Abstract
Background
There are few studies describing periodic limb movement syndrome (PLMS) in rapid eye movement (REM) sleep in patients with narcolepsy, restless legs syndrome, REM sleep behavior disorder, and spinal cord injury, and to a lesser extent, in insomnia patients and healthy controls, but no published cases in multiple sclerosis (MS). The aim of this study was to investigate PLMS in REM sleep in MS and to analyze whether it is associated with age, sex, disability, and laboratory findings.
Methods
From a study of MS patients originally published in 2011, we retrospectively analyzed periodic limb movements (PLMs) during REM sleep by classifying patients into two subgroups: PLM during REM sleep greater than or equal to ten per hour of REM sleep (n=7) vs less than ten per hour of REM sleep (n=59). A univariate analysis between PLM and disability, age, sex, laboratory findings, and polysomnographic data was performed.
Results
MS patients with more than ten PLMs per hour of REM sleep showed a significantly higher disability measured by the Kurtzke expanded disability status scale (EDSS) (P=0.023). The presence of more than ten PLMs per hour of REM sleep was associated with a greater likelihood of disability (odds ratio 22.1; 95% confidence interval 3.5–139.7; P<0.0001), whereas there were no differences in laboratory and other polysomnographic findings.
Conclusion
PLMs during REM sleep were not described in MS earlier, and they are associated with disability measured by the EDSS.
Introduction
Periodic limb movement syndrome (PLMS) can occur in a wide range of sleep disorders such as restless legs syndrome (RLS), periodic limb movement disorder (PLMD), narcolepsy, and rapid eye movement (REM) sleep behavior disorder (RBD). PLMS has been reported in healthy subjects without any complaints as well.Citation1–Citation3 The International Classification of Sleep Disorders–Third Edition (ICSD-3), describes PLMS as periodic episodes of repetitive, highly stereotyped limb movements that occur during sleep, most frequently in the lower extremities and during nonrapid eye movement (NREM) sleep.Citation1 The ICSD-3 states that PLMS is normally absent during REM sleep (except for RBD) as REM sleep is associated with atonia and an inhibition of motor phenomena.
If the periodic limb movement index (PLMI; the number of PLMs per hour of sleep) exceeds 15 per hour of sleep (in adults) and leads clinically to sleep disturbances, it is called PLMD.Citation1 Approximately 70%–80% of patients with RLS show PLMS in a single-night polysomnography.Citation1 RLS is defined as an urge to move the legs, usually accompanied by or thought to be caused by uncomfortable and unpleasant sensations in the legs, which worsen during periods of rest, are relieved by movement, and occur exclusively or predominantly in the evening or night.Citation1
PLMD and RLS are two different sleep disorders, but both are associated with PLMS and react favorably to dopaminergic medication, and it is assumed that they share a similar etiology.Citation1,Citation4,Citation5 PLMD is considered to be an endophenotype of RLS,Citation6 and PLMS-related gene variants are correlated with an increased risk of developing RLS.Citation7 The etiology of idiopathic RLS and PLMD is not yet completely understood, but genetic predisposition and brain iron deficiency with abnormal dopaminergic consequences seem to play a crucial role.Citation1,Citation8 A large genome-wide association study in Iceland identified a genetic variant in patients with PLMS (without RLS) which was associated with decreased ferritin serum levels, and the authors discussed the possible involvement of iron depletion in the pathogenesis of PLMD.Citation9
The investigation of intracortical inhibition (ICI) by transcranial magnetic stimulation in patients with RLS and PLMS points to a dysfunction of the motor system at a subcortical and supraspinal level.Citation5,Citation10 ICI is not altered by dysfunction of the corticospinal tract or spinal motoneurons,Citation5 and a cortical origin of PLMS is unlikely.Citation4 Studies using single-photon emission tomography showed a decrease in central D2-receptor occupancy and a lower binding of iodobenzamide – especially in the striatal structures – compared to controls.Citation11 Studies using functional magnetic resonance imaging (fMRI) in PLMS patients showed an activation in the red nuclei, brainstem, and cerebellum and thalamus.Citation12 However, the cerebellar and thalamic activation may occur due to sensory leg discomfort, whereas the red nucleus and brainstem are involved in the generation of PLMS. Therefore, it has been suggested that these regions play a crucial role in the etiology of PLMS and RLS, but different studies showed inconsistent findings about the concise subcortical region.Citation13,Citation14
Rijsman et al described abnormal H-reflexes in PLMD suggesting a causative spinal disinhibition due to a dysfunction of descending and segmental neural pathways changing the excitability of the spinal motoneurons.Citation4 In summary, even if the pathophysiology of PLMS is still unknown, the data actually suggest that PLMS may be generated in subcortical brain regions with disinhibited subcortical inputs to the cortex and simultaneous disinhibition of the spinal cord.
