Abstract
Purpose
The nonmotor symptoms (NMS) of Parkinson’s disease (PD) are important factors for quality of life (QoL). Few studies on NMS have been conducted in Asian PD patients. Additionally, effects of anti-PD drugs on risk of NMS are still controversial. We therefore conducted this hospital-based cross-sectional study to examine the clinical factors, including concomitant anti-PD medication use, on the occurrence of NMS and QoL in Taiwanese PD patients.
Patients and methods
PD patients who received long-term follow-up in the movement disorders clinics were enrolled and received NMS questionnaire (NMSQuest) and the 39-item Parkinson’s Disease Questionnaire (PDQ-39). Spearman’s rank correlation coefficient was checked for the correlation between clinical factors and NMSQT/PDQSI. Multiple linear regressions were applied to assess the influence of clinical factors on NMSQT/PDQSI.
Results
A total of 210 PD patients (mean age 66.1±9.86 years, Hoehn and Yahr stage 2.2±0.9) were included in this study. Up to 98% of patients reported at least one symptom of NMS. The most prevalent symptom was urinary complaints (56%), followed by memory/apathy (30%) and depression/anxiety (28%). The correlation between NMSQT and PDQSI was strong (rs=0.667), especially the item of depression/anxiety (rs=0.607). The regression model for NMSQT indicated that disease duration and severity, but not pharmacological therapy, were major predictors of NMS.
Conclusion
Our data indicated a high prevalence rate of NMS in PD patients. Among symptoms of NMS, depression and anxiety had the greatest impact on QoL. Concomitant anti-PD medication use did not affect the occurrence of NMS and QoL.
Introduction
The nonmotor symptoms (NMS) of Parkinson’s disease (PD) are recognized as important disability-causing factors. NMS include autonomic dysfunction,Citation1 neuropsychological problems,Citation2 sleep disturbance,Citation3,Citation4 and sensory symptoms.Citation5 These symptoms constitute a burden on the caregiver and the public health system. In the PRIAMO study,Citation6 up to 98.6% of PD patients had NMS. Recently, the correlation between NMS and PD patients’ health-related quality of life (HRQoL) has been emphasized. Several studies indicate that NMS, rather than motor symptoms, are a major cause of poor HRQoL.Citation7–Citation9 The NMS screening questionnaire (NMSQuest), a self-completed questionnaire comprising 30 items, is a rapid screening tool for the early detection of patients’ NMS.Citation10,Citation11 Previous studies have compared NMS in different countries and showed more prevalent gastrointestinal symptoms in Asian countries, probably due to ethnic and economic differences.Citation12,Citation13 However, the NMSQuest is still not routinely used for Taiwanese PD patients. Demographic data regarding NMS in Taiwanese PD patients remain unknown.
The pathophysiology of NMS is suspected to involve dopamine, noradrenaline, and serotonin. A previous review indicated that current dopaminergic anti-Parkinson medication has a limited effect on NMS.Citation14 Although dopamine agonists reportedly have some antidepression effects,Citation15,Citation16 they also precipitate other NMS, such as orthostatic hypotension, hallucination, and impulse control diorder.Citation17–Citation19 Thus, whether dopamine agonists improve patients’ NMS and HRQoL remains a point of controversy.
The purpose of our study is to investigate the prevalence of NMS in PD patients in Taiwan and the impact of NMS on HRQoL since few studies on NMS have been conducted in Asian PD patients and the effects of anti-PD drugs, especially dopamine agonists, on risk of NMS are still controversial. We therefore conducted this hospital-based cross-sectional study to examine the clinical factors, including concomitant anti-PD medication use, on the occurrence of NMS and HRQoL in Taiwanese PD patients. We used the NMSQuest and 39-item Parkinson’s Disease Questionnaire (PDQ-39) to explore the correlation among NMS, clinical factors, pharmacological therapy, and HRQoL.
