Abstract
Numerous studies have shown that autophagy failure plays a critical role in the pathogenesis of Alzheimer’s disease, including increased expression of beta-amyloid (Aβ) protein and the dysfunction of Aβ clearance. To further evaluate the role of autophagy in Alzheimer’s disease, the present study was implemented to investigate the effects of autophagy on α-secretase, β-secretase, or γ-secretase, and observe the effects of autophagy on autophagic clearance markers. These results showed that both autophagy inhibitor and inducer enhanced the activity of α-, β-, and γ-secretases, and Aβ production. Autophagy inhibitor may more activate γ-secretase and promote Aβ production and accumulation than its inducer. Both autophagy inhibitor and inducer had no influence on Aβ clearance. Hence, autophagy inhibitor may activate γ-secretase and promote Aβ production and accumulation, but has no influence on Aβ clearance.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease, of which cognitive impairment gradually worsens over time.Citation1–Citation3 As AD advances through the brain, it eventually affects all aspects of a person’s life including mental abilities, emotions and moods, behavior, and the ability to carry out daily activities like eating and grooming.Citation4,Citation5 It is well known that there are three consistent neuro-pathological hallmarks associated with AD, including extracellular amyloid-rich senile plaques, intracellular neurofibrillary tangles and neuronal degeneration of basal forebrain cholinergic neurons that innervate the hippocampus and the cortex.Citation6–Citation8
Autophagy is one of the major degradation pathway characterized by a ubiquitous cellular process responsible for the bulk degradation of long-lived proteins and organelles through an autophagosome-lysosomal pathway.Citation9,Citation10 The word autophagy is derived from the Greek words auto (meaning “self”) and phage (meaning “eat”), of which the main function of autophagy refers to clear abnormal or obsolete cellular proteins.Citation11 There are at least three processes by which intracellular constituents enter lysosomes for degradation distinguishable by their mechanisms: macro-autophagy (the most prevalent form), micro-autophagy, and chaperone-mediated autophagy. Autophagy exists in both normal cellular homeostasis and disease states. Increasing findings have demonstrated that autophagosome-lysosomal dysfunction contributes to severe neurodegenerative disorders related to accumulations of lysosomes and autophagic vacuoles (AVs).Citation11 Compelling research studies have supported that the pivotal role of autophagy in the clearance of aggregate-prone proteins is responsible for several neurodegenerative disorders,Citation12,Citation13 which are implicated in the pathogenesis of AD,Citation13,Citation14 Parkinson’s disease,Citation15,Citation16 Huntington’s disease,Citation17,Citation18 and other related disorders.
An autophagosome, a spherical structure with double layer membranes, is a cellular vesicle that ingests cellular debris and transports the debris to lysosomes. Growing evidence indicates that the rate of autophagosome formation and maturation and the efficiency of autophagosome/lysosome fusion decline in neurodegenerative diseases with age.Citation19–Citation21 A growing number of studies have shown that dysfunction of autophagy plays a critical role in the pathogenesis of AD, including senile plaques, neurofibrillary tangles, and neuronal degeneration.Citation13,Citation22 Moreover, it has been found that immature AVs accumulate during the early evolution of pathology in a dendrite in the PS1-APP (amyloid protein precursor) mouse model of AD while pathological AVs’ accumulation is associated with inhibited retrograde AVs’ transport and impaired autophagosome/lysosome fusion.Citation23–Citation25 Furthermore, a link between autophagy dysfunction and beta-amyloid (AP) generation and clearance has been reported to occur in AD.Citation26,Citation27 A number of papers have investigated the precise role of autophagy in the Aβ generation and clearance. However, understanding the exact mechanism may help to design more effective therapeutic strategies to prevent neuronal degeneration and death. It is well known that Aβ production and deposition represent a key feature and is thought as the classic pathological hallmarks in AD. Aβ is generated from APP by the sequential actions of two proteolytic enzymes: β-secretase (beta-site APP cleavage enzyme, BACE) and γ-secretase complex.Citation28,Citation29 In addition, APP undergoes another cleavage: the non-amyloidogenic processing by α-secretase and γ-secretase complex to release membrane-anchored carboxy-terminal fragments that may be associated with apoptosis.Citation30,Citation31 Therefore, it is possible that autophagy regulated Aβ generation via controlling the activity of α-, β-, or γ-secretases. The present study was implemented to investigate the effects of autophagy on α-, β-, and γ-secretase, and the level of Aβ, and to observe the effects of autophagy on autophagic clearance markers. The aim is to further evaluate the role of autophagy in the neurodegenerative process of AD.