Little is known about the etiology of symptomatic PLMS and RLS in multiple sclerosis (MS). The prevalence of RLS in MS patients – especially in those with severe pyramidal and sensory disability – is four times higher than in control subjects,Citation15 and studies using conventional and diffusion tensor magnetic resonance imaging (MRI) show a significantly reduced cervical cord average fractional anisotropy in MS patients with RLS (compared to those without RLS),Citation16 whereas there was no difference between patients with and without RLS in the whole brain, cerebellum, and brainstem. It is worth noting that the MS patients with RLS showed a higher expanded disability status scale (EDSS)Citation17 score than MS patients without RLS in this study.
In our recent cross-sectional polysomnographic study of patients with MS, we observed that PLMS occurred not only in NREM sleep but in some MS patients during REM sleep as well.Citation18
PLMS in REM sleep has only been described in a few conditions. Telles et al reported PLMS in NREM and REM sleep in patients with spinal cord injury (SCI), but they did not mention the PLMI.Citation19 Yokota et al investigated PLMS in ten patients with spinal cord damage due to SCI, cervical spondylosis, MS, and spinal vascular attack (SVA).Citation20 The clinical manifestation and polysomnographic findings in these patients did not show any difference from typical PLMS as described in the literature, except that PLMs were not suppressed in REM sleep in two patients with complete transection of spinal cord due to SCI and SVA.
Outside the context of MS, SCI, and spinal cord damage, PLMS in REM sleep is described only in a few studies: In the study by Ferri et al,Citation21 PLMI during REM sleep (REM-PLMI) was very low in normal controls (1.9 per hour, standard deviation [SD] 3.8) but higher in patients with narcolepsy (13.9 per hour, SD 14.8) and in patients with RLS (27.6 per hour, SD 35.3). Allena et al investigated 14 patients with bad sleep quality (only patients with PLMS in both NREM and REM were included) compared to 14 healthy controls of similar age.Citation22 The mean REM-PLMI was 10.95 per hour (SD 8.51) in the PLMS group and 0.36 per hour (SD 0.93) in the control group. In all patients, the PLMI was higher in NREM than in REM. Another study described PLMS during REM sleep in insomnia patients as well (REM-PLMI in insomnia patients: 7.6 per hour, SD 10.9; REM-PLMI in healthy controls: 5.7 per hour, SD 8.2; REM-PLMI in RLS patients: 19.7 per hour, SD 39.1).Citation23 All patients in this study showed substantially more PLMS during NREM sleep compared with REM sleep.
In the aforementioned studies, healthy controls and insomnia patients showed a mean REM-PLMI of <10 per hour, whereas in patients with narcolepsy and RLS, the mean REM-PLMI was >10 per hour. Moreover, in all patients, the REM-PLMI was lower than the PLMI during NREM sleep.
The aim of the present work is to describe REM-associated PLMs in MS patients as a previously unpublished pathology and to investigate whether they are associated with age, sex, disability, and laboratory findings.
Materials and methods
The data for this retrospective analysis came from our original cross-sectional studyCitation18 of 66 consecutive MS patients, investigated by two consecutive home-based polysomnographies.