Materials and methods
Patients
This cohort study was conducted in the neurology department of National Taiwan University Hospital (NTUH), a tertiary referred medical center in Taipei, Taiwan. From February 2014 to December 2014, PD patients who fulfilled the United Kingdom Parkinson’s Disease Brain Bank CriteriaCitation20 and received long-term follow-up in NTUH movement disorder clinics were recruited. Patients who were diagnosed with atypical parkinsonism or secondary parkinsonism were excluded. Since the National health insurance of Taiwan pays the cost of the anti-cholinesterase inhibitor, rivastigmine, for subjects with PD with dementia, we therefore excluded subjects with concomitant use of rivastigmine to exclude participants with PD with dementia.Citation21 All patients in this study provided written informed consent. The study was approved by the Ethical Research Committee of NTUH.
Measures
Patients who fulfilled the diagnostic criteria were asked to complete the Chinese version of the NMSQuestCitation22 and PDQ-39Citation23 while they were in the waiting room of the clinic. Previous studies have validated the Chinese versions of these two tests and the reliability of each domain of these two tests in the Chinese version are between 71% and 95%, which represents an acceptable psychometric property. If necessary, aid from patients’ caregivers or a nurse was acceptable. Demographic data, including patients’ age, sex, age at onset, duration, Hoehn and Yahr (H&Y) stage, disease type, and current medication, were collected from medical records. Patients’ disease type was classified as tremor-at-onset type or bradykinesia/rigidity-at-onset type by review of their medical record, depending on their initial presentation at their first visit to our clinics.Citation24,Citation25 Individuals with resting tremor predominant as initial presentation were classified as tremor-at-onset type; others were classified as bradykinesia/rigidity-at-onset type.
The NMSQuest is a validated self-completed yes–no-type questionnaire to assess patients’ NMS. This 30-items questionnaire measure nine domains of NMS: digestive, urinary, sexual, cardiovascular, memory/apathy, hallucination/delusion, depression/anxiety, sleep, and miscellany.Citation10 Positive responses are summed up to yield a total score (NMSQT) that ranges from 0 to 30. Higher scores indicate worse NMS condition.Citation11 The prevalence of each item was calculated on the total 210 samples by computing the number of positive responses and transforming them into a percentage. Positive answers in each domain were summed to obtain the score for the domain. The standardized prevalence of each domain was calculated by the number of positive responses divided by the total number of responses in the domain and transforming this values into a percentage.
Patients’ HRQoL was assessed by the PDQ-39,Citation7 which contains 39 items. Each item was rated by the patients using one of five categories, from 0 (never) to 4 (very frequent). PDQ-39 summary index (PDQSI) was calculated by dividing the sum of the total raw score by the maximum possible score (156 or 152 points, depending on the patient’s marriage status) and multiplying by 100. In this study, we used PDQSI as a standardized index for representing PD patients’ life quality.
Statistical analysis
Spearman’s rank correlation coefficient was calculated to check the correlation between clinical factors, pharmacological therapy, NMSQT, and PDQSI. P-values of less than 0.05 were accepted as significant. The strength of the association for correlation coefficients was interpreted as follows: ≤0.19, negligible correlation; 0.20–0.39, weak correlation; 0.40–0.59, moderate correlation; 0.60–0.79, strong correlation; and ≥0.80, very strong correlation.Citation26 A stepwise multiple linear regression analysis was calculated to demonstrate which clinical factors contributed significantly to NMSQT. Age, sex, age at PD onset, duration of PD symptoms, H&Y stage, levodopa dosage, and dopamine agonist dosage (transformed into levodopa-equivalent dosage)Citation27 were involved as independent variables. Owing to collinearity between patients’ current age and their age at disease onset (r=0.92), age at PD onset was transformed into dummy variables (<50 years =0; ≥50 years =1). Stepwise multiple linear regression analysis was also applied to assess the contribution of clinical factors to the PDQSI. For the regression model of PDQSI, we involved the aforementioned factors and NMSQT as independent variables.