These results noted that the both autophagy inhibitor and inducer enhanced Aβ1–42 expression while the level of Aβ1–42 peptide was more remarkably increased by the autophagy inhibitor than by the autophagy inducer. Both autophagy inhibitor and inducer increased the activity of α-, β-, and γ-secretases while the components of the γ-secretase complex (Presenilin 1, Nicastrin, and presenilin enhancer 2 [Pen-2]) were more activated by autophagy inhibitor, compared with the inducer treatment. However, this study revealed that there was no difference between the treatment of the autophagy inhibitor and autophagy inducer. Our study suggests that autophagy inhibitor may activate γ-secretase and promote Aβ accumulation, but has no influence on Aβ clearance.
Materials and methods
Cell culture
SH-SY5Y, a human-derived neuroblastoma cell line, is thrice-cloned originally from SK-N-SH and widely used in the scientific research of neurodegenerative disorders.Citation32 SH-SY5Y was grown in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA) and 1% penicillin/streptomycin. Cells were maintained in a humidified atmosphere at 37°C with 5% CO2. The autophagy inhibitor (3-methyladenine, 3-MA, Santa Cruz Biotechnology, Dallas, TX, USA) and inducer (STF-62247, Selleck Chemicals, Houston, TX, USA) were dissolved in dimethyl sulfoxide and used at the following concentrations: the inhibitor (3-MA), 10 mM; the inducer (STF-62247), 10 μM. The cells were treated with the autophagy inhibitor and inducer without fetal bovine serum for 24 hours.
RNA extraction and real-time quantitative PCR
Total cellular RNA was extracted using the Trizol reagent (Invitrogen), according to the manufacturer’s instruction. cDNAs were synthesized and real-time PCR was performed using the GoTaq® 2-Step RT-qPCR System (Promega, Madison, WI, USA) in an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Gene expression data were normalized to the geometric mean of the β-actin, housekeeping gene, to control for variability in expression levels and calculated as 2−[(Ct of gene)−(Ct of β-actin)], where Ct represents the threshold cycle for each transcript. The used primers in this study were the following: BACE1 (forward: 5′-AGGCAGCTGTCCAGCACATACC-3′, reverse: 3′-TAGCCAGCTGGTGCAGGAGAT-5′), a disintegrin and metalloprotease 17 (ADAM17) (forward: 5′-AGCAGGTGTCGTTGTTCAGA-3′, reverse: 3′-GTGGCGATCACGAGAACAAT-5′), Presenilin 1 (forward: 5′-CAGGTGCTATAAGGTCATCC-3′, reverse: 3′-GTCCACAGCAACGTTATAGG-5′), Presenilin 2 (forward: 5′-CAGCTCATCTACACGCCATT-3′, reverse: 3′-CAGCAGCATCAGTGAAGACA-5′), Nicastrin (forward: 5′-TACGGAACCAGGTGGAGGAT-3′, reverse: 3′-GAAGGCACCAGAGTGGTCAG-5′), APH-1 (forward: 5′-TCCTGACTTCAGCCTTTCTGAC-3′, reverse: 3′-CAAGAGGCTGCGCTGAATAC-5′), Pen-2 (forward: 5′-GCCAAATCAAAGGCTATGTCTG-3′, reverse: 3′-ATGGTGAAGGAGAGGTAGTC-5′), β-actin (forward: 5′-TGGCACCCAGCACAATGAA-3′, reverse: 3′-CTAAG TCATAGTCCGCCTAGAAGCA-5′).