Polysomnography
In this study, performed between 2007 and 2009, we used the Rechtschaffen and Kales criteriaCitation24 for classification of sleep stages, the Coleman criteriaCitation25 for classifying PLM, and the diagnostic guidelines of the Task Force of the American Academy of Sleep Medicine for respiratory events.Citation26 In the present study, we retrospectively analyzed the polysomnographic data of the second night: PLMI, REM-PLMI, PLM arousal index per hour of sleep (PLMI-A), sleep efficiency (percentage of total sleep time per time spent in bed [TiB]), percentage of slow wave sleep time (NREM 3 and 4) per TiB, percentage of NREM 1 and 2 sleep time per TiB, percentage of REM sleep time per TiB, sleep latency in minutes, wake after sleep onset in minutes, number of awakenings, apnea–hypopnea index per hour of sleep (AHI).
Ethical committee
The original study was approved by the local ethics committees, and all participants gave written informed consent prior to the assessment.
Laboratory investigations and EDSS values
Blood samples were analyzed for vitamin B12, ferritin, iron, folic acid, creatinine, thyroid-stimulating hormone, and glycated hemoglobin. Disability was measured with the EDSS.Citation17
Statistical analysis
The results were expressed as mean, SD, and range.
The correlation between PLMI and EDSS values was calculated using the Spearman’s coefficient. Furthermore, differences in EDSS values between patients with or without PLMS were analyzed. Patients were classified into two subgroups by REM-PLMI (≥10 vs <10 PLMs per hour of REM sleep).
Following an exploratory analysis of the data and a (negative) check for normality of the underlying distributions, differences between the two subgroups regarding polysomnographic parameters, age, EDSS, and laboratory findings were (univariately) analyzed using the Mann–Whitney U-test.
This analysis was performed in two steps: first, a univariate analysis with all predictor variables; subsequently, all significant variables remained in the final model of multiple logistic regression. Odds ratios (ORs) were calculated. Statistical significance was established at P<0.05.
Regarding EDSS values, a receiver operating characteristic (ROC) analysis was performed. Due to the results of the ROC analysis, we introduced the dichotomous variable: EDSS ≥4.0 vs <4.0 in order to compute the OR. Afterward, a logistic regression was performed in order to calculate the outcome of this dependent variable.
All tests should be understood as constituting exploratory data analysis, such that neither previous power calculations nor subsequent adjustments for multiple testing have been made. Analysis was performed with SPSS software (IBM© SPSS© Statistics, Version 21, ©Copyright 1989, 2010 SPSS Inc., an IBM Company).
Results
Correlation between EDSS values and PLMI
We did not find a relationship between the PLMI (in NREM and REM sleep) and EDSS values (P=0.137). Moreover, patients suffering from PLMS with more than or equal to 15 periodic leg movements per hour of sleep did not show a higher disability measured with the EDSS compared to patients with less than 15 periodic leg movements per hour of sleep (P=0.636).
Classification into different subgroups with respect to the REM-PLMI
displays the demographic and polysomnographic data. Sixty-six MS patients (21 men and 45 women) aged 20–66 years were classified into two subgroups according to the REM-PLMI <10 per hour (group 1) or REM-PLMI ≥10 per hour (group 2). Fifty-nine patients were classified into group 1 (14 out of these 59 patients were taking antidepressants, and ten of them were suffering from RLS). The remaining seven patients were classified into group 2 (none of these seven patients were taking antidepressants or dopaminergic therapy; two out of these seven patients were suffering from comorbid RLS).
Table 1 Differences between the two subgroups: demographic and polysomnographic parameters
Comparison of the different subgroups
Group 2 showed significantly higher EDSS values compared with group 1 (mean 4.9 [SD 2.5] vs 2.4 [SD 1.6]; P=0.023). However, there were no differences between the two groups with regard to the AHI, laboratory findings or age, and sex.
Polysomnographic data
The PLMI (NREM and REM) and the PLMI-A were significantly higher in group 2 compared with group 1 (mean PLMI 63.5 per hour of sleep [SD 49.4] vs 18.3 per hour of sleep [SD 22.8]; P=0.008; mean PLMI-A 6.6 per hour of sleep [SD 5.1] vs 2.3 per hour of sleep [SD 2.3]; P=0.030).
shows typical REMs in electrooculograms (EOGs) in a 30-second epoch with REM sleep atonia in the chin electromyogram (EMG), whereas PLMs are present in the right anterior tibial muscle (marked with a black arrow) during the corresponding time period.