To compare our prevalence of each symptom with others’, we chose two international studies (one included only Europe and USA, the other included Europe, USA, Israel, and Japan) and one study from People’s Republic of China. χ2 test was used for comparison ().Citation10,Citation28,Citation29 SPSS version 22.0 was used for the statistical analyses.
Results
We collected data from 210 patients (110 males, 100 females; mean age: 66.1±9.86 years; median: 67 years; mode: 63 years; age range: 33–86 years; mean age at onset: 60±10.53 years; median: 61 years; mode: 61 years; onset age range: 26–83; disease duration 6.11±4.13 years; and H&Y stage 2.2±0.9). Demographic data are shown in . Half of our subjects were in H&Y stage 2. Most of our patients were using levodopa (97.6%) and dopamine agonists (81%).
Table 1 Demographic, clinical, and medical characteristics for our subjects
The result of NMSQuest and PDQ-39 are shown in . The mean NMSQT was 7.77±4.74, which was considered as moderate in severity according to a previous study.Citation11 The prevalence of NMS was 98.57%. Only three patients had no NMS. Among 30 NMS, nocturia was the most frequently reported NMS (prevalence =62.86%). Constipation was the second most prevalent problem among patients (prevalence =50.95%). The least frequent complaint was bowel incontinence, which happened in 2.86% of patients (). Of the nine NMS subdomains, the urinary domain had the highest prevalence (56.19%), followed by memory/apathy (30.48%) and depression/anxiety (27.86%), while hallucination/delusion had the lowest prevalence (9.76%). The mean PDQSI was 16.13±15.61.
Table 2 Descriptive statistics of the NMSQT and PDQ-39 rating scales in 210 Taiwanese patients with Parkinson’s disease
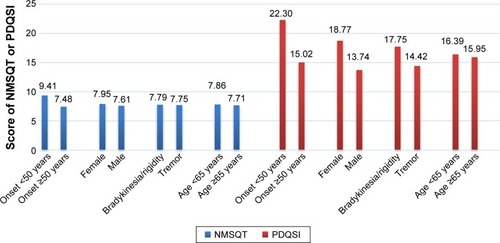
Spearman’s rank correlation coefficients indicated that the NMSQT and all subdomains correlated with the PDQSI (). NMSQT had stronger correlation with PDQSI than H&Y stage (rs of NMSQT =0.667; rs of H&Y stage =0.503). Among nine NMS subdomains, depression/anxiety correlated most strongly with PDQSI (rs=0.607), while the urinary domain exhibited the weakest correlation (rs=0.172). Age and disease types at onset did not significantly correlate with NMSQT and PDQSI, but there was significant difference between sexes in some NMS subdomains (). Females had higher scores in memory/apathy and depression/anxiety (prevalence of memory/apathy, female vs male: 57% vs 47%, average score: 1.1 vs 0.75; prevalence of depression/anxiety, female vs male: 42% vs 29%, average score: 0.69 vs 0.44). Males had higher a score in sexual symptoms (prevalence of sexual dysfunction, female vs male: 26% vs 41%, average score: 0.34 vs 0.6). The PDQSI, NMSQT, and almost all NMS subdomains were correlated positively with disease duration, H&Y stage, and levodopa dosage. However, dopamine agonist dosage did not correlate significantly with the PDQSI, NMSQT, or almost all subdomains.
Table 3 Correlations of scores of NMSQuest, Hoehn and Yahr stage, and PDQSI
The regression model for the NMSQT indicated that duration of disease and H&Y stage were independent variables (). The regression model for the PDQSI revealed that independent variables included NMSQT, H&Y stage, age at onset, and sex ().