Western blotting
Cells were harvested in sampling buffer (62.5 mmol/L Tris-HCl [pH 6.8], 10% glycerol, 2% SDS) and heated for 5 minutes at 100°C. The concentration of extracted proteins was determined by the Bradford assay using a commercial kit (Bio-Rad, Berkeley, CA, USA). Equal quantities of protein were separated by electrophoresis on 12% SDS/polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Roche, Indianapolis, IN, USA). The membranes were then probed with rabbit primary antibodies against anti-BACE1, anti-ADAM17, anti-Presenilin 1, anti-Presenilin 2, anti-Nicastrin, anti-APH-1, anti-Pen-2, neprilysin (NEP), insulin-degrading enzyme (IDE), endothelin-converting enzyme 1 (ECE-1), and ECE-2 (1:1,000; Santa Cruz Biotechnology). The protein expression was detected with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000; Amersham Pharmacia Biotech, Piscataway, NJ, USA) and an enhanced chemiluminescence kit (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. Anti-β-actin mouse monoclonal antibody (1:1,000; Cell Signaling, Danvers, MA, USA) acted as a loading control.
Transmission electron microscopy
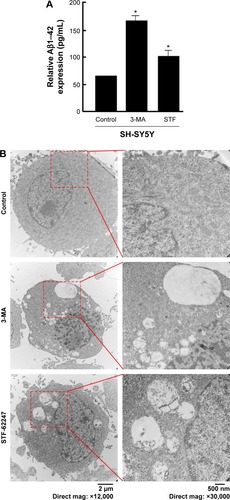
SH-SY5Y treated with 3-MA and STF-62247 for 24 hours was fixed for 2 hours at room temperature with 2.5% glutaraldehyde in phosphate-buffered saline (pH 7.4) and, subsequently, with 1% OsO4 in 50 mM sodium cacodylate buffer (pH 7.3), dehydrated in an ethanol series and embedded into epon (catalyst). Ultrathin sections of 50 nm were contrasted with uranyl acetate and lead citrate and analyzed in a Tecnai Spirit transmission electron microscope (FEI) with an ORIUS CCD camera (Gatan).
Aβ1–42 measurement
Cells were seed at a constant density to obtain identical experimental conditions in the different tests and to achieve a high accuracy of the measurements. Aβ1–42 levels were determined in the culture supernatant using an ELISA kit (Uscn Life). The assays were performed according to manufacturer’s guidelines. Results were expressed as pg/mL.
Statistical analysis
Student’s t-test was used to evaluate the significant difference between two groups of data in all the pertinent experiments. Data were represented as the mean ± standard error of the mean. P-value <0.05 (using a two-tailed paired t -test) was considered as statistically significant.
Ethics
This study was approved by the Ethics committee of Renmin Hospital, Hubei University of Medicine.
Results
Inhibiting autophagy pathway increased Aβ expression
Mounting hypothesis has shown that extracellular Aβ plays an important role in AD pathogenesis.Citation22,Citation33,Citation34 Consistent with other research results, the level of Aβ1–42 peptide was remarkably increased by the autophagy inhibitor (3-MA), compared with control and the autophagy inducer (STF-62247) (P<0.001) (). Different from the other research results, both the autophagy inhibitor (3-MA) and inducer (STF-62247) enhanced Aβ1–42 expression whereas the level of Aβ1–42 stimulated by 3-MA was higher than that by STF-62247 (P<0.005) ().
Figure 1 Modulation of autophagy affects Aβ secretion.
Abbreviations: Aβ, beta-amyloid; 3-MA, 3-methyladenine; mag, magnification.