Figure 1 Typical examples of PLM during REM sleep.
Abbreviations: PLM, periodic limb movement; REM, rapid eye movement; EEG, electroencephalography; EMG, electromyogram; EOG, electrooculogram.
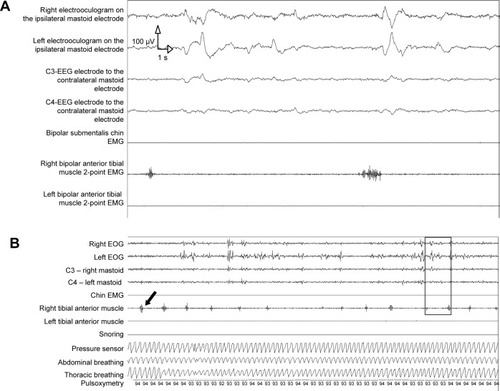
shows 5 minutes of polysomnography with EOGs, chin EMG, both anterior tibial muscles and abdominal and thoracic breathing, pulsoxymetry, and snoring. The black frame highlights the smaller 30-second epoch as shown in . The EOG show REMs. The EEG shows alpha, beta, and desynchronized waves. Please note the atonia in the chin EMG. There are simultaneous PLMs in the right anterior tibial muscle (marked with a black arrow). Recordings are from a 39-year-old male patient with relapsing–remitting MS for 10 years and spasticity of both legs (EDSS =7 points). Prior to and following this epoch, PLMs in the left tibial anterior muscle were also detected.
There were no further differences between the two groups regarding the other polysomnographic data (sleep efficiency, slow wave sleep, NREM 1 and 2, REM sleep, sleep latency, wake after sleep onset, number of awakenings, AHI).
As only the EDSS values showed a significant difference between the two groups, we could not compute a multiple logistic regression.
Classification into two subgroups with respect to EDSS values
We performed an ROC analysis in order to define an appropriate EDSS cutoff value investigating a possible relationship between disability and REM-PLMI ≥10 per hour. Of all patients with an EDSS >4.0, 89.4% showed an REM-PLMI ≥10 with a sensitivity of 71.4% and a specificity of 89.8%. The presence of more than ten PLMs per hour of REM sleep was in line with a greater risk of disability in EDSS (OR 22.1; 95% confidence interval 3.5–139.7; P<0.0001). In sum, patients with EDSS values ≥4 showed a higher probability of presenting PLM during REM sleep (high REM-PLMI).
Discussion
The most important finding of this study is that PLMS during REM sleep may also occur in MS patients – and is associated with disability.
REM sleep is associated with the suppression of postural muscle tone (REM atonia). Whereas muscle tone and movement are largely absent in REM sleep, the overall brain activity, even in locomotor-generating regions, is maximal.Citation27 Atonia is mediated by direct inhibition and passive disfacilitation (reduced motor facilitation) of spinal motor neurons.Citation27–Citation29 In the past, an aminergic–cholinergic hypothesis of REM sleep onset and maintenance due to a reciprocal inhibitory interaction between cholinergic active neurons and monoaminergic neurons has been replaced by the GABAergic–glutamatergic hypothesis.Citation30 Brainstem glutamatergic and GABAergic neurons play a key role in the genesis of REM sleep, and brainstem mechanisms play a crucial role,Citation23,Citation31 but antagonism of glycine and GABAA receptors does not prevent or even reverse REM atonia.Citation32 Multiple inhibitory mechanisms seem to be responsible for triggering loss of activity and muscle tone during REM sleep.Citation32
However, glutamatergic neurons of the sublaterodorsal tegmental nucleus generate REM sleep by their descending projections to medullary or spinal glycinergic/GABAergic premotoneurons and play a key role in the induction of muscle atonia during REM sleep,Citation33 and in the animal model, pontine lesions and lesions of the medial medulla lead to REM sleep without atonia.Citation34
In MS, spinal cord damage (especially in MS patients suffering from RLS) and spinal lesions are associated with disability quantified by the EDSS.Citation16,Citation35 The detection of REM-PLMS in more disabled MS patients argues for a symptomatic etiology of PLMS in these patients and against idiopathic PLMS.
