Abstract
Human sexuality is contingent upon many biological and psychological factors. Such factors include sexual drive (libido), physiological arousal (lubrication/erection), orgasm, and ejaculation, as well as maintaining normal menstrual cycle. The assessment of sexual dysfunction can be difficult due to the intimate nature of the problem and patients’ unwillingness to discuss it. Also, the problem of dysfunction is often overlooked by doctors. Atypical antipsychotic treatment is a key component of mental disorders’ treatment algorithms recommended by the National Institute of Health and Clinical Excellence, the American Psychiatric Association, and the British Society for Psychopharmacology. The relationship between atypical antipsychotic drugs and sexual dysfunction is mediated in part by antipsychotic blockade of pituitary dopamine D2 receptors increasing prolactin secretion, although direct correlations have not been established between raised prolactin levels and clinical symptoms. Variety of mechanisms are likely to contribute to antipsychotic-related sexual dysfunction, including hyperprolactinemia, sedation, and antagonism of a number of neurotransmitter receptors (α-adrenergic, dopaminergic, histaminic, and muscarinic). Maintaining normal sexual function in people treated for mental disorders can affect their quality of life, mood, self-esteem, attitude toward taking medication, and compliance during therapy.
Introduction
Sexual dysfunction is a common problem in people with mood disorders,Citation1 schizophrenia, and other psychotic disorders, with reported prevalence rates of 30%–80% in women and 45%–80% in men.Citation2–Citation4 In clinical practice, sexual disorders are found as a complication in people treated with antipsychotics. The prevalence rate of sexual disorders in men treated with antipsychotics is estimated to be 54% (). Among them, 38% have problems in achieving erection, 42% with maintaining erection, at least 19% have ejaculation disorders (reduced volume or no ejaculation), and 58% have reduced orgasm intensity. Furthermore, in men, priapism, galactorrhea (3%), and gynecomastia (6%) may also be observed. Sexual disorders are found in about 30% of women treated with antipsychotics. According to the results obtained by different authors, reduced orgasm quality (33%), reduced ability to reach orgasm (22%), and pain during orgasm (7%) were the most common problems in women treated with antipsychotics. However, hyperprolactinemia, galactorrhea (5%–19%), and breast swelling (3%) are more frequently found in women than in men. Furthermore, irregular (78%) or complete suppression of menstrual cycles (22%–50%) was observed in women.Citation5 In people receiving psychiatric treatment, the possibility of other concurring factors should also be considered (chronic metabolic diseases and other medications) that can affect sexual function. Sexual dysfunction can also be related to thyroid disorders.Citation6 The concurrence of thyroid disease in mentally ill patients may significantly delay the onset of pharmacological treatment, including treatment with antipsychotic drugs, and delay the use of slow transcranial magnetic stimulationCitation7 and electroconvulsive therapy.Citation8 Thus, a detailed medical history to confirm or exclude sexual disorders before commencing psychiatric treatment is extremely important. The sexual function of the patient should therefore be evaluated in the premorbid period, during the period from illness onset to treatment initiation, after treatment initiation, and also during exacerbation of mental disease and remission.Citation9
Atypical antipsychotics (AAPs) are recommended for the treatment of schizophrenia and other psychotic disorders, including those resulting from organic diseases. These drugs have a more extensive influence on the range of schizophrenia symptoms (deficits, affective, and cognitive) than typical antipsychotic drugs.Citation10 Moreover, AAPs rarely cause side effects, such as neuroleptic malignant syndrome, versus typical antipsychotic drugs.Citation11,Citation12 AAPs may be considered as atypical when characterized by Melzert’s constant (their affinity for 5-HT2A and dopamine D2 receptors [D2R] should exceed or be equal to 1.12). Another distinct feature of atypical drugs is the D2R-blocking time, which is directly correlated to the dissociation constant (Kd) of the ligand–receptor complex. AAP drugs form a ligand–receptor complex with a shorter lifespan than typical antipsychotics. Furthermore, the values of their dissociation constant are lower than Kd for dopamine.Citation13 AAPs are also used in the treatment of bipolar disorder due to their mood stabilizing properties. They also enhance the antidepressant influence of drugs that are used in the treatment of other affective diseases such as atypical depression,Citation14 drug-resistant depression,Citation15 and depression resulting from organic disease.
