Abstract
Objective
The aim of this study is to explore the resting-state functional connectivity (FC) differences between the paired default mode network (DMN) subregions in patients with primary insomnia (PIs).
Methods
Forty-two PIs and forty-two age- and sex-matched good sleepers (GSs) were recruited. All subjects underwent the resting-state functional magnetic resonance imaging scans. The seed-based region-to-region FC method was used to evaluate the abnormal connectivity within the DMN subregions between the PIs and the GSs. Pearson correlation analysis was used to investigate the relationships between the abnormal FC strength within the paired DMN subregions and the clinical features in PIs.
Results
Compared with the GSs, the PIs showed higher Pittsburgh Sleep Quality Index score, Hamilton Anxiety Rating Scale score, Hamilton Depression Rating Scale score, Self-Rating Depression Scale score, Self Rating Anxiety Scale score, Self-Rating Scale of Sleep score, and Profile of Mood States score (P<0.001). Compared with the GSs, the PIs showed significant decreased region-to-region FC between the medial prefrontal cortex and the right medial temporal lobe (t=−2.275, P=0.026), and between the left medial temporal lobe and the left inferior parietal cortices (t=−3.32, P=0.001). The abnormal FC strengths between the DMN subregions did not correlate with the clinical features.
Conclusion
PIs showed disrupted FC within the DMN subregions.
Introduction
Primary insomnia (PI), one of the most prevalent health complaints worldwide, characterized by difficulties in initiating or maintaining sleep, or non-restorative sleep in the absence of any related medical or psychiatric condition, has been associated with cognitive deficits, including the attention, memory, decision making, and executive function.Citation1,Citation2 In spite of a recent increase in the neuroimaging research into the PI, it has yet to establish a consistent conclusion about its neuropathology,Citation3 especially the structural studies of the brain volume alterations.Citation4–Citation10 On the functional imaging aspects, the studies are too few and diverse in methodology to yield any general conclusions. Altena et al concluded that patients with PI (PIs) are cognitively compromised, as shown by hypoactivation in the left prefrontal cortex and left inferior frontal gyrus during task performance.Citation11 Drummond et alCitation12 further investigated the cognitive impairments in the PIs, they found less activation in the thalamus, fronto-parietal cortex, and cerebellum, and these brain regions normally associated with the working memory and the motor and visual processing during the cognitive tasks compared with the good sleepers (GSs).Citation12 Previous study found that the aberrant activation of the insula, which integrates temporal and bodily states, in arousal networks may underlie the misperception of sleep quality and subjective distress in insomnia.Citation13 Our previous study found that both female and male PIs showed different regional homogeneity in the temporal cortex, cingulate cortex, cerebellum, and frontal gyrus.Citation2 Although these studies provided insights into the neural events occurring in the PIs, there was far less evidence for the neuromechanism changes.
It is proposed that the resting-state functional magnetic resonance imaging (rs-fMRI), one of the hot areas in neuroimaging and one that is suitable for the mechanism research of central nervous system, can detect the spontaneous neuronal activity of the human brain and provide new insights into the pathophysiology of disease, because of its advantages in not requiring exposure to radioactive tracers, accurate positioning, and ease of combining functional imaging with structural imaging. Functional connectivity (FC) that is an important part of the rs-fMRI has been widely used as a technique for unbiased analysis of the brain’s functional connectome.Citation14,Citation15 Previous studies demonstrated that the PIs relative to the GSs showed reduced FC between the left orbitofrontal cortex and the left caudate head,Citation16 and between the parietal lobe and the frontal lobe.Citation17 Huang et al found decreased FC mainly between the amygdala and the insula, striatum, and thalamus, and increased FC mainly between the amygdala and the premotor cortex and sensorimotor cortex in the PIs compared with the GSs.Citation18
The “default-mode network” (DMN) is an organized functional network of several brain regions. Anatomically, the DMN spans the bilateral inferior parietal cortices (IPC), posterior cingulate cortex, precuneus, medial prefrontal cortex (MPFC), retrosplenial cortex, and parts of the hippocampal formation and medial temporal lobe (MTL).Citation19,Citation20 Recent researches have suggested that the DMN might be associated with the collection and evaluation of information,Citation21 self-referential mental activity,Citation22 extraction of episodic memory,Citation23 emotion and anxiety,Citation24,Citation25 mind wandering or daydreaming,Citation26 and autobiographical memory retrieval and envisioning the future when individuals are not focusing on the external environment.Citation27–Citation29 More and more researches have been devoted to exploring the DMN changes in various kinds of diseases, including the sleep deprivation,Citation30,Citation31 cognitive impairment,Citation32 and autism.Citation33 However, far less is still known about the effect of PIs on the intrinsic DMN node connectivity. In this study, we examined the FC changes of the paired DMN subregions in the PIs using the seed-based region-to-region FC method to explore its possible neural mechanisms.
