Abstract
This open-label, single-arm, multicenter, 13-week, prospective study explored the efficacy, safety, and tolerability of paliperidone palmitate (150 milligram equivalents [mg eq] [day 1], 100 mg eq [day 8], both deltoid injections; 75–150 mg eq, deltoid/gluteal injection) in Chinese patients with acute schizophrenia (Positive and Negative Syndrome Scale [PANSS] total score ≥70), who previously had unsatisfactory therapeutic effect following oral antipsychotic treatment (without washout period). Primary efficacy endpoint was percentage of patients with ≥30% improvement in the PANSS total score at the end of 13 weeks. Secondary efficacy endpoints included change from baseline to end of week 13 in PANSS total score, PANSS subscale scores, Marder factor scores, Clinical Global Impressions–Severity score, and Personal and Social Performance Scale scores. Overall, 477/610 enrolled patients (full analysis set, 78.2%) completed the study (men: 55.1%; women: 44.9%; mean age: 31.5 years). Total, 443/610 (72.6%, full analysis set) patients achieved primary endpoint (mean [standard deviation] change from baseline: –30.9 [19.51]). All secondary endpoints demonstrated significant improvement at the end of 13 weeks. One death occurred during this acute phase. The most common (>5%) treatment-emergent adverse events were extrapyramidal disorders (8.4%). The efficacy and safety data are consistent with other short-term, placebo-controlled studies of paliperidone palmitate conducted in similar populations.
Keywords:
Introduction
Though second generation oral antipsychotics are a critical modality in the treatment of schizophrenia, partial or total nonadherence to oral antipsychotics is known to cause treatment failure, relapse, and hospitalization.Citation1 Long-acting injectable (LAI) antipsychotics enhance adherence, maximize pharmacokinetic coverage, and minimize antipsychotic withdrawal symptoms, potentially enhancing treatment outcomes in patients with schizophrenia.Citation2–Citation4
Paliperidone palmitate (PP), an LAI antipsychotic, is approved for acute and maintenance treatment of schizophrenia in adults in the United States, Europe, People’s Republic of China, Korea, and several other countries.Citation5,Citation6 An earlier study with PP demonstrated that once-monthly treatment with PP is rapid-acting in the acute phase and effectively prevents relapse during long-term maintenance.Citation6 Most of these studies were performed using fixed dosages of PP to treat well-defined groups of patients with schizophrenia and typically included an initial washout period.Citation7–Citation10 A few of these studies were performed using flexible dosing of PP, which better reflects how dosing is implemented in real-life clinical settings with more diverse patient populations (eg, having higher comorbidities, using more comedications, or containing a mix of disease severities).Citation6,Citation11–Citation13 However, there remains lack of data on PP’s use as a flexible-dose treatment in patients with schizophrenia who are directly switched from oral antipsychotic drugs without any washout period, as may occur in a clinical setting. Because comparative information regarding the efficacy and safety between PP and other treatment drugs is available from a series of randomized, double-blind, placebo-controlled, Phase III clinical studies on PP in both global populationCitation8,Citation9,Citation14,Citation15 as well as Chinese populationCitation14 with schizophrenia, the objective of the current exploratory study was to determine the efficacy of flexibly dosed PP in Chinese patients with acute schizophrenia who were previously unsatisfactorily treated with oral antipsychotics.
Methods
Study participants
The acute phase of the flexible-dose, open-label, single-arm, multicenter, prospective study was conducted from October 2012 to November 2013 at 22 sites in the People’s Republic of China. Men and women (both inpatients as well as outpatients), aged 18–65 years (both inclusive), with a diagnosis of acute schizophrenia based on the Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision (DSM-IV-TR; Positive and Negative Syndrome Scale [PANSS] score ≥70), who had a PANSS total score between 70 and 120 at screening and baseline were enrolled. Enrolled patients could have either had prior antipsychotic treatment or not, but all lacked a satisfactory therapeutic effect (defined as patients with PANSS score above 70 in spite of previous treatment, and who were willing to switch to another antipsychotic).