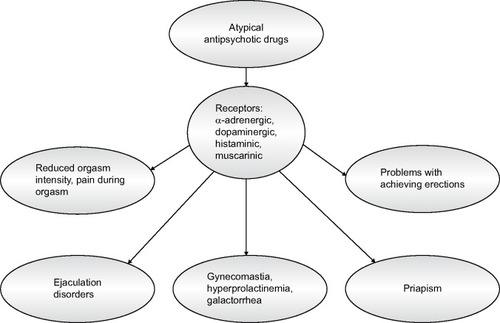
AAPs can affect sexual function in the following ways: (1) through their specific central effect of blocking dopaminergic receptors in the hypothalamus, contributing to hyperprolactinemia, galactorrhea, menstruation, or erection disorders and reduced libido; (2) through their peripheral effect of blocking α-adrenergic receptors, responsible for dilation of the arteries in the penis; (3) through an unspecific central sedative effect leading to reduced sexual activity.Citation15
The influence of AAPs on the central nervous system
The influence of AAPs on sexual function is related to the activation of neurotransmitters such as dopamine and serotonin in the central nervous system. This process involves the brain’s dopaminergic and serotonergic structures.
The influence of dopamine on sexual function
The dopaminergic structures involved in sexual motivation include the nucleus accumbens, which is a part of the mesolimbic pathway and the medial preoptic region of the hypothalamus. Stimulation of dopaminergic receptors of the paraventricular nucleus in the hypothalamus is important for causing erection. The serotonergic system (hippocampus and amygdala) has an inhibiting influence on sexual motivation, orgasm, and ejaculation. However, stimulation of individual serotonergic receptors in the central nervous system can cause various effects. The serotonergic receptors 5-HT2 and 5-HT3 have an inhibiting influence on sexual activity, while the stimulation of 5-HT1A receptors stimulates these functions.Citation16,Citation17
AAPs have an influence on the synthesis and circulation of dopamine, which has an impact on libido, erection, and ejaculation. Dopamine is the most important hypothalamic prolactin (PRL)-inhibiting factor. Blocking dopamine secretion leads to hyperprolactinemia, which can inhibit sexual function, particularly libido and erection, by increasing gamma-aminobutyric acid (GABA)-ergic activity and opioid levels.Citation18 Dopamine has an inhibiting influence on PRL secretion mainly via two pathways: the tuberoinfundibular dopaminergic (TIDA) system and the tuberohypophysial pathway. The TIDA system, formed by an aggregation of dopaminergic neurons found in the arcuate nucleus of the hypothalamus, plays the most important role in PRL release in humans. These dopaminergic neurons release dopamine into the perivascular space of the median eminence. Subsequently, dopamine is transported to the anterior lobe of the pituitary gland. The second inhibitory dopamine pathway, the tuberohypophyseal pathway, projects to the intermediate and posterior lobes of the pituitary gland. Dopamine released into the blood reaches the lactotroph cells and binds to the D2R on the membrane of these cells. D2R stimulation inhibits the synthesis and release of PRL, as well as lactotroph proliferation. The TIDA network is partially regulated by autocrine-negative feedback of PRL. An increase in circulating PRL levels results in higher activity of TIDA neurons, whereas a decrease in circulating PRL levels reduces their activity. Thus, PRL regulates its own release by acting directly on the hypothalamic dopaminergic neurons. PRL probably regulates tyrosine hydroxylase activity, the rate-limiting enzyme in dopamine synthesis. A blockade of D2R counteracts the tonic inhibitory effect on PRL secretion. Therefore, the stronger the dopamine blockade, the higher the increase in PRL.Citation19,Citation20
PRL influence on sexual function
Antipsychotics have a D2-blocking effect and can therefore increase PRL secretion. The normal reference values of PRL in the blood are 10–20 ng/mL in men and 10–25 ng/mL in women.Citation21 PRL secretion increases during sleep and is at its highest during the rapid eye movement stage sleep. PRL levels increase to 30 ng/mL between 4 am and 6 am. Current research has revealed that galactorrhea, impotence, azoospermia, and lack of libido are observed when PRL levels are >60 ng/mL.Citation22 Hyperprolactinemia can also lead to reduced blood testosterone levels and can indirectly contribute to reduced sexual activity.Citation18,Citation20 