Materials and methods
Forty-two PIs (15 males and 27 females; mean age: 49.24±12.26) who had sleep onset and/or maintenance insomnia were recruited from the Psychiatry Department of our Hospital, and forty-two GSs (18 males and 24 females; mean age: 49.14±10.20) who were age, sex, and education status-matched to the PIs were recruited from the community via a newspaper advertisement. Twenty-three PIs (seven males and 16 females) were not the first-time visitors and had taken hypnotic medications or psychoactive medications before, the other nineteen PIs (eight males and eleven females) were first-time visitors and had never taken medications before. The medication history duration was from 1 month to 5 years. Before the tests, the PIs were asked to stop taking any medications for at least 2 weeks before the data collection and for the duration of the study; however, three PIs only stopped taking agents 2–4 days before the test.
The PIs met the following criteria as in our previous study where the regional homogeneity results of a group of 24 PIs from the 42 PIs in our study were reported:Citation2 conformity to the pertinent diagnostic criteria as defined by the International Classification of Sleep Disorder-2,Citation34 higher duration of insomnia than 2 months, higher score of Pittsburgh Sleep Quality Index (PSQI) than 5, a sleep diary for at least 2 weeks’ duration, and right-handedness. All GSs met the following criteria: a good sleeping habit and good sleep onset and/or maintenance, a regular dietary habit, no consumption of any stimulants, medications, tea or coffee for at least 3 months before the study, lower score of PSQI than five, and lower score of Hamilton Depression Rating Scale (HAMD) and Hamilton Anxiety Rating Scale (HAMA) scores <7.
The exclusion criteria for both groups comprised pathological brain MRI findings, inborn or other acquired diseases, any foreign implants in the body, present, or past psychiatric or central nervous system disorders, substance dependency or substance abuse (including heroin, nicotine, or alcohol addiction for GSs), foreign implants in the body, any history of swing shift, shift work, sleep complaints or other sleep disorder, including the hypersomnia, parasomnia, sleep-related breathing disorder, sleep-related movement disorder, or circadian rhythm sleep disorder.
Research design and procedures
An experienced psychiatrist evaluated the PIs with the Diagnostic and Statistical Manual of Mental Disorders, version 4 (DSM-IV)Citation35 for the life history of psychiatric disorders, as well as an unstructured clinical interview for the history of medical and sleep disorders. To evaluate the sleep status, the PIs were asked to wear a Fitbit Flex tracker (http://help.fitbit.com) for two consecutive nights and the GSs for 1 week. During the time, the total sleep time, sleep onset latency, sleep efficiency, and number of awakenings were recorded.
The volunteers were instructed to wear black blinders and sponge earplugs, and fix the head, to avoid audiovisual stimulus during the rs-fMRI scans. They were told to relax and not to think of anything, and not to fall asleep in particular. A simple questionnaire was administered immediately after the scans to determine whether the subjects were awake during the session. The data of the subjects who were asleep during the scans were excluded. This study was approved by The Human Research Ethics Committee of our Hospital. All volunteers participated voluntarily and were informed of the purposes, methods, and the potential risks, and all signed an informed consent form.
Questionnaires
All volunteers were asked to complete a number of questionnaires, including the PSQI,Citation36 Insomnia Severity Index,Citation37 HAMD,Citation38 HAMA,Citation39 Self-Rating Depression Scale,Citation40 Self Rating Anxiety Scale,Citation41 Self-Rating Scale of Sleep, and Profile of Mood States.Citation42,Citation43
MRI parameters
MRI scanning was performed on a 3-Tesla MR scanner (Trio, Siemens, Erlangen, Germany). High-resolution T1-weighted images were acquired with a three-dimensional spoiled gradient-recalled sequence in an sagittal orientation: 176 images (repetition time =1,900 ms, echo time =2.26 ms, thickness =1.0 mm, gap =0.5 mm, acquisition matrix =256×256, field of view =250 mm ×250 mm, flip angle =9°) were obtained. Finally, an 8-minute rs-fMRI scan was obtained with eyes closed. A total of 240 functional images (repetition time =2,000 ms, echo time =30 ms, thickness =4.0 mm, gap =1.2 mm, acquisition matrix =64×64, flip angle =90°, field of view =220 mm ×220 mm, 29 axial slices with Gradient-Recalled Echo-Planar Imaging pulse sequence) covering the whole brain were obtained.