Outside of the specific context of MS, the relationship between PLMS and cortical arousal in idiopathic PLMD or RLS has been intensely discussed.Citation22,Citation36 It is generally accepted that PLMS is associated with cortical arousals, but the temporal relationship between cortical arousals and PLMS is still a matter of debate.Citation37 PLMS seems not to be the trigger phenomenon of cortical arousal or cerebral activation, and several studies argue for a central (subcortical) oscillatory mechanism inducing cerebral activation and PLMS.Citation22 In our previously published article, the increased number of PLMS in those MS patients suffering from PLMD or RLS has not been connected with an increase in PLM-related arousal.Citation18 Further studies should investigate the relationship between PLMS and cortical arousals in symptomatic PLMD/RLS in MS. At least the presumed pathophysiological mechanism of symptomatic PLMD/RLS in MS patients could explain the lack of PLMS-related arousal in this issue (because they are caused not only by a subcortical generator but also by spinal cord damage by spinal or brainstem lesions). In addition, a disconnection between the brain and the spinal cord could account for an absence of PLMS-related arousal. To date, however, we do not have exact data about this issue, and further studies combining polysomnography and MRI investigations are needed.
Methodical limitations
This is a retrospective data analysis. As the original study was performed from 2007 to 2009, we did not use the new 2007 scoring rules from the American Academy of Sleep Medicine.Citation31 Prospective polysomnographic studies using these scoring rules and combining the polysomnographic data with high-resolution MRI are needed. Another methodical limitation of this study is the small number of patients in group 2 (n=7) compared to group 1 (n=59). However, the value of the present work is to point out that the examination of PLM during REM sleep in patients with MS remains an important and under-recognized issue.
Conclusion
PLMs during REM sleep are associated with higher disability scores in MS patients, and they could represent a clinical feature of symptomatic PLMS. Further studies using MRI and polysomnography are needed.
Acknowledgments
The authors thank Dr Gosia Sullivan for reviewing the manuscript. This is a retrospective analysis of a cross-sectional study published in Multiple Sclerosis Journal in 2011, which was supported by the German Research Foundation (DFG Exc 257 to FP). For the original work, Weinmann Medical Technology (Hamburg, Germany) provided two of the three polysomnography systems used in the study. The results have been presented as a poster at the conference organized by the European Sleep Research Society in Tallinn.
Disclosure
The authors report no conflicts of interest in this work.
References
- American Academy of Sleep MedicineInternational classification of sleep disorders Diagnostic and Coding Manual3rd edDarien, IllAmerican Academy of Sleep Medicine2014
- HornyakMFeigeBRiemannDVoderholzerUPeriodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatmentSleep Med Rev200610316917716762807
- CarrierJFrenetteSMontplaisirJPaquetJDrapeauCMorettiniJEffects of periodic leg movements during sleep in middle-aged subjects without sleep complaintsMov Disord20052091127113215884036
- RijsmanRMStamCJde WeerdAWAbnormal H-reflexes in periodic limb movement disorder; impact on understanding the pathophysiology of the disorderClin Neurophysiol2005116120421015589198
- TergauFWischerSPaulusWMotor system excitability in patients with restless legs syndromeNeurology19995251060106310102429
- WinkelmanJWPeriodic limb movements in sleep – endophenotype for restless legs syndrome?N Engl J Med200735770370517634452
- WinkelmannJSchormairBLichtnerPGenome-wide association study of restless legs syndrome identifies common variants in three genomic regionsNat Genet20073981000100617637780
- DauvilliersYWinkelmannJRestless legs syndrome: update on pathogenesisCurr Opin Pulm Med201319659460024048084
- StefanssonHRyeDBHicksAA genetic risk factor for periodic limb movements in sleepN Engl J Med2007357763964717634447