Table 4 Multiple linear regression analysis for NMSQT and PDQSI
Discussion
This was the first study, to our knowledge, to use NMSQuest to evaluate NMS in Taiwanese PD patients. In our study of 210 PD patients, we observed a high prevalence rate of NMS (98.57%). Among nine subdomains, the most prevalent domains were urinary complaints, followed by memory/apathy and depression/anxiety. NMSQT and all subdomains were correlated with the PDQSI. Among those subdomains, depression/anxiety had the strongest correlation coefficient with the HRQoL of PD patients. The regression model indicated that disease duration and severity were major predictors of NMS. Concomitant use of anti-PD medication, including dopamine agonist, was not correlated with the occurrence of NMS or HRQoL of patients with PD.
The general prevalence of NMS in our study was close to that of previous studies in other countries ().Citation10,Citation13,Citation28–Citation38 This indicates that NMS are very frequent and universal concerns in PD patients.Citation6 For each item in NMSQuest, we compared our study with the studies of Chaudhuri et al,Citation10 Martinez-Martin et al,Citation28 and Gan et al.Citation29 The results showed that there were no significant differences in most NMS rates between studies (). The three most common symptoms in our study were nocturia (62.85%), constipation (50.95%), and urgency (49.52%), and the least common symptom was bowel incontinence (2.86%). This result was the same as ChaudhuriCitation10 and Martinez-Martin’s studiesCitation28 in Europe and USA. In contrast, studies in the People’s Republic of ChinaCitation29–Citation32 revealed that the most common symptom was easily forgetting (prevalence: 56.1%–68.56%) (). This suggests that factors other than race, such as age, disease duration, treatment strategy, education, and economical status, may be more influential on NMS. Further study for comparison among groups is needed. Previous studies have suggested that Asian PD patients may have higher rates of constipation or gastrointestinal problems.Citation12,Citation13 However, our study and one recent large-scale study from the People’s Republic of ChinaCitation30 showed that constipation rates were not higher than Western groups (). Ethnic effect on NMS is still controversial.
Although urinary symptoms were the most common in patients, the correlation between urinary symptoms and HRQoL was lowest (rs=0.176). This may be due to the fact that urinary symptoms rarely induce motor disturbance and psychosocial problems, which are mainly evaluated in PDQ-39. Measurement bias should be carefully considered when evaluating the impact of urinary symptoms on patients’ life quality. Neuropsychological symptoms exerted the greatest influence over our patients’ quality of life; among those, depression/apathy correlated most strongly with PDQSI (rs=0.607). This finding is consistent with previous studies indicating that depression has the greatest impact on PD patients.Citation8,Citation12,Citation26,Citation28,Citation39 In our study, 30% of patients self-reported having depressed mood, which was compatible with the newest meta-analysis on the prevalence of depression among individuals with a PD diagnosis (36.6%).Citation40 Depression is two- to threefold more prevalent among PD patients compared to healthy people, and can precede the diagnosis of PD by 4–6 years.Citation41 This evidence suggests an underlying neurodegenerative process for depression in PD patients. Braak et al’s pathology study demonstrated that the degeneration of PD is not limited to the substantia nigra; it also affects the locus coeruleus and raphe nucleus before the beginning of PD motor symptoms.Citation42 This finding indicates that noradrenaline and serotonin were also involved in the pathology of depression in PD. Positron emission tomography has provided evidence of decreasing dopamine and noradrenaline transporter levels in the locus coeruleus and several regions of limbic system.Citation43 18F-fluorodeoxyglucose positron emission tomography has shown decreasing availability of the serotonergic 1A receptor in the limbic and orbitofrontal regions.Citation44 Several studies that employed resting-state functional magnetic resonance imaging have revealed reduced functional connectivity in the prefrontal–limbic network in PD patients with depression.Citation45–Citation47
Pramipexole was found to improve depression in 12 weeks in 323 depressive PD patients.Citation15 In that study, the Beck Depression Inventory score decreased by 5.9 points in the pramipexole group, compared with 4.0 points in the placebo group. In another study that involved only 44 patients, the Hamilton Anxiety Scale and the Montgomery–Asberg Depression Rating Scale also dropped in PD patients using ropinirole.Citation48 However, in the present study, we did not find a significant correlation between calculated dopamine agonist dosage and NMSQT (including all subdomains) (). In addition, we compared NMSQT, score of depression/anxiety subdomain, and PDQSI between the patients with and without using dopamine agonists. None of those showed significant difference (Mann–Whitney test; NMSQT: P=0.766; depression/anxiety: P=0.111; PDQSI: P=0.406). The negative results indicate that dopamine agonists did not eliminate depression and might not improve patients’ life quality. However, only a yes/no question can be answered for each symptom in NMSQuest. Dopamine agonists cannot completely eliminate depressive symptoms but they may partially relieve the depression symptoms. The Non-Motor Symptoms Scale,Citation49 another assessment tool which is able to evaluate the severity of each symptom, should be applied for further evaluation of dopamine agonists’ antidepression effects.