According to the research, 80% of men with PRL blood levels >50 ng/mL had reduced libido and suffered from impotence.Citation18,Citation20 AAPs as a group cause less hyperprolactinemia than conventional antipsychotics; yet, there is considerable variation among specific drugs.Citation5 Of the AAPs, risperidone, amisulpiride, and zotepine have the strongest stimulating effect on PRL synthesis. Asmal et alCitation23 showed in their study that PRL levels were increased at the beginning of treatment and were significantly higher in patients treated with risperidone. The average increase in PRL levels was higher in women than in men. However, Suzuki et alCitation24 showed that the highest PRL levels (higher in the group of studied women vs men) were measured in the blood of patients treated with risperidone or olanzapine as monotherapy, versus patients taking aripiprazole, quetiapine, and perospirone combined. Furthermore, Jeong et alCitation25 found that increased PRL levels during treatment with risperidone was accompanied by sexual dysfunction, especially problems with sexual arousal and penile erection, which were measured by using the Arizona Sexual Experience Scale.Citation26 The symptoms of sexual dysfunction disappeared after risperidone withdrawal and the commencement of aripiprazole. The influence of risperidone on the synthesis of PRL and sexual function are combined with its strong affinity for the D2 receptors and its simultaneous effect on the 5-HT2 receptors. Peuskens et alCitation21 found that among men receiving risperidone at a dose of 1–16 mg/day, 4.2%–17.7% had problems with erection and 3.6%–17.7% had a problem with ejaculation. The authors suggested that hyperprolactinemia could be the cause of these dysfunctions. Nevertheless, Jummani and CoffeyCitation27 observed mild hyperprolactinemia (11–21 ng/mL), premature ejaculation, and impotence in patients treated with risperidone at a dose of 3.5 mg/day. Other AAPs such as clozapine and quetiapine do not display an influence on PRL secretion.Citation23 However, Serretti and ChiesaCitation28 showed that sexual dysfunction (desire, arousal, and orgasm dysfunction) occurred during treatment with quetiapine and aripiprazole, although PRL blood levels were within the normal range. These observations indicate that other factors such as chronic stress and metabolic diseases (hypertension and diabetes) can be responsible for sexual dysfunction.
Olanzapine is another AAP that influences PRL levels and sexual function.Citation29 This results in olanzapine binding with a wide range of dopaminergic (D1, D2, D3, D4) and serotonergic (5-HT2A, 5-HT3, 5-HT6, 5-HT2C) receptors. According to many studies, olanzapine is characterized by having a lower and more selective affinity for dopaminergic receptors (mainly the mesolimbic pathway) than risperidone. Konarzewska et alCitation30 in their own research found a temporary increase in PRL levels in patients treated with olanzapine. This increase was lower in the group of patients treated with olanzapine versus patients treated with risperidone. Similar results were obtained by Kim et alCitation31 who observed a lower increase in PRL levels and sexual dysfunction following the change in treatment from risperidone to olanzapine. BaggaleyCitation32 noticed that risperidone showed a significantly higher influence on the development of sexual dysfunction versus olanzapine, quetiapine, and aripiprazole. There are also reports concerning sexual dysfunction and treatment with sertindole, in which orgasm without ejaculation was observed, which was associated with the peripheral α1-adrenergic antagonism of this drug, and also orgasm with ejaculation of reduced ejaculate volume.Citation33 Sertindole is a second-generation antipsychotic drug with a high affinity for dopaminergic D2, serotonergic 5-HT2A, 5-HT2C, and α1-adrenergic receptors and a low affinity for other receptors. Muscatello et al,Citation34 Lewis et al,Citation35 and Jukić et alCitation36 in their studies found that sertindole can cause sexual dysfunction independent of increased PRL levels, more commonly in men than in women.