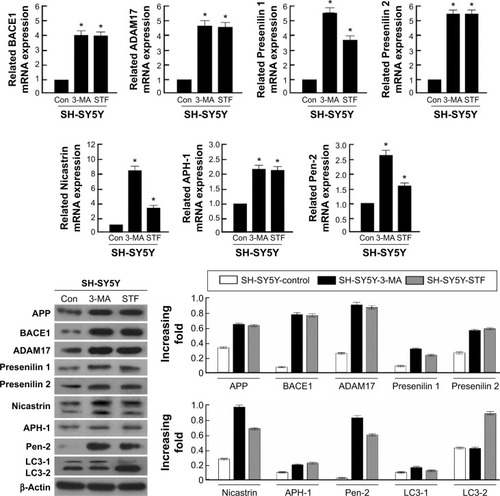
Autophagy dysfunction augmented the activity of γ-secretase complex
APP is cleaved sequentially at the extracellular site by β-secretase (BACE) and γ-secretase to release Aβ peptides.Citation35–Citation37 APP can be also cleaved by α-and γ-secretases to produce P3. The α-secretase represents two members of the family of ADAM: tumor necrosis factor-converting enzyme (ADAM17) and ADAM10. β-Secretase, a membrane-bound aspartic protease, is also called BACE.Citation36,Citation38 γ-Secretase is a multi-subunit protease complex, consisting of four individual proteins: Presenilin, Nicastrin, APH-1 (anterior pharynx-defective 1), and Pen-2.Citation35,Citation38
In the present study, the glioma cell line SH-SY5Y was treated with autophagy inhibitor (3-MA) and autophagy inducer (STF-62247). Then, the cell harvested to analyze by real-time quantitative PCR and Western blotting. These results demonstrated that α- and β-secretases, respectively exhibited as BACE1 and ADAM 17, were not different between 3-MA and STF-62247 treatments. The components of the γ-secretase complex (Presenilin 1, Nicastrin, and Pen-2) were activated by 3-MA, compared with the STF-62247 treatment (P<0.001) (). Both autophagy inhibitor and inducer improved the activity of α-, β-, and γ-secretases (P<0.005).
Figure 2 Effect of autophagy on APP-related cleavage secretases.
Abbreviations: Aβ, beta-amyloid; ADAM17, a disintegrin and metalloprotease 17; APH-1, anterior pharynx-defective 1; APP, amyloid protein precursor; Bace1, beta-site APP cleavage enzyme; 3-MA, 3-methyladenine; Con, control, LC3, microtubule-associated protein 1A/1B-light chain 3.
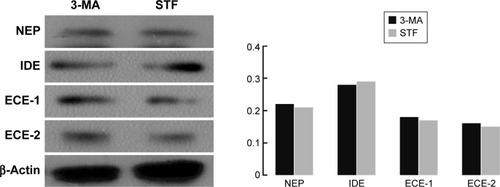
Autophagy had no effect on the Aβ clearance markers
Aβ production and failure of Aβ clearance are key factors in the development of AD. An overall impairment in Aβ clearance has been found where clearance rates for both Aβ1–42 and Aβ1–40 were impaired in AD.Citation35,Citation39 Several Aβ-degrading enzymes such as NEP, IDE, ECE-1, and ECE-2 are critical in Aβ accumulation determined in part by the imbalance between the production of Aβ and its removal from the brain.Citation35,Citation40,Citation41 Therefore, the present study further investigated the influence of autophagy on the Aβ clearance markers (NEP, IDE, ECE-1, and ECE-2) although several research supported that the activation of autophagy benefited Aβ clearance. However, these findings revealed that there was no difference between the treatment of the autophagy inhibitor (3-MA) and autophagy inducer (STF-62247) (P>0.05) ().
Figure 3 Influence of autophagy on the Aβ clearance markers.
Abbreviations: Aβ, beta-amyloid; ECE-1, endothelin-converting enzyme 1; ECE-2, endothelin-converting enzyme 2; IDE0, insulin-degrading enzyme; NEP, neprilysin; 3-MA, 3-methyladenine.
Discussion
Autophagy is one major cellular pathway associated with the removal of aggregated proteins. A few studies elucidated that autophagy plays a critical role in multiple pathological lesions of AD, such as dysregulating APP turnover and enhancing the activity of β- and/or γ-secretases.Citation42–Citation44 Autophagy affects an array of molecular pathways that may play a role in both Aβ generation and Aβ clearance.Citation22,Citation45,Citation46 However, the precise mechanism or role of autophagy in Aβ generation and Aβ clearance remained unclear.Citation22 We found that both autophagy inhibitor and inducer are associated with Aβ generation via regulating α-, β-, and/or γ-secretases. The relationship between autophagy inhibitor and the level of Aβ is robust enough for autophagy inducer, which heightened Aβ expression through upregulating α-, β-, and/or γ-secretases. Nevertheless, there was no divergence in Aβ clearance under the treatment of autophagy inhibitor and inducer.