Our regression model demonstrates that only disease severity and duration are independent variables for NMSQT. In contrast to Martinez-Martin et al’s study,Citation28 our regression model does not reveal age of onset as an independent predictor. In our correlation study, patients with young-onset PD (YOPD) surprisingly had higher NMSQT and PDQSI (ie, worse HRQoL), possibly because our YOPD group featured longer disease duration (YOPD vs late-onset PD [LOPD]: 7.9 years vs 5.8 years). The other regression model for PDQSI indicates that NMSQT, H&Y stage, age at onset, and sex are independent values, in which NMSQ plays the most important role. This result is quite similar to previous studies’ findings that NMS are the major predictor for patients’ quality of life, suggesting that NMS cause more disability than motor symptoms.Citation7–Citation9 In our regression model for PDQSI, sex and age at onset were also independent values, although they have minor roles. Looking at our data (), YOPD and female patients had similar NMSQT (YOPD vs LOPD: 9.41 vs 7.48, P=0.238; female vs male: 7.95 vs 7.61, P=0.784). However, they had slightly higher PDQSI (YOPD vs LOPD: 22.3 vs 15.02, P=0.008; female vs male: 18.77 vs 13.74, P=0.202). This may indicate that, although they had similar number of NMS, they still subjectively felt that they had a poor life quality. In Dubayova et al’s study,Citation50 females also had slightly higher PDQ-39 scores although there was no significant difference. In female patients, age and neuroticism were more significantly correlated with PDQ-39 score than they were in males. In Nutt et al’s study,Citation51 multiple linear regression showed age and sex were independent variables contributing to PDQSI.
Figure 1 Comparison of NMSQT and PDQSI between different groups of onset age, sex, disease at onset, and current age.
There are several limitations in the current study. Although our sample number is relatively small, it is comparable to other studies (). In addition, another limitation of our study is that few YOPD patients were involved (n=32), which may partly explain why age at onset did not play an important role in our study. Although YOPD predicts higher NMSQT in Martinez-Martin et al’s study,Citation28 another study indicated that patients with LOPD have more gastrointestinal and urinary symptoms, dementia, and psychosis, which influence patients’ quality of life more.Citation52 To better understand the differences in NMS between YOPD and LOPD patients, more YOPD patients should be included in future studies.
Conclusion
Our findings indicate that NMS are common in PD patients and determine the life quality of PD patients. NMS severity is positively correlated with disease severity and duration. Among NMS subdomains, depression and other neuropsychological symptoms most influence patients’ quality of life. However, concomitant anti-PD medication use did not affect the occurrence of NMS and quality of life. Further study for treatment of NMS is mandatory to improve the life quality of patients.
Acknowledgments
The authors thank all of those who participated in this study. This study was supported by Grant NTUH 101-S1889 and 103-S2485 from National Taiwan University Hospital, Taiwan.