The influence of serotonin and noradrenaline on sexual function
After dopamine, serotonin is the second most important neurotransmitter to influence sexual function.Citation37 Serotonin mainly inhibits sexual function by stimulating postsynaptic 5-HT2A and 5-HT2C receptors, while the stimulation of presynaptic 5-HT1A autoreceptors increases sexual activity, reducing serotonin secretion from the nerve termini. Furthermore, reduction of the negative symptoms of schizophrenia is combined with the serotonergic system, particularly with antagonism of the 5-HT2A and/or 5-HT1A receptors.Citation38,Citation39 By blocking these receptors located on dopaminergic neurons, dopaminergic transmission in the mesocortical pathway is intensified, and as a result, cognitive (such as focusing attention and working memory) and executive functions are improved. On the other hand, an increase in transmission in the nigrostriatal pathway reduces the risk of neuroleptic malignant syndrome through disinhibition. Noradrenaline, another neurotransmitter, increases the ability for arousal through its influence on central receptors and inhibits erection by binding with peripheral α1 receptors.Citation40
The peripheral influence of AAP drugs
The adrenergic system is one of the peripheral mechanisms that has an influence on penile erection. It is accompanied by the relative functional prevalence of the parasympathetic (β2-adrenergic activation) over the sympathetic (α1-adrenergic inhibition) system, necessary to dilate penile arteries and for blood to flow into the cavernous bodies of the penis.Citation41 One related sexual dysfunction is priapism. In this disorder, a prolonged, painful erection of the penis occurs, which is unrelated to sexual arousal or stimulation. Priapism can be caused by the blocking of α1-adrenergic receptors by AAP drugs. According to numerous studies, most of the AAP drugs, such as olanzapine, risperidone, and amisulpiride can cause priapism. It is worth noting that olanzapine can cause both reversible and irreversible priapism.Citation42 The symptoms of reversible priapism after 10 days of olanzapine treatment at a dose of 10 mg/day were observed by Doufik et al.Citation43 Priapism symptoms disappeared when the treatment was changed from olanzapine to amisulpiride at a dose of 400 mg/day. Furthermore, reversible priapism with olanzapine was also described by Penaskovic et al.Citation44 This sexual dysfunction appeared after 7 days of olanzapine treatment at a dose of 15 mg/day. However, Jagadheesan et alCitation45 described irreversible priapism after treatment with olanzapine. The symptoms of priapism appeared on the sixth day of treatment with olanzapine at a dose of 5 mg/day. Reversible priapism was also observed after treatment with other neuroleptics such as risperidone and ziprasidone. Ginory and NguyenCitation46 observed priapism after a month’s therapy with risperidone at a dose of 6 mg/day. Furthermore, Cruzado and VallejosCitation47 described priapism that developed during monotherapy with risperidone at a dose of 3 mg/day, after 3 years of using the drug. Due to this adverse effect, risperidone was withdrawn and quetiapine at a dose of 450 mg/day was prescribed for the patient and the side effect disappeared. Karamustafalioglu et alCitation48 observed signs of priapism caused by ziprasidone at a dose of 40 mg/day on the seventh day of treatment. The patient felt uncomfortable and stated that the condition had lasted for nearly 8 hours and was not associated with sexual stimulation or desire. Recurring priapism during treatment with ziprasidone at a total daily dose of 160 mg (40 mg bid + 80 mg qhs) was described by Kaufman et alCitation49 and with a dose of 140 mg/day (ziprasidone with food, 80 mg at bedtime and 60 mg in the morning) was described by Denton et al.Citation50 Ziprasidone can not only cause priapism but also cause spontaneous orgasm. AAP drugs may also cause very painful priapism in children. There is one case of a 12-year-old boy treated with risperidone at a dose of 4 mg/day for schizophrenia, reported by Prabhuswamy et alCitation51 and a second case of a 14-year-old boy treated with olanzapine at a dose of 5 mg/day, reported by Husár and Zerhau.Citation52