- HanajimaRUgawaYTeraoYOgataKKanazawaIIpsilateral cortico-cortical inhibition of the motor cortex in various neurological disordersJ Neurol Sci19961401–21091168866435
- StaedtJStoppeGKöglerANocturnal myoclonus syndrome (periodic movements in sleep) related to central dopamine D2-receptor alterationEur Arch Psychiatry Clin Neurosci199524518107786913
- BucherSFSeelosKCOertelWHReiserMTrenkwalderCCerebral generators involved in the pathogenesis of the restless legs syndromeAnn Neurol19974156396459153526
- BucherSFTrenkwalderCOertelWHReflex studies and MRI in the restless legs syndromeActa Neurol Scand19969421451508891061
- BriellmannRSRöslerKMHessCWBlink reflex excitability is abnormal in patients with periodic leg movements in sleepMov Disord19961167107148914098
- ManconiMFerini-StrambiLFilippiMthe Italian REMS Study GroupMulticenter case-control study on restless legs syndrome in multiple sclerosis: the REMS StudySleep200831794495218655317
- ManconiMRoccaMAFerini-StrambiLRestless legs syndrome is a common finding in multiple sclerosis and correlates with cervical cord damageMult Scler2008141869317942519
- KurtzkeJFRating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS)Neurology19833311144414526685237
- VeauthierCRadbruchHGaedeGFatigue in multiple sclerosis is closely related to sleep disorders: a polysomnographic cross-sectional studyMult Scler201117561362221278050
- TellesSCAlvesRCChadiGPeriodic limb movements during sleep and restless legs syndrome in patients with ASIA A spinal cord injuryJ Neurol Sci20113031–211912321257181
- YokotaTHiroseKTanabeHTsukagoshiHSleep-related periodic leg movements (nocturnal myoclonus) due to spinal cord lesionJ Neurol Sci1991104113181919596
- FerriRZucconiMManconiMDifferent periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndromeSleep200629121587159417252889
- AllenaMCampusCMorroneEPeriodic limb movements both in non-REM and REM sleep: relationships between cerebral and autonomic activitiesClin Neurophysiol200912071282129019505849
- FerriRGschliesserVFrauscherBPoeweWHöglBPeriodic leg movements during sleep and periodic limb movement disorder in patients presenting with unexplained insomniaClin Neurophysiol2009120225726319109055
- RechtschaffenAKalesAA Manual of Standardized Terminology Techniques and Scoring System for Sleep Stages of Human SubjectsLos Angeles, CAUS Department of Health, Education, and Welfare, Public Health Services-National Institutes of Health, National Institute of Neurological Diseases and Blindness, Neurological Information Network1968
- ColemanRMGuilleminaultCPeriodic movement in sleep (nocturnal myoclonus) and restless legs syndromeGuilleminaultCSleeping and Waking Disorders: Indications and TechniquesNew YorkAddison-Wesley1982265295
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task ForceSleep19992266768910450601
- PeeverJControl of motoneuron function and muscle tone during REM sleep, REM sleep behavior disorder and cataplexy/narcolepsyArch Ital Biol2011149445446622205591
- ChaseMHMoralesFRThe atonia and myoclonia of active (REM) sleepAnnu Rev Psychol1990415575841968326
- LaiYYKodamaTSiegelJMChanges in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis studyJ Neurol Sci2001211873847391
- LuppiPHGervasoniDVerretLParadoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesisJ Physiol Paris20061005–627128317689057
- LuppiPHClementOSapinEBrainstem mechanisms of paradoxical (REM) sleep generationPflugers Arch20124631435222083642
- BrooksPLPeeverJHUnraveling the mechanisms of REM sleep atoniaSleep200831111492149719226735
- ClémentOSapinEBérodAFortPLuppiPHEvidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergicSleep201134441942321461384
- SchenkelESiegelJMREM sleep without atonia after lesions of the medial medullaNeurosci Lett19899821591652565566
- WeierKMazraehJNaegelinYBiplanar MRI for the assessment of the spinal cord in multiple sclerosisMult Scler201218111560156922539086
- ManconiMFerriRZucconiMDissociation of periodic leg movements from arousals in restless legs syndromeAnn Neurol201271683484422718547
- FerriRManconiMAricòDPunjabiNMZucconiMExperimentally induced arousals do not elicit periodic leg motor activity during sleep in normal subjectsSleep Med2013141859023127585