Supplementary materials
Table S1 Comparison of the prevalence of each nonmotor symptom with other studies
Table S2 Correlations of clinical factors with NMSQT, subdomains, and PDQSI
Table S3 NMS prevalence and rate of constipation and memory symptoms in other studies
References
- Martinez-MartinPSchapiraAHStocchiFPrevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patientsMov Disord200722111623162917546669
- ChaudhuriKRMartinez-MartinPSchapiraAHInternational multicenter pilot study of the first comprehensive self-completed non-motor symptoms questionnaire for Parkinson’s disease: the NMSQuest studyMov Disord200621791692316547944
- GanJZhouMChenWLiuZNon-motor symptoms in Chinese Parkinson’s disease patientsJ Clin Neurosci201421575175424411328
- WuYGuoXYWeiQQNon-motor symptoms and quality of life in tremor dominant vs postural instability gait disorder Parkinson’s disease patientsActa Neurol Scand Epub2015720
- ZhangNLiuWYeMCohenADZhangYThe heterogeneity of non-motor symptoms of Parkinson’s diseaseNeurol Sci201536457758425376559
- LiDWGuZWangCNon-motor symptoms in Chinese Parkinson’s disease patients with and without LRRK2 G2385R and R1628P variantsJ Neural Transm2015122566166725062988
- CheonSMHaMSParkMJKimJWNonmotor symptoms of Parkinson’s disease: prevalence and awareness of patients and familiesParkinsonism Relat Disord200814428629018042421
- TsuboiYYamadaTChaudhuriRKComparison profile of non motor symptoms in Japanese patients with PD with European patients and healthy controls. Extension of the NMSQuest studyMov Disord200621suppl15S648
- Rukmini MridulaKBorgohainRJabeenSAComparison of frequencies of non motor symptoms in Indian Parkinson’s disease patients on medical management versus deep brain stimulation: A case-control studyIran J Neurol2015142869326056553
- CosentinoCNuñezYTorresLFrequency of non-motor symptoms in Peruvian patients with Parkinson’s diseaseArq Neuropsiquiatr201371421621923588282
- KhedrEMEl FetohNAKhalifaHAhmedMAEl BehKMPrevalence of non motor features in a cohort of Parkinson’s disease patientsClin Neurol Neurosurg2013115667367722902078
- BostantjopoulouSKatsarouZKarakasisCPeitsidouEMilioniDRossopoulosNEvaluation of non-motor symptoms in Parkinson’s Disease: An underestimated necessityHippokratia201317321421924470730
- CrosiersDPickutBTheunsJNon-motor symptoms in a Flanders-Belgian population of 215 Parkinson’s disease patients as assessed by the Non-Motor Symptoms QuestionnaireAm J Neurodegener Dis20121216016723383390
Disclosure
The authors report no financial disclosures or any conflicts of interest in this work.
References
- GoldsteinDSDysautonomia in Parkinson diseaseCompr Physiol20144280582624715569
- de la RivaPSmithKXieSXWeintraubDCourse of psychiatric symptoms and global cognition in early Parkinson diseaseNeurology201483121096110325128183
- JahanIHauserRASullivanKLMillerAZesiewiczTASleep disorders in Parkinson’s diseaseNeuropsychiatr Dis Treat2009553554019898667
- YuRLTanCHWuRMThe impact of nocturnal disturbances on daily quality of life in patients with Parkinson’s diseaseNeuropsychiatr Dis Treat2015112005201226273203
- SkogarOFallPAHallgrenGParkinson’s disease patients’ subjective descriptions of characteristics of chronic pain, sleeping patterns and health-related quality of lifeNeuropsychiatr Dis Treat2012843544223091387
- BaronePAntoniniAColosimoCThe PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s diseaseMov Disord200924111641164919514014
- Martinez-MartinPRodriguez-BlazquezCKurtisMMChaudhuriKRNMSS Validation GroupThe impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s diseaseMov Disord201126339940621264941