The sedative influence of AAP drugs leading to reduced sexual activity
AAP drugs can adversely influence sexual function due to their sedative effect. This process involves the blocking of dopaminergic and 5-HT2A serotonergic receptors, antihistaminergic effects (blocking of H1 receptors), and an anticholinergic effect (blocking of muscarinic receptors). During optimalization of AAP treatment with regard to their antipsychotic influence, sedative symptoms leading to reduced sexual activity must be considered. When introducing AAP treatment, knowledge of the pharmacokinetics of the prescribed drug is important. It is recommended to administer the whole dose of the drug in the evening, yet sufficiently early enough so that the peak concentration of the drug in the blood decreases at the moment of falling asleep (medicine can be administered even 5–6 hours before sleep).Citation53,Citation54 By dosing olanzapine this way, the sedative effect and adverse influence on sexual function can be minimalized. During treatment with clozapine or quetiapine, these drugs should be administered in two daily doses due to their short half-life and in order to avoid sedative effects during the day. Also, the evening dose of the drug should be higher than that given in the morning.Citation55,Citation56 Aripirazole, sertindole, amisulpiride, and ziprasidone do not show sedative effects as they do not have any antihistaminergic and anticholinergic effects. Therefore, they do not have a negative effect on sexual function.Citation57–Citation59
Conclusion
Problems during therapy with AAP drugs include a lack of feedback from patients about the adverse effects caused by the drugs, particularly when they are combined with sexual dysfunction. Patients who do not tolerate these adverse effects may have problems with compliance during treatment. In conclusion, current knowledge about the mechanisms of sexual dysfunction caused by AAP drugs indicates the important role of dopaminergic blockade as a factor in reducing sexual drive as well as the ability to achieve erection and ejaculation, directly and as a result of hyperprolactinemia. Also, the blockade of alpha-1 and probably α2-adrenergic and muscarinic receptors is related to some disorders (impotence, priapism), although its final effects seem to depend on the location (ie, central, peripheral) of the target receptors to a larger extent, as well as on the overlapping, “modulating” receptor effects of a drug. The anti-serotonergic properties of AAP drugs can to some extent prevent sexual dysfunction.
Author contribution
MJJ contributed in the conception of the study, interpretation, preparation of manuscript, editing, revising, and recruitment.
Acknowledgments
The work was carried out at the Department of General Surgery, Municipal Hospital in Piekary Slaskie, Poland. Professional language editing has been performed by a native English speaker – Peter Kośmider-Jones. This research has received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The author reports no conflicts of interest in this work.
References
- GroverSGhoshASarkarSChakrabartiSAvasthiASexual dysfunction in clinically stable patients with bipolar disorder receiving lithiumJ Clin Psychopharmacol201434447548224781439
- HamiltonLDMestonCMChronic stress and sexual function in womenJ Sex Med201310102443245423841462
- AgmoAOn the intricate relationship between sexual motivation and arousalHorm Behav201159568168820816969
- BoinACNozoeKTPoleselDNAndersenMLTufikSThe potential role of sleep in sexual dysfunction in patients with schizophreniaSchizophr Res20141541–312612724556470
- KopecekMBaresMSvarcJDockeryCHoracekJHyperprolactinemia after low dose of amisulprideNeuro Endocrinol Lett200425641942215665803