Exclusion criteria of the study were active DSM-IV-TR axis I diagnosis other than schizophrenia (dissociative disorder, bipolar disorder, major depressive disorder, schizoaffective disorder, schizophreniform disorder, autistic disorder, primary substance-induced psychotic disorder); a DSM-IV-TR diagnosis of active substance dependence (excluding nicotine and caffeine dependence) within 6 months before screening; history of suicide or imminent risk of suicide or violent behavior; first episode patients of schizophrenia; encephalopathic syndrome; mild-moderate or severe mental retardation; risk factors for prolonged QT interval; presence of circumstances that may increase the risk of the occurrence of torsade de pointes or sudden death; pregnancy; and lactating women. Patients treated with clozapine for treatment refractory or treatment resistant schizophrenia, monoamine oxidase inhibitor antidepressants within 30 days before screening, and depot antipsychotic drugs including PP within six injection interval or electroconvulsive therapy within 30 days before screening were all excluded from the study. Stable doses of antidepressants started 30 days before the study were continued throughout. Oral lorazepam or other short-acting benzodiazepines were permissible for agitation or anxiety. Previously used oral antipsychotic drugs were to be gradually reduced and stopped within 2 weeks after the first PP injection.
The independent ethics committee or institutional review board at each study site approved the protocol, and the study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices and applicable regulatory requirements. All patients provided written informed consent before enrollment.
Study drug
Because doses of PP can be expressed both in terms of milligram equivalents (mg eq) of the pharmacologically active fraction, ie, paliperidone, and in milligrams of PP, the doses expressed as PP 75, 100, and 150 mg eq equate to 117, 156, and 234 mg, respectively, of PP. The study medication PP was provided as 75, 100, and 150 mg eq injectable suspensions (supplied as 156 mg/mL).
Study design
The study consisted of three phases: screening (1 week), acute treatment phase (13 weeks), and follow-up phase (1 year). Patients without documented previous exposure to oral risperidone, paliperidone extended release, or risperidone depot suspension were administered risperidone (at least 1 mg/day, ≥3 days) or paliperidone extended release (at least 3 mg/day, ≥3 days). Patients were enrolled only if they demonstrated tolerability to these medications. During the acute treatment phase, patients received intramuscular injection of 150 mg eq dose of PP on day 1 and 100 mg eq PP dose on day 8 (both deltoid), followed by a monthly maintenance dose between 75 and 150 mg eq on days 36, 64, and 92 (deltoid or gluteal). Baseline values were taken on the day when the first dose of PP (150 mg eq) was given. Follow-up was conducted at the end of weeks 26, 39, 52, and 65. Data from the follow-up phase are reported separately.
Efficacy assessments
The primary efficacy endpoint was the percentage of patients with ≥30% improvement in the PANSS total score from baseline to end of 13 weeks. The PANSS scale is a 30-item scale with each item rated on a scale of 1 (absent) to 7 (extreme). It provides a total score (range 30–210) and scores for the following three subscales: positive subscale (range 7–49): sum of items P1 to P7 in the positive subscale; negative subscale (range 7–49): sum of items N1 to N7 in the negative subscale; general psychopathology (range 16–112): sum of items G1 to G16 in the general psychopathology subscale. Secondary endpoints included change from baseline to end of week 13 in PANSS total score, PANSS subscale scores, and Marder factor scores (positive symptoms [range 8–56]: sum of delusions, hallucinatory behavior, grandiosity, suspiciousness [items P1, P3, P5, and P6 in the positive subscale], stereotyped thinking [item N7 in the negative subscale], somatic concern, unusual thought content, lack of judgment/insight [items G1, G9, and