- ValkovicPHarsanyJHanakovaMMartinkovaJBenetinJNonmotor symptoms in early- and advanced-stage Parkinson’s disease patients on dopaminergic therapy: how do they correlate with quality of life?ISRN Neurol2014201458730224729891
- Rodríguez-ViolanteMCervantes-ArriagaACoronaTMartínez-RamírezDMorales-BriceñoHMartínez-MartínPClinical determinants of health-related quality of life in Mexican patients with Parkinson’s diseaseArch Med Res201344211011423376054
- ChaudhuriKRMartinez-MartinPSchapiraAHInternational multicenter pilot study of the first comprehensive self-completed non-motor symptoms questionnaire for Parkinson’s disease: the NMSQuest studyMov Disord200621791692316547944
- ChaudhuriKRSauerbierARojoJMThe burden of non-motor symptoms in Parkinson’s disease using a self-completed non-motor questionnaire: a simple grading systemParkinsonism Relat Disord201521328729125616694
- AzminSKhairul AnuarAMTanHJNonmotor symptoms in a Malaysian Parkinson’s disease populationParkinsons Dis2014201447215724800102
- TsuboiYYamadaTChaudhuriRKComparison profile of non motor symptoms in Japanese patients with PD with European patients and healthy controls. Extension of the NMSQuest studyMov Disord200621suppl15S648
- ChaudhuriKRHealyDGSchapiraAHNational Institute for Clinical ExcellenceNon-motor symptoms of Parkinson’s disease: diagnosis and managementLancet Neurol20065323524516488379
- BaronePPoeweWAlbrechtSPramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trialLancet Neurol20109657358020452823
- Ray ChaudhuriKMartinez-MartinPAntoniniARotigotine and specific non-motor symptoms of Parkinson’s disease: post hoc analysis of RECOVERParkinsonism Relat Disord201319766066523557594
- ChaudhuriKRAutonomic dysfunction in movement disordersCurr Opin Neurol200114450551111470968
- PolettiMPerugiGLogiCDopamine agonists and delusional jealousy in Parkinson’s disease: a cross-sectional prevalence studyMov Disord201227131679168223150469
- ShapiroMAChangYLMunsonSKThe four As associated with pathological Parkinson disease gamblers: anxiety, anger, age, and agonistsNeuropsychiatr Dis Treat20073116116719300546
- HughesAJDanielSEKilfordLLeesAJAccuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 casesJ Neurol Neurosurg Psychiatry19925531811841564476
- KuLJPaiMCUse of cognitive enhancers and associated medical care costs among patients with dementia: a nationwide study in TaiwanInt Psychogeriatr201426579580424429098
- LiHJZhangMFChenMXValidation of the nonmotor symptoms questionnaire for Parkinson’s disease: results from a Chinese pilot studyInt J Neurosci Epub20141218
- MaHIHwangWJChen-SeaMJReliability and validity testing of a Chinese-translated version of the 39-item Parkinson’s Disease Questionnaire (PDQ-39)Qual Life Res200514256556915892447
- HersheyLAFeldmanBJKimKYCommichauCLichterDGTremor at onset. Predictor of cognitive and motor outcome in Parkinson’s disease?Arch Neurol19914810104910511929897
- DeweyRBJrTanejaAMcClintockSMMotor symptoms at onset of Parkinson disease and risk for cognitive impairment and depressionCogn Behav Neurol201225311512022960435
- LiHZhangMChenLNonmotor symptoms are independently associated with impaired health-related quality of life in Chinese patients with Parkinson’s diseaseMov Disord201025162740274620945434
- TomlinsonCLStoweRPatelSRickCGrayRClarkeCESystematic review of levodopa dose equivalency reporting in Parkinson’s diseaseMov Disord201025152649265321069833
- Martinez-MartinPSchapiraAHStocchiFPrevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patientsMov Disord200722111623162917546669
- GanJZhouMChenWLiuZNon-motor symptoms in Chinese Parkinson’s disease patientsJ Clin Neurosci201421575175424411328
- WuYGuoXYWeiQQNon-motor symptoms and quality of life in tremor dominant vs postural instability gait disorder Parkinson’s disease patientsActa Neurol Scand Epub2015720