- OglodekESzotaAAraszkiewiczAElectroconvulsive therapy in a patient with drug-resistant depression and thyroid hormone imbalanceAust N Z J Psychiatry2014481969723814070
- OglodekEWiczkowskiASieronABilska-UrbanAMośDThe effect of extremely low-frequency magnetic fields on the morphology of thyroid gland cells in female ratsPol J Environ Stud2008175757763
- KristensenDHagemanIBauerJJørgensenMBCorrellCUAntipsychotic polypharmacy in a treatment-refractory schizophrenia population receiving adjunctive treatment with electroconvulsive therapyJ ECT201329427127623859980
- MillierAAmriIBoyerLAuquierPToumiMUtility decrements associated with side effects in schizophreniaJ Med Econ2014171285386125211094
- ConnollyATaylorDFactors associated with non evidence-based prescribing of antipsychoticsTher Adv Psychopharmacol20144624725625489476
- OglodekESzotaAAraszkiewiczAOlanzapine-induced neuroleptic malignant syndrome after 10 years of treatmentAust N Z J Psychiatry2013471097223630396
- SzotaAOgłodekEAraszkiewiczAFever development in neuroleptic malignant syndrome during treatment with olanzapine and clozapinePharmacol Rep201365227928723744413
- GrundmannMKacirovaIUrinovskaRTherapeutic drug monitoring of atypical antipsychotic drugsActa Pharm201464438740125531781
- SzotaAOglodekEAraszkiewiczAA female patient with depression and conversion disorder following brain tumor surgeryAust N Z J Psychiatry201347121213121423975697
- SzotaAOgłodekEAraszkiewiczABipolar disorder: mixed episodes concomitant with gambling addictionAust N Z J Psychiatry201348658658724366857
- GründerGWetzelHSchlösserRNeuroendocrine response to antipsychotics: effects of drug type and genderBiol Psychiatry199945189979894580
- BevanJSInterpreting prolactin levels: implications for the management of large pituitary lesionsBr J Neurosurg199151362021431
- FreemanMEKanyicskaBLerantANagyGProlactin: structure, function, and regulation of secretionPhysiol Rev20008041523163111015620
- HaddadPMWieckAAntipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and managementDrugs200464202291231415456328
- KinonBJGilmoreJALiuHHalbreichUMPrevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidonePsychoneuroendocrinology200328suppl 2556812650681
- PeuskensJPaniLDetrauxJDe HertMThe effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive reviewCNS Drugs201428542145324677189
- WeizmannAWeizmannRHartJMaozBWijsenbeekHBen DavidMThe correlation of increased serum prolactin levels with decreased sexual desire and activity in elderly menJ Am Geriatr Soc19833184854886875154
- AsmalLFlegarSJWangJRummel-KlugeCKomossaKLeuchtSQuetiapine versus other atypical antipsychotics for schizophreniaCochrane Database Syst Rev201311CD00662524249315
- SuzukiYSugaiTFukuiNDifferences in plasma prolactin levels in patients with schizophrenia treated on monotherapy with five second-generation antipsychoticsSchizophr Res20131451–311611923375624
- JeongHGLeeMSLeeHYKoYHHanCJoeSHChanges in sexual function and gonadal axis hormones after switching to aripiprazole in male schizophrenia patients: a prospective pilot studyInt Clin Psychopharmacol201227417718322407277
- BuckleyPFGoldsteinJMEmsleyRAEfficacy and tolerability of quetiapine in poorly responsive, chronic schizophreniaSchizophr Res2004662–314315015061246
- JummaniRCoffeyBJHyperprolactinemia in an adolescent with psychotic disorder on risperidoneJ Child Adolesc Psychopharmacol200919679179420035598
- SerrettiAChiesaAA meta-analysis of sexual dysfunction in psychiatric patients taking antipsychoticsInt Clin Psychopharmacol201126313014021191308
- DavidSRTaylorCCKinonBJBreierAThe effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophreniaClin Ther20002291085109611048906
- KonarzewskaBWołczyńskiSSzulcAGalińskaBPopławskaRWaszkiewiczNEffect of risperidone and olanzapine on reproductive hormones, psychopathology and sexual functioning in male patients with schizophreniaPsychoneuroendocrinology200934112913918838228
- KimKSPaeCUChaeJHEffects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidoneJ Clin Psychiatry200263540841312019665