G12 in the general psychopathology subscale]; negative symptoms [range 7–49]: sum of blunted affect, emotional withdrawal, poor rapport, passive social withdrawal, lack of spontaneity [items N1, N2, N3, N4, and N6 in the negative subscale], motor retardation, and active social avoidance [items G7 and G16 in the general psychopathology subscale]; disorganized thoughts [range 7–49]: sum of conceptual disorganization [item P2 in the positive subscale], difficulty in abstract thinking [item N5 in the negative subscale], mannerisms and posturing, disorientation, poor attention, disturbance of volition, and preoccupation [items G5, G10, G11, G13, and G15 in the general psychopathology subscale]; uncontrolled hostility/excitement [range 4–28]: sum of excitement, hostility [items P4 and P7 in the positive subscale], uncooperativeness, and poor impulse control [items G8 and G14 in the general psychopathology subscale]; anxiety/depression [range 4–28]: anxiety, guilt feelings, tension, and depression [items G2, G3, G4, and G6 in the general psychopathology subscale]), Clinical Global Impressions–Severity (CGI-S) score (7-point scale ranging from 1 [not ill] to 7 [extremely severe]), Personal and Social Performance scale (PSP) score (a score between 71 and 100 indicated a mild degree of difficulty, a score between 31 and 70 indicated a moderate degree of dysfunction, and a score of 30 or less indicated functioning so poor that the patient required intensive supervision), medication satisfaction questionnaire, involvement evaluation questionnaire, medication adherence rating scale score, and medication preference questionnaire (MPQ) scores.
Safety assessments
Safety assessments included treatment-emergent adverse events (TEAEs), clinical laboratory tests, 12-lead electrocardiograms, vital signs measurement, and physical examination findings.
Statistical methods
The efficacy analysis was conducted in the full analysis set, which included all patients who received at least one dose of PP and had at least one postbaseline efficacy evaluation. The safety analysis set included all patients who received at least one dose of PP. Based on findings of an earlier study,Citation16 the expected percentage of patients with at least 30% improvement in total PANSS was 40%. The percent change of PANSS total score was defined as (100× change)/(baseline −30), as 30 is the lowest value of PANSS. Using the large sample normal approximation, it was estimated that at least 93 patients were required to reach a 95% confidence interval (CI) for a single proportion that maximally extended 10% from the observed proportion. Considering the analysis of two subgroups (prior antipsychotics; recently diagnosed or not recently diagnosed based on the disease diagnosis time of 3 years), approximately 600 patients were planned to be included in the study. Paired t-test or Wilcoxon signed-rank test were used to analyze the changes before and after the treatment for all efficacy parameters. Safety was summarized using descriptive statistics.
Results
Study participants
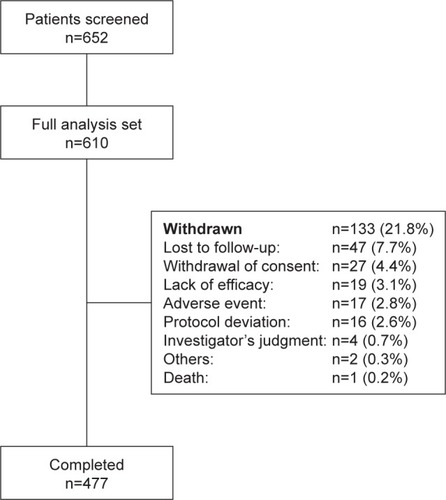
Overall, 616 (99.4%) out of 620 enrolled patients were included in the safety analysis set and 610 (98.4%) patients were included in the full analysis set. There was a slightly higher number of men than women (336 [55.1%] men and 274 [44.9%] women); mean standard deviation (SD) age was 31.5 (10.9) years. The majority (70.2%) of the patients were diagnosed with paranoid schizophrenia. The mean (SD) baseline PSP score was 44.9 (13.6), and the PANSS total score was 91.8 (12.5) (). Overall, 263 patients (60.5% men; mean age: 31.3 years) had prior risperidone or paliperidone treatment, while 52 patients (73.1% men; mean age: 29.9 years) were treated previously with olanzapine and 293 (47.4% men; mean age: 32.0 years) with other antipsychotic treatments. Of the total, 477 (78.2%) patients completed the acute treatment phase (). The most common reasons (>4% patients) for discontinuation were lost to follow-up (n=47, 7.7%) and withdrawal of consent (n=27, 4.4%) ().