- ZhangNLiuWYeMCohenADZhangYThe heterogeneity of non-motor symptoms of Parkinson’s diseaseNeurol Sci201536457758425376559
- LiDWGuZWangCNon-motor symptoms in Chinese Parkinson’s disease patients with and without LRRK2 G2385R and R1628P variantsJ Neural Transm2015122566166725062988
- CheonSMHaMSParkMJKimJWNonmotor symptoms of Parkinson’s disease: prevalence and awareness of patients and familiesParkinsonism Relat Disord200814428629018042421
- Rukmini MridulaKBorgohainRJabeenSAComparison of frequencies of non motor symptoms in Indian Parkinson’s disease patients on medical management versus deep brain stimulation: A case-control studyIran J Neurol2015142869326056553
- CosentinoCNuñezYTorresLFrequency of non-motor symptoms in Peruvian patients with Parkinson’s diseaseArq Neuropsiquiatr201371421621923588282
- KhedrEMEl FetohNAKhalifaHAhmedMAEl BehKMPrevalence of non motor features in a cohort of Parkinson’s disease patientsClin Neurol Neurosurg2013115667367722902078
- BostantjopoulouSKatsarouZKarakasisCPeitsidouEMilioniDRossopoulosNEvaluation of non-motor symptoms in Parkinson’s Disease: An underestimated necessityHippokratia201317321421924470730
- CrosiersDPickutBTheunsJNon-motor symptoms in a Flanders-Belgian population of 215 Parkinson’s disease patients as assessed by the Non-Motor Symptoms QuestionnaireAm J Neurodegener Dis20121216016723383390
- SlawekJDerejkoMLassPFactors affecting the quality of life of patients with idiopathic Parkinson’s disease – a cross-sectional study in an outpatient clinic attendeesParkinsonism Relat Disord200511746546816154794
- ChenHZhaoEJZhangWMeta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosisTransl Neurodegener201541125671103
- IshiharaLBrayneCA systematic review of depression and mental illness preceding Parkinson’s diseaseActa Neurol Scand2006113421122016542159
- BraakHDel TrediciKRübUde VosRAJansen SteurENBraakEStaging of brain pathology related to sporadic Parkinson’s diseaseNeurobiol Aging200324219721112498954
- RemyPDoderMLeesATurjanskiNBrooksDDepression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic systemBrain2005128Pt 61314132215716302
- BallangerBKlingerHEcheJRole of serotonergic 1A receptor dysfunction in depression associated with Parkinson’s diseaseMov Disord2012271848921994070
- IwabuchiSJKrishnadasRLiCAuerDPRaduaJPalaniyappanLLocalized connectivity in depression: a meta-analysis of resting state functional imaging studiesNeurosci Biobehav Rev201551778625597656
- LuoCChenQSongWResting-state fMRI study on drug-naive patients with Parkinson’s disease and with depressionJ Neurol Neurosurg Psychiatry201485667568324227759
- ShengKFangWSuMAltered spontaneous brain activity in patients with Parkinson’s disease accompanied by depressive symptoms, as revealed by regional homogeneity and functional connectivity in the prefrontal-limbic systemPLoS One201491e8470524404185
- RektorovaIBalazMSvatovaJEffects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter studyClin Neuropharmacol200831526126618836343
- ChaudhuriKRMartinez-MartinPBrownRGThe metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot studyMov Disord200722131901191117674410
- DubayovaTNagyovaIHavlikovaENeuroticism and extraversion in association with quality of life in patients with Parkinson’s diseaseQual Life Res2009181334218989757
- NuttJGSiderowfADGuttmanMMobility, mood and site of care impact health related quality of life in Parkinson’s diseaseParkinsonism Relat Disord201420327427924182524
- SpicaVPekmezovićTSvetelMKostićVSPrevalence of non-motor symptoms in young-onset versus late-onset Parkinson’s diseaseJ Neurol2013260113113722820720