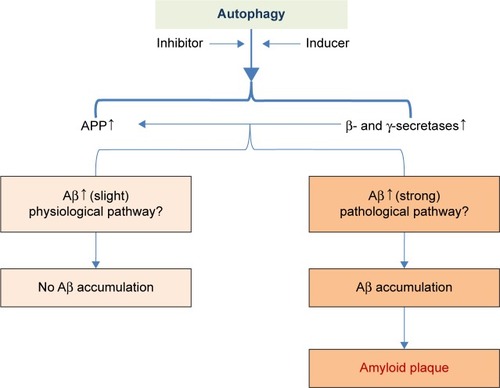
Our study has certain distinctive outcome. Formerly, inducing autophagy pathway keeps a foothold to restrain Aβ generation whereas inhibiting autophagy pathway is a friend for Aβ generation. Yet, the present results were different from the past whereas this study illustrated that both autophagy inhibitor and inducer account for Aβ generation. Consistent with the former findings, inhibiting autophagy pathway was a more strongly enhancer to the event of Aβ generation. Thus, it seems that our results were self-contradictory and paradox. It may be reasonable to take into consideration that Aβ plays a dual role as both physical and pathological substance. Functional Aβ is abundant in most environmental biofilms. Several potential activities have been discovered for Aβ, such as activating kinase enzymes, protection against oxidative stress, regulation of cholesterol transport, functioning as a transcription factor, and anti-microbial activity.Citation47–Citation50 Intracellular Aβ may impel a variety of cellular events such as protein degradation, axonal transport, neuronal firing, and autophagy to apoptosis.Citation47,Citation51–Citation53 Therefore, it is indicated that autophagy plays dual roles in the degradation and secretion of Aβ since inducing and inhibiting autophagy pathway shares a common access to heighten Aβ production under the Aβ physical process ().
Figure 4 The hypothesis of the role of autophagy in the Aβ pathophysiology process.
Abbreviations: Aβ, beta-amyloid; APP, amyloid protein precursor.
It is well known that Aβ is generated from APP by the sequential cleavage of two proteolytic enzymes: β-(BACE) and γ-secretase.Citation54,Citation55 A few studies demonstrated that the role of autophagy has been linked to Aβ generation and aberrant processing of APP.Citation56,Citation57 Autophagy plays an important role in Aβ generation via regulating APP turnover and stimulating the activity of β- and γ-secretases.Citation58,Citation59 However, the key role of autophagy in AD development is still under consideration today.Citation60 Whether autophagy regulates Aβ by influencing the expression of these associated secretases (α-, β-, and/or γ-secretases)? In controversy with the other findings which indicate that autophagy inducer can inhibit the expression of β- and γ-secretases, and Aβ generation, it was occurred that both autophagy inhibitor and inducer increased the activity of α-, β-, and γ-secretases in the present study. In consideration to the physiological function of APP, α-, β-, and γ-secretases,Citation49,Citation61 it may be a normal response to the stimulation of autophagy inducer and acute stress.
Importantly, this study demonstrated that autophagy inhibitor augmented the activity of γ-secretase complex by upregulating its components (Presenilin 1, Nicastrin, and Pen-2), compared with autophagy inducer. Accordingly, it was inferred that autophagy failure contributed to Aβ generation via activating γ-secretase complex ().
Several studies have supported that failure of Aβ clearance in the brains of patients with AD has been involved in autophagy dysfunction.Citation62,Citation63 The Aβ deposition and formation of Aβ plaques will be accelerated because of defects in its removal, mediated through a combination of diffusion along perivascular extracellular matrix, transport across vessel walls into the blood stream, and enzymatic degradation.Citation44,Citation64–Citation66 Several Aβ-degrading enzymes such as NEP, IDE, ECE-1, and ECE-2 are critical in Aβ clearance, most of which are produced by neurons and glial cells.Citation40,Citation41 Scientific evidence has revealed the role of autophagy in Aβ clearance involved in Aβ-degrading enzymes.Citation40,Citation44 However, the clearance markers of Aβ NEP, IDE, ECE-1, and ECE-2) were not different between autophagy inducer and inhibitor treatment. These results showed that, autophagy inhibitor may activate γ-secretase and promote Aβ generation and accumulation, but has no influence on Aβ clearance.