Table 1 Demographic and baseline characteristics (full analysis set)
The most commonly received concomitant medications (more than five patients) included risperidone (n=215, 35.2%), trihexyphenidyl hydrochloride (n=135, 22.1%), herbal preparation (n=81, 13.3%), lorazepam (n=71, 11.6%), clonazepam (n=59, 9.7%), oral paliperidone (n=58, 9.5%), olanzapine (n=54, 8.9%), and quetiapine fumarate (n=41, 6.7%).
Among all patients, mean (SD) per dose of PP received by each patient was 114.7 (23.41) mg eq (excluding the first and second injection dose, third injection dose: 114.6 [24.93] mg eq; fourth injection dose: 114.8 [26.22] mg eq; fifth injection dose: 114.7 [26.32] mg eq; final dose: 114.8 [26.26] mg eq). Mode dose received was 114.7 (25.91) mg eq. Median duration of the study drug exposure was 94 days (range: 1–153 days).
Primary efficacy
A total 443 patients (72.6%) out of 610 patients (full analysis set) achieved ≥30% decrease in PANSS total score from baseline to end of 13 weeks following PP treatment (last observation carried forward, 95% CI: 68.90–76.13) (). Of the 444 patients in the per protocol analysis set, 364 patients (84.5%) achieved ≥30% decrease in PANSS total score, 391 patients (90.7%) achieved ≥20% decrease in PANSS total score, and 274 patients (63.6%) achieved ≥50% decrease in PANSS total score at the end of 13 weeks.
Table 2 Therapeutic response from baseline to week 13 (full analysis set)
Secondary efficacy
The PANSS total score significantly improved (P<0.001) over time with a mean (SD) change from baseline of −9.7 (10.60) at the end of week 1 to −30.9 (19.51) at the end of week 13. All the PANSS Marder factor scores significantly improved (P<0.001) from baseline to end of 13 weeks following PP treatment, with the greatest improvement occurring in positive symptoms (mean [SD] change from baseline: −10.7 [7.1]) (). The mean (SD) change from baseline to end of 13 weeks in the CGI-S scores was −1.8 (1.3) and PSP score was 19.3 (16.3) (P<0.001 for both).
Table 3 Change in secondary efficacy measures from baseline to week 13 (full analysis set)
The involvement evaluation questionnaire subscale and total scores, the MPQ scores, and medication adherence rating scale scores showed significant improvement from baseline to end of 13 weeks (P<0.001) following PP treatment (). Overall, 78% of patients preferred injections over tablets at the end of 13 weeks versus 60% patients who preferred injections at baseline. More than 50% of patients responded that injections were not complicated, and 48.4% of patients claimed that they did not have to worry about remembering to take their medication. Overall, 32% of patients noted they experienced fewer adverse effects when using injections (versus oral medications) and also considered the injections as more effective for their symptoms (eg, auditory hallucination). In terms of injection site, 70% of patients preferred the deltoid site for injection with almost 50% of the patients noting that deltoid injections were simpler; 45.6% of patients (n=278) felt less embarrassed to receive deltoid injections, indicating a slight majority felt comfortable with gluteal injections ().
Table 4 Changes of MARS score and MPQ scores from baseline to the end of week 13 (full analysis set)
Safety
Overall, 198 (32.1%) out of 616 patients (safety analysis set) experienced at least one TEAE. The most common (>2% incidence) TEAEs included extrapyramidal symptoms (EPS) (8.4%), insomnia (4.7%), constipation (4.4%), upper respiratory tract infection (4.1%), and akathisia (2.3%) (). There was one death and 14 patients had serious TEAEs during the study. These serious TEAEs included worsening of schizophrenia (n=8), EPS (n=3), and auditory hallucination, depression, lung infection, and hematuria (n=1 each). One patient was pregnant when exposed to PP. Seventeen patients (2.8%) experienced TEAEs that resulted in termination of treatment. The TEAEs that lead to permanent discontinuation of treatment included worsening of schizophrenia (n=6), EPS (n=3), followed by insomnia, auditory hallucination, bradycardia, depression, sinus bradycardia, akathisia, increased alanine transaminase and aspartate transaminase levels, lung infection, hematuria, and acne (n=1, each). The most common categories of EPS-related TEAEs reported included parkinsonism (n=55, 8.9%) followed by hyperkinesia (n=18, 2.9%), dystonia (n=2; 0.3%), tremor (n=2; 0.3%), and dyskinesia (n=1, 0.2%) ().