- BaggaleyMSexual dysfunction in schizophrenia: focus on recent evidenceHum Psychopharmacol200823320120918338766
- Martin-DuPRBaumannPSexual dysfunctions induced by antidepressants and antipsychoticsRev Med Suisse2008415075876218476641
- MuscatelloMRBrunoAPandolfoGMicòUSettineriSZoccaliREmerging treatments in the management of schizophrenia – focus on sertindoleDrug Des Devel Ther20104187201
- LewisRBagnallALeitnerMSertindole for schizophreniaCochrane Database Syst Rev20002CD00171510796657
- JukićMKDrmićSMimicaNEjaculatory dysfunction in patient with schizophrenia on sertindolePsychiatr Danub201022112813120305609
- CrawfordAMBeasleyCMJrTollefsonGDThe acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentrationsSchizophr Res199726141549376336
- OgłodekEASzotaAJustMJMośDAraszkiewiczAComparison of chemokines (CCL-5 and SDF-1), chemokine receptors (CCR-5 and CXCR-4) and IL-6 levels in patients with different severities of depressionPharmacol Rep201466592092625150002
- OgłodekEASzotaAMJustMJMośDMAraszkiewiczAThe MCP-1, CCL-5 and SDF-1 chemokines as pro-inflammatory markers in generalized anxiety disorder and personality disordersPharmacol Rep2015671858925560580
- KleinbergDLDavisJMde CosterRVan BaelenBBrecherMProlactin levels and adverse events in patients treated with risperidoneJ Clin Psychopharmacol199919157619934944
- BreierAFMalhotraAKSuTPPinalsDAElmanIAdlerCMClozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine responseAm J Psychiatry199915622942989989566
- Pérez-IglesiasRMataIMartínez-GarcíaOLong-term effect of haloperidol, olanzapine, and risperidone on plasma prolactin levels in patients with first-episode psychosisJ Clin Psychopharmacol201232680480823131886
- DoufikJOthemanYKhaliliLGhanmiJOuanassAAntipsychotic-induced priapism and management challenges: a case reportEncephale201440651852124709224
- PenaskovicKMHaqFRazaSPriapism during treatment with olanzapine, quetiapine, and risperidone in a patient with schizophrenia: a case reportPrim Care Companion J Clin Psychiatry2010125PCC.09100939
- JagadheesanKThakurAAkhtarSIrreversible priapism during olanzapine and lithium therapyAust N Z J Psychiatry200438538115144518
- GinoryANguyenMA case of priapism with risperidoneCase Rep Psychiatry2014201424157325379316
- CruzadoLVallejosCEPriapism associated with risperidone use: report of one caseRev Med Chil2012140111445144823677191
- KaramustafaliogluNKaleliogluTTanrioverOGungorFGencAA case report of priapism caused by ziprasidonPsychiatry Investig2003104425427
- KaufmanKRSternLMohebatiAOlsavskyAHwangJZiprasidone-induced priapism requiring surgical treatmentEur Psychiatry2006211485016356688
- DentonKKolliVSharmaAZiprasidone-induced ischemic priapism requiring surgical intervention: a case reportPrim Care Companion CNS Disord2013151PCC.12101443
- PrabhuswamyMSrinathSGirimajiSSeshadriSRisperidone-induced priapism in a 12-year-old boy with schizophreniaJ Child Adolesc Psychopharmacol200717453954017822349
- HusárMZerhauPPriapism in childhood-case report of 14-year-old boyRozhl Chir200685732933017044274
- BrichartNDelavierreDPeneauMIbrahimHMallekAPriapism associated with antipsychotic medications: a series of four patientsProg Urol2008181066967318971111
- ReevesRRKimbleRProlonged erections associated with ziprasidone treatment: a case reportJ Clin Psychiatry2003641979812590634
- MitchellJEPopkinMKAntipsychotic drug therapy and sexual dysfunction in menAm J Psychiatry198213956336376122381
- SongerDABarclayJCOlanzapine-induced priapismAm J Psychiatry2001158122087208811729037
- ShahSKA comparative study of sexual dysfunction in schizophrenia patients taking aripiprazole versus risperidoneKathmandu Univ Med J20131142121125
- AndersohnFSchmedtNWeinmannSWillichSNGarbeEPriapism associated with antipsychotics: role of alpha1 adrenoceptor affinityJ Clin Psychopharmacol2010301687120075651
- OgłodekESzotaAJustMMośDAraszkiewiczAThe role of the neuroendocrine and immune systems in the pathogenesis of depressionPharmacol Rep201466577678125149980