In the current study, both autophagy inhibitor and inducer increased the activity of α-, β-, and γ-secretases, and enhanced Aβ production. Moreover, autophagy inhibitor may more activate γ-secretase and promote Aβ production and accumulation, compared with its inducer. Both autophagy inhibitor and inducer had no influence on Aβ clearance. However, other results indicate that inducing autophagy pathway keeps a foothold to restrain Aβ generation whereas inhibiting autophagy pathway is a friend for Aβ generation. Aberrant autophagy induction leads into a concentration of AVs rich in APP, Aβ and the elements crucial for its formation while the dysfunction of autophagic clearance plays an important role in AD pathogenesis.Citation60 Overall, the precise role of in AD pathogenesis is still under contention.Citation11 Therefore, it is indispensable to further elucidate the potential routes for autophagy-mediated Aβ production and clearance in the AD pathophysiology mechanisms.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (81070878/H0902) and Nature Science Foundation of Guangdong (S20120200-10867) to Professor Bin Zhao, the Hubei Province Health and Family Planning Scientific Research Project (WJ2015MB219), the Hubei Provincial Nature Science Foundation (2015CFB260), and the Shiyan Nature Science Foundation (15K70) to Dr Zhiyou Cai.
Disclosure
The authors report no conflicts of interest in this work.
References
- MhatreSDMichelsonSJGomesJTabbLPSaundersAJMarendaDRDevelopment and characterization of an aged onset model of Alzheimer’s disease in Drosophila melanogasterExp Neurol2014261C77278125173219
- FargoKBleilerLAlzheimer’s Association reportAlzheimers Dement201410e47e9224818261
- ChoiSJJungSSYouYSPrevalence of Alzheimer’s dementia and its risk factors in community-dwelling elderly KoreansPsychiatry Investig200857885
- HsiehSHodgesJRPiguetORecognition of positive vocalizations is impaired in behavioral-variant frontotemporal dementiaJ Int Neuropsychol Soc20131948348723369869
- TeriLLogsdonRYesavageJMeasuring behavior, mood, and psychiatric symptoms in Alzheimer diseaseAlzheimer Dis Assoc Disord199711Suppl 650599437448
- HartigWKaczaJPaulkeBRIn vivo labelling of hippocampal beta-amyloid in triple-transgenic mice with a fluorescent acetylcholinesterase inhibitor released from nanoparticlesEur J Neurosci2010319910920092557
- HeeseKAkatsuHAlzheimer’s disease – an interactive perspectiveCurr Alzheimer Res2006310912116611011
- da Cruz e SilvaOAHenriquesAGDominguesSCda Cruz e SilvaEFWnt signalling is a relevant pathway contributing to amyloid beta-peptide-mediated neuropathology in Alzheimer’s diseaseCNS Neurol Disord Drug Targets2010972072620942790
- MelendezALevineBAutophagy in C. elegansPasadena (CA)Worm Book2009126
- ChaumorcelMSouquereSPierronGCodognoPEsclatineAHuman cytomegalovirus controls a new autophagy-dependent cellular antiviral defense mechanismAutophagy20084465318340111
- FunderburkSFMarcellinoBKYueZCell “self-eating” (autophagy) mechanism in Alzheimer’s diseaseMt Sinai J Med201077596820101724
- ZhouXYangCLiuYLipid rafts participate in aberrant degradative autophagic-lysosomal pathway of amyloid-beta peptide in Alzheimer’s diseaseNeural Regen Res201499210025206748
- YangYChenSZhangJStimulation of autophagy prevents amyloid-beta peptide-induced neuritic degeneration in PC12 cellsJ Alzheimers Dis20144092993924531159
- MoreiraPISiedlakSLWangXIncreased autophagic degradation of mitochondria in Alzheimer diseaseAutophagy2007361461517786024
- ArduinoDMEstevesARCardosoSMMitochondria drive autophagy pathology via microtubule disassembly: a new hypothesis for Parkinson diseaseAutophagy2013911211423075854
- YangQMaoZParkinson disease: a role for autophagy?Neuroscientist20101633534120360601