Table 5 TEAEs reported in ≥2% of patients and EPSs-related TEAEs (safety analysis set)
Nine patients experienced prolactin-related TEAEs in the acute treatment phase. Of these, 5 patients had increased prolactin levels, 2 women had delayed menses and 1 patient each had oligomenorrhea, irregular menstruation, and menstrual disorder. The TEAEs related to suicidality were reported in one patient. Overall, 170 patients (27.6%) had abnormalities in ECG readings values at baseline; at the end of 13 weeks, 83 patients (13.5%) had abnormalities in ECG readings. Increased body weight was reported as a TEAE in 7 patients. The mean (SD) body weight of patients treated with PP increased from 64.42 (12.44) kg (baseline) to 65.5 (12.20) kg at the end of 13 weeks. A total of 48 patients (7.8 %) had significant weight gain (increased ≥7% from baseline) while 16 patients (2.6%) had significant weight loss (decreased ≥7% from baseline) at the end of 13 weeks.
Discussion
This open-label, exploratory study is the first to examine the efficacy and safety of flexibly dosed PP in Chinese patients with acute schizophrenia who were previously unsatisfactorily treated and switched directly from oral antipsychotics. The study demonstrated that at the end of 13 weeks, PP (75, 100, and 150 mg eq) treatment significantly improved schizophrenia symptoms in these patients.
A PANSS total score decrease of ≥30% from baseline was used as a criterion for a clinically significant improvement in these patients. Consistent with the findings of global studies,Citation8,Citation9,Citation14,Citation15 the current study demonstrated that 72.6% of Chinese patients achieved this criterion at the end of 13 weeks, with 51.5% of patients achieving ≥30% decrease in PANSS total score at week 5. Furthermore, 52.8% of patients achieved significant improvement of clinical symptoms (≥50% decrease in PANSS total score from baseline to end of 13 weeks). This was corroborated by improvements in all psychiatric symptoms (as assessed by the Marder factor scores, PANSS subscale scores, CGI-S scores, and PSP scores) following PP treatment at the end of 13 weeks. Our results are consistent with a previous randomized, controlled study conducted in Chinese patients, which showed significant improvement in psychiatric symptoms at the end of a 13-week fixed-dose PP treatment.Citation17
The results of the MPQ scores, which assessed the attitude and preference to PP, are consistent with global findingsCitation18 and indicate that patients’ preference to PP over oral antipsychotics may be higher than physicians’ expectations. The current study also demonstrated significant improvement in compliance to treatment adherence at 13 weeks of PP treatment. This is of clinical relevance since nonadherence to antipsychotic medication is very common in patients with schizophrenia, which ultimately lead to increased relapse rates and poorer long-term outcomes.Citation19,Citation20
Because most LAIs cannot rapidly achieve the efficacy needed for improving acute symptoms (and may require oral supplementation at initiation of dosing), LAI treatment is generally introduced at the maintenance phase of schizophrenia treatment. An earlier study in a Chinese population had demonstrated PP treatment to be efficacious in treating patients with acute symptoms.Citation17 However, unlike the earlier studyCitation14 that had a definite washout period before switching treatments, the current study enrolled patients who were directly switched from the oral antipsychotic drugs without any washout period in the acute treatment phase.
