuropharmacological properties of khatProg Neuropsychopharmacol Biol Psychiatry2008321147116618561890
- Kotb El-SayedMIAminHAEffect of Catha edulis on insulin, resistin and cortisol levels in type-2 diabetics and non-diabeticsAm J Biochem Biotechnol20128157163
- RothmanRBRothBLHufeisenSJIn vitro characterization of ephedrine-related stereoisomer at biogenic amine transporters and the receptors revealed selective actions as norepinephrine transporter substratesJ Pharmacol Exp Ther200330713814512954796
- BanjawMSchmidtWCatha edulis extract and its active principle cathinone induce ipsilateral rotation in unilaterally lesioned ratsBehav Pharmacol20061761562017021394
- SchotteAJanssenPFMegensAALeysenJEOccupancy of central neurotransmitter receptors by risperidone, clozapine and haloperidol, measured ex vivo by quantitative autoradiographyBrain Res19936311912027510574
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull1987132612763616518
- World Health OrganizationThe ICD-10 Classification of Mental and Behavioral Disorders-Diagnostic Criteria for ResearchGenevaWHO2010
- DethySLauteMAVan BlercomNDamhautPGoldmanSHildebrandJMicrodialysis-HPLC for plasma levodopa and metabolites monitoring in parkinsonian patientsClin Chem19974357407449166225
- GlennonRALiebowitzSMSerotonin receptor affinity of cathinone and related analoguesJ Med Chem1982253933977069716
- CalcagnettiDJSchechterMDIncrease locomotor activity of rat after intracerebral administration of cathinoneBrain Res Bull1992298438461473016
- RobinsonTEBeckerJBEnduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosisBrain Res Rev198611157198
- KalivasPNeurotransmitter regulation of dopamine neurons in the ventral tegmental areaBrain Res Brain Res Rev199318751138096779
- BanjawMYMiczekKSchmidtWJRepeated Catha edulis oral administration enhances the baseline aggressive behaviour in isolated ratsJ Neural Transm200611354355616082505
- KrauseKHDreselSHKrauserJKungHFTatschKIncreased striatal dopamine transporter in adult patients with attention deficit hyperactive disorder: effects of methylphenidate as measured by single photon emission computed tomographyNeurosci Lett200028510711010793238
- KehrJIchinoseFYoshitakeSMephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake ratsBr J Pharmacol20111641949195821615721
- EllisonGNeural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitryEur Neuropsychopharmacol20021228729712126867
- ZelgerJSchornoHCarlinEBehavioural effects of cathinone: an amphetamine obtained from Catha edulis: comparisons with amphetamine, norpseudoephedrine, apomorphine and nomifensineBull Narc19803267816911034
- PantelisCHindlerCTaylorJUse and abuse of khat (Catha edulis): a review of the distribution, pharmacology, side effects and a description of psychosis attributed to khat chewingPsychol Med1989196576682572026
- WarfaNKleinABhuiKLeaveyGCraigTStansfeldSKhat use and mental illness: a critical reviewSoc Sci Med20076530931817544193
- BhuiKCraigTMohamudSWarfaNStansfeldSAThornicroftGMental disorders among Somali refugees developing culturally appropriate measures and assessing socio-cultural risk factorsSoc Psychiatry Psychiatr Epidemiol20064140040816520881
- GrazianiMMilellaMNenciniPKhat chewing from the pharmacological point of view: an updateSubst Use Misuse20084376278318473221
- BarrAMarkouAPhillipsAA‘crash’ course on psychostimulant withdrawal as a model of depressionTrends Pharmacol Sci20022347548212368072
- ZwebenJCohenJChristianDPsychiatric symptoms in methamphetamine usersAm J Addict20041318119015204668
- CaiHLFangPLiHDAbnormal plasma monoamine metabolism in schizophrenia and its correlation with clinical responses to risperidone treatmentPsychiatry Res201118819720221146875
- BennettMROne hundred years of adrenaline: the discovery of autoreceptorsClin Auton Res1999914515910454061
- FlettJColwellCSerotonin modulation of calcium transients in cells in the suprachiasmatic nucleusJ Biol Rhythms19991435436310511003
- AronsonJWhere name and image meet″ – the argument for ″adrenalineBr Med J200032050650910678871