- KogaHMartinez-VicenteMAriasEKaushikSSulzerDCuervoAMConstitutive upregulation of chaperone-mediated autophagy in Huntington’s diseaseJ Neurosci201131184921850522171050
- Martinez-VicenteMTalloczyZWongECargo recognition failure is responsible for inefficient autophagy in Huntington’s diseaseNat Neurosci20101356757620383138
- WongYCHolzbaurELThe regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradationJ Neurosci2014341293130524453320
- RajawatYSHiliotiZBossisIAging: central role for autophagy and the lysosomal degradative systemAgeing Res Rev2009819921319427410
- TanCCYuJTTanMSJiangTZhuXCTanLAutophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapyNeurobiol Aging20143594195724360503
- NixonRAAlzheimer neurodegeneration, autophagy, and Abeta secretion: the ins and outs (comment on DOI:10.1002/bies.201400002)Bioessays20143654724819351
- LiLZhangSZhangXAutophagy enhancer carbamazepine alleviates memory deficits and cerebral amyloid-beta pathology in a mouse model of Alzheimer’s diseaseCurr Alzheimer Res20131043344123305067
- YuWHCuervoAMKumarAMacroautophagy – a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s diseaseJ Cell Biol2005171879816203860
- LeeJKJinHKParkMHAcid sphingomyelinase modulates the autophagic process by controlling lysosomal biogenesis in Alzheimer’s diseaseJ Exp Med20142111551157025049335
- WolfeDMLeeJHKumarALeeSOrensteinSJNixonRAAutophagy failure in Alzheimer’s disease and the role of defective lysosomal acidificationEur J Neurosci2013371949196123773064
- NixonRAAutophagy, amyloidogenesis and Alzheimer diseaseJ Cell Sci20071204081409118032783
- AllsopDYamamotoTKametaniFMiyazakiNIshiiTAlzheimer amyloid beta/A4 peptide binding sites and a possible ‘APP-secretase’ activity associated with rat brain cortical membranesBrain Res1991551191913140
- HataSFujishigeSArakiYAlcadein cleavages by amyloid beta-precursor protein (APP) alpha- and gamma-secretases generate small peptides, p3-Alcs, indicating Alzheimer disease-related gamma-secretase dysfunctionJ Biol Chem2009284360243603319864413
- DengYWangZWangRAmyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1 (BACE1), and shifting the cleavage site to Aβ Aspl contributes to Alzheimer pathogenesisEur J Neurosci2013371962196923773065
- FahrenholzFAlpha-secretase as a therapeutic targetCurr Alzheimer Res2007441241717908044
- CaiZLiBLiKZhaoBDown-regulation of amyloid-β through AMPK activation by inhibitors of GSK-3β in SH-SY5Y and SH-SY5Y-AβPP695 cellsJ Alzheimers Dis201229899822214783
- FernandezMAKlutkowskiJAFreretTWolfeMSAlzheimer presenilin-1 mutations dramatically reduce trimming of long amyloid β-peptides (Aβ) by gamma-secretase to increase 42-to-40-residue AβJ Biol Chem201428945310433105225239621
- SwomleyAMForsterSKeeneyJTAbeta, oxidative stress in Alzheimer disease: evidence based on proteomics studiesBiochim Biophys Acta201418421248125724120836
- CaiZZhaoBLiKMammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer’s disease?J Neurosci Res2012901105111822344941
- CaiZZhaoBRatkaAOxidative stress and beta-amyloid protein in Alzheimer’s diseaseNeuromolecular Med20111322325021901428
- CaiZYanLJLiKQuaziSHZhaoBRoles of AMP-activated protein kinase in Alzheimer’s diseaseNeuromolecular Med20121411422367557
- CaiZZhaoYZhaoBRoles of glycogen synthase kinase 3 in Alzheimer’s diseaseCurr Alzheimer Res2012986487922272620
- MawuenyegaKGSigurdsonWOvodVDecreased clearance of CNS beta-amyloid in Alzheimer’s diseaseScience2010330177421148344
- CaiZHussainMDYanLJMicroglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s diseaseInt J Neurosci201412430732123930978
- YanLJXiaoMChenRCaiZMetabolic dysfunction of astrocyte: an initiating factor in beta-amyloid pathology?Aging Neurodegener2013171424443714
- ShinJYParkHJKimHNMesenchymal stem cells enhance autophagy and increase beta-amyloid clearance in Alzheimer disease modelsAutophagy201410324424149893