The incidence of early discontinuations (21.8%) was much less in the current study compared with the discontinuation rates (45%–60%) reported in other studies in schizophrenia,Citation8 including other PP studies.Citation2,Citation7,Citation9 This may be likely because the study allowed gradual withdrawal of previous antipsychotic treatment within 2 weeks after the first PP injection and thus avoided the deterioration of symptoms usually observed immediately during the washout of previous antipsychotic treatment. The rapid achievement of therapeutic plasma levels of PP and consequent higher efficacy may have also led to the lower early discontinuations in these patients. This is consistent with an earlier double-blind, randomized, placebo-controlled relapse prevention study, which demonstrated 15% treatment discontinuation in patients treated with PP.Citation21
The safety profile was comparable with previous clinical studies of PP in the Chinese population.Citation17 The incidence of EPS-related events reported during this study were higher compared with previous global studies (with a majority white population);Citation7 however, the Chinese population has comparatively lower body weights than the western population, which might result in relatively higher drug exposures following PP treatment, contributing to the increased EPS-related events. It should also be noted that the dose used in the current study was higher compared with the dose used in earlier studies, which may have also contributed to the higher incidence of EPS events in this population. Overall, the local injection site tolerability was good and comparable with previous studies.Citation7,Citation14 The incidence of potentially prolactin-related TEAEs (<1%) in this study was also consistent with the incidences seen in other PP studies.Citation6,Citation7,Citation14
The open-label design of this study, wherein patients were aware that they are being treated with active products, may have resulted in some bias with regards to efficacy or adverse events reporting. This study was an exploratory trial, in which the objective was not to compare the efficacy and safety difference between PP and other treatments (such as active control, placebo). Because comparative information is available from a series of randomized, double-blind, placebo- or active-drug controlled Phase III clinical studies of PP,Citation22,Citation23 the current study mainly aimed to explore the efficacy of flexibly dosed PP in a Chinese patient population. The findings of the current study are of particular clinical relevance as the study design is more consistent with the type of treatment administered in clinical practice, where flexible dosing is usually applied. These findings may aid physicians in treating patients with schizophrenia after directly switching them from different previous oral antipsychotics. Further studies on symptomatic remission are warranted, however, to better understand the improvements in functional recovery in these patients.
Conclusion
The efficacy data are consistent with other short-term, placebo-controlled studies of PP, conducted in similar populations. Safety and tolerability of all PP doses tested were consistent with the global safety profile and known pharmacology of paliperidone. No new safety signals and no noteworthy tolerability issues related to the mode of administration of PP as a monthly injectable were detected in this study.
Author contributions
Tianmei Si, Kerang Zhang, Jisheng Tang, Maosheng Fang, and Keqing Li were the principal investigators for the study. They conducted the study and interpreted the results. Jianmin Zhuo and Yu Feng were involved in study design and the interpretation of results. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Dr Shruti Shah (SIRO Clinpharm Pvt Ltd) for writing assistance and Dr Wendy Battisti (Janssen Research and Development, LLC) for additional editorial assistance. The authors also thank the study participants, without whom this study would not have been accomplished, as well as the following investigators for their participation in this study: Tianmei Si, Fude Yang, Hezhao Deng, Jinbei Zhang, Chunlian Liang, Zhengwan Qu, Bin Feng, Qiyi Mei, Jianxiong Fan, Binhong Wang, Kerang Zhang, Honggeng Zhang, Maosheng Fang, Jingtao Zheng, Jisheng Tang, Jicheng Dong, Keqing Li, Huichun Li, Jianchu Zhou, Huaqing Meng, Hongjun Tian, and Yi Cao.
This study was funded by Xi’an Janssen Pharmaceutical Ltd (Beijing, People’s Republic of China) who was responsible for study design and data collection, analysis, and interpretation. The sponsor was also responsible for deciding to publish the data and provided a formal review of the manuscript.
Disclosure
Jianmin Zhuo and Yu Feng are employees of Janssen Research and Development, LLC. All authors meet International Committee of Medical Journal Editors criteria, and all those who fulfilled those criteria are listed as authors. All authors had access to the study data and made the final decision about where to publish these data. The authors report no other conflicts of interest in this work.