- SteeleJWGandySLatrepirdine (Dimebon(R)), a potential Alzheimer therapeutic, regulates autophagy and neuropathology in an Alzheimer mouse modelAutophagy2013961761823380933
- NixonRAWegielJKumarAExtensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy studyJ Neuropathol Exp Neurol20056411312215751225
- JaegerPAWyss-CorayTBeclin 1 complex in autophagy and Alzheimer diseaseArch Neurol2010671181118420937944
- LingDSalvaterraPMA central role for autophagy in Alzheimer-type neurodegenerationAutophagy2009573874019395865
- TorresMJimenezSSanchez-VaroRDefective lysosomal proteolysis and axonal transport are early pathogenic events that worsen with age leading to increased APP metabolism and synaptic Abeta in transgenic APP/PS1 hippocampusMol Neurodegener201275923173743
- GhavamiSShojaeiSYeganehBAutophagy and apoptosis dysfunction in neurodegenerative disordersProg Neurobiol2014112244924211851
- KupersteinFBrandAYavinEAmyloid Abeta1-40 preconditions non-apoptotic signals in vivo and protects fetal rat brain from intrauterine ischemic stressJ Neurochem20049196597415525350
- AnsariNKhodagholiFMolecular mechanism aspect of ER stress in Alzheimer’s disease: current approaches and future strategiesCurr Drug Targets20131411412223016524
- VagnoniAGlennonEBPerkintonMSGrayEHNobleWMillerCCLoss of c-Jun N-terminal kinase-interacting protein-1 does not affect axonal transport of the amyloid precursor protein or Aβ productionHum Mol Genet2013224646465223825109
- VagnoniAPerkintonMSGrayEHFrancisPTNobleWMillerCCCalsyntenin-1 mediates axonal transport of the amyloid precursor protein and regulates Aβ productionHum Mol Genet2012212845285422434822
- VosselKAZhangKBrodbeckJTau reduction prevents Abeta-induced defects in axonal transportScience201033019820829454
- WintonMJLeeEBSunEIntraneuronal APP, not free Aβ peptides in 3xTg-AD mice: implications for tau versus Aβ-mediated Alzheimer neurodegenerationJ Neurosci2011317691769921613482
- GeorgeAJHolsingerRMMcLeanCAAPP intracellular domain is increased and soluble Abeta is reduced with diet-induced hypercholesterolemia in a transgenic mouse model of Alzheimer diseaseNeurobiol Dis20041612413215207269
- SonSMSongHByunJAccumulation of autophagosomes contributes to enhanced amyloidogenic APP processing under insulin-resistant conditionsAutophagy201281842184422931791
- MakiokaKYamazakiTKakudaSOkamotoKVariations in the effects on synthesis of amyloid beta protein in modulated autophagic conditionsNeurol Res20093195996819215663
- TungYTWangBJHuMKAutophagy: a double-edged sword in Alzheimer’s diseaseJ Biosci20123715716522357213
- TamboliIYHampelHTienNTSphingolipid storage affects autophagic metabolism of the amyloid precursor protein and promotes Abeta generationJ Neurosci2011311837184921289194
- Ulamek-KoziolMFurmaga-JablonskaWJanuszewskiSNeuronal autophagy: self-eating or self-cannibalism in Alzheimer’s diseaseNeurochem Res2013381769177323737325
- ChunYSParkYOhHGO-GlcNAcylation promotes non-amyloidogenic processing of amyloid-beta protein precursor via inhibition of endocytosis from the plasma membraneJ Alzheimers Dis201544126127525208619
- HungSYHuangWPLiouHCFuWMAutophagy protects neuron from Abeta-induced cytotoxicityAutophagy2009550251019270530
- RajadasJSunWLiHEnhanced Aβ(1–40) production in endothelial cells stimulated with fibrillar Aβ(1–42)PLoS One20138e5819423505467
- CecariniVBonfiliLCuccioloniMWild type and mutant amyloid precursor proteins influence downstream effects of proteasome and autophagy inhibitionBiochim Biophys Acta2014184212713424215712
- LiWPoteetEXieLLiuRWenYYangSHRegulation of matrix metalloproteinase 2 by oligomeric amyloid beta proteinBrain Res2011138714114821376707
- KnoblochMKonietzkoUKrebsDCNitschRMIntracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta miceNeurobiol Aging2007281297130616876915