References
- ChuePEmsleyRLong-acting formulations of atypical antipsychotics: time to reconsider when to introduce depot antipsychoticsCNS Drugs200721644144817521224
- KaneJMEerdekensMLindenmayerJPKeithSJLesemMKarcherKLong-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychoticAm J Psychiatry200316061125113212777271
- KeithSJPaniLNickBPractical application of pharmacotherapy with long-acting risperidone for patients with schizophreniaPsychiatr Serv2004559997100515345759
- NasrallahHAThe case for long-acting antipsychotic agents in the post-CATIE eraActa Psychiatr Scand2007115426026717355516
- Invega® Sustenna® (paliperidone palmitate) extended-release injectable suspension [prescribing information]Titusville, NJJanssen Pharmaceuticals, Inc.2014
- KramerMLitmanRHoughDPaliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety studyInt J Neuropsychopharmacol201013563564719941696
- HoughDLindenmayerJPGopalSSafety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophreniaProg Neuropsychopharmacol Biol Psychiatry20093361022103119481579
- GopalSHoughDWXuHEfficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response studyInt Clin Psychopharmacol201025524725620389255
- NasrallahHAGopalSGassmann-MayerCA controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophreniaNeuropsychopharmacology201035102072208220555312
- GopalSVijapurkarULimPMorozovaMEerdekensMHoughDA 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophreniaJ Psychopharmacol201125568569720615933
- SliwaJKBossieCAMaYWAlphsLEffects of acute paliperidone palmitate treatment in subjects with schizophrenia recently treated with oral risperidoneSchizophr Res20111321283421775106
- FleischhackerWWGopalSLaneRA randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophreniaInt J Neuropsychopharmacol201215110711821777507
- PandinaGLaneRGopalSA double-blind study of paliperi-done palmitate and risperidone long-acting injectable in adults with schizophreniaProg Neuropsychopharmacol Biol Psychiatry201135121822621092748
- HoughDGopalSVijapurkarULimPMorozovaMEerdekensMPaliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled studySchizophr Res20101162–310711719959339
- PandinaGJLindenmayerJPLullJA randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of pali-peridone palmitate in adults with acutely exacerbated schizophreniaJ Clin Psychopharmacol201030323524420473057
- SchreinerABergmansPCherubinPA prospective flexible-dose study of paliperidone palmitate in nonacute but symptomatic patients with schizophrenia previously unsuccessfully treated with oral antip-sychotic agentsClin Ther2014361013721388.e125444566
- LiHRuiQNingXXuHGuNA comparative study of pali-peridone palmitate and risperidone long-acting injectable therapy in schizophreniaProg Neuropsychopharmacol Biol Psychiatry20113541002100821315787
- GeertsPMartinezGSchreinerAAttitudes towards the administration of long-acting antipsychotics: a survey of physicians and nursesBMC Psychiatry2013135823414331
- TandonRBridging the efficacy-effectiveness gap in the antipsychotic treatment of schizophrenia: back to the basicsJ Clin Psychiatry20147511e1321e132225470099
- KaneJMKishimotoTCorrellCUAssessing the comparative effectiveness of long-acting injectable vs oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatryJ Clin Epidemiol2013668 SupplS37S4123849151
- MarkowitzMFuDJLevitanBGopalSTurkozIAlphsLLong-acting injectable paliperidone palmitate versus oral paliperidone extended release: a comparative analysis from two placebo-controlled relapse prevention studiesAnn Gen Psychiatry20131212223845018
- GopalSGassmann-MayerCPalumboJSamtaniMNShiwachRAlphsLPractical guidance for dosing and switching paliperidone palmitate treatment in patients with schizophreniaCurr Med Res Opin201026237738720001492
- SamtaniMNGopalSGassmann-MayerCAlphsLPalumboJMDosing and switching strategies for paliperidone palmitate: based on population pharmacokinetic modelling and clinical trial dataCNS Drugs2011251082984521936586