Data preprocessing
Functional data were checked by MRIcro software (www.MRIcro.com) to exclude the defective data. The first ten time points of the functional images were discarded due to the possible instability of the initial MRI signal and the participants’ adaptation to the scanning environment. On the basis of MATLAB2010a (Mathworks, Natick, MA, USA), the rest of the data pre-processing was performed by the DPARSFA (http://rfmri.org/DPARSF) software, including digital imaging and communications in medicine form transformation, slice timing, head-motion correction, spatial normalization, and smooth with a Gaussian kernel of 6×6×6 mm3 full-width at half-maximum. The participants who had more than 1.5 mm maximum translation in x, y, or z and 1.5° of motion rotation were rejected. The Friston six head-motion parameters were used to regress out head-motion effects based on recent work, showing that higher-order models were more effective in removing head-motion effects.Citation44,Citation45 Linear regression was also applied to remove other sources of spurious covariates along with their temporal derivatives, including the signal from a ventricular regions of interest, and the signal from a region centered in white matter.Citation27 Of note, the global signal was not regressed out in the present data, as in our previous studies,Citation2,Citation46,Citation47 for the reason that there is still a controversy concerning removing the global signal in the preprocessing of resting-state data.Citation27,Citation48 After the head-motion correction, the align fMRI images were spatially normalized to the Montreal Neurological Institute space and re-sampled at a resolution of 3 mm ×3 mm ×3 mm. After the pre-processing, the time series for each voxel was linearly detrended and filtered (bandpass 0.01–0.08 Hz) to reduce the low-frequency drift, high-frequency physiological respiratory and cardiac noise.Citation49
Definition of DMN seed regions
According to the previous studies,Citation50–Citation52 we defined the eight canonical core regions within the DMN: posterior cingulate cortex, MPFC, bilateral hippocampal formation, bilateral MTL, and bilateral IPC (, ). The average time courses of these eight regions were defined by placing spherical seeds (r=6 mm) and then extracted from each subject.
Figure 1 Regions of interest within the DMN.
Abbreviations: PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex; HF, hippocampus formation; IPC, inferior parietal cortices; MTL, medial temporal lobe; DMN, default mode network; MNI, Montreal Neurological Institute.
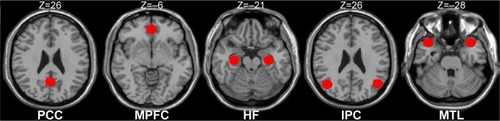
Table 1 The coordinate of the definition of the DMN subregions
FC analysis
For each subject, a correlation map was produced by computing the correlation coefficient (r score) between each pair of the DMN regions, totally yielding the 28 paired connectivity. Correlation coefficients were then converted to z-values using Fisher’s r-to-z transform to standardize the statistic analysis because the correlation coefficient r is not normally distributed.
Correlation analysis
To investigate the relationships between the clinical measures and the FC strength of the region-to-region within the DMN in PIs, the z-values of the temporal correlation coefficients of the different paired connectivity between the PIs and the GSs were correlated with the clinical questionnaires using the Pearson correlation analysis. The threshold was set at a significance level of P<0.05.
Statistical analysis
For the clinical measures, two-sample Student’s t-test (homoscedasticity) and Mann–Whitney U-test (heteroscedasticity) were used for the continuous data. All the results are quoted as two-tailed P-values. P<0.05 was considered as statistically significant. All the statistical analyses were performed using IBM SPSS version 21.0 statistical software.
Within each group, a random-effect one-sample t-test was performed on the individual z-value maps of the 28 pairs of DMN subregions. A corrected significance level of P<0.05, using an false-positive adjustment, was performed for the multiple comparison corrections.Citation53,Citation54 The two-sample t-test was performed on the individual z-value maps of the 28 pairs of DMN subregions to determine the FC differences between the PIs and the GSs with the age, sex, and education as covariates. A corrected significance level of individual voxel P<0.05, using the false-positive adjustment, was used to determine the statistical significance.
Results
Demographic and clinical questionnaires
Demographic and clinical questionnaires of each group are summarized in . No significant differences were found in age (t=−0.996; P=0.322), sex (χ2=0.645; P=0.422), and education (t=−0.408; P=0.684) between the PIs and the GSs (P>0.05). Compared with the GSs, the PIs showed higher PSQI score (P<0.001), shorter total sleep time (P<0.001) and lower sleep efficiency (P<0.001), had worse subjective sleep estimate as measured by the Self-Rating Scale of Sleep (P<0.001), and demonstrated disturbed mood state as measured by the HAMA (P<0.001), HAMD (P<0.001), Self Rating Anxiety Scale (P<0.001), Self-Rating Depression Scale (P<0.001), and Profile of Mood States (P<0.001). The Fitbit Flex tracker found that the no significant difference in the total sleep time (P=0.862) between the PIs and the GSs, but the PIs showed lower sleep efficiency (P<0.001), longer sleep onset latency (P<0.001), and more number of awakenings (P<0.001) compared with the GSs.
Table 2 Demographics and characteristics of the PIs and GSs
FC results
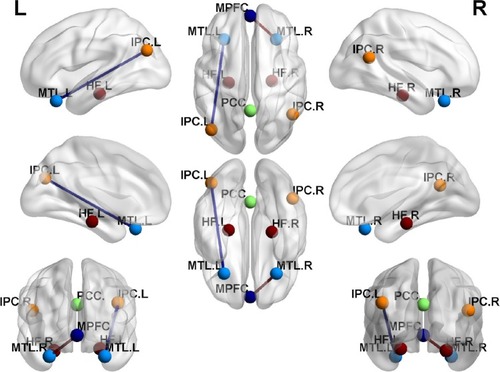
shows the FC results of the 28 pairs of DMN subregions in the PIs and the GSs, respectively. In each group, all these DMN regions were strongly connected to each other. Compared with the GSs, the PIs demonstrated two significant decreased FC regions, that is, between the MPFC and the right MTL (MTL.R) (t=−2.275, P=0.026), and between the left MTL (MTL.L) and the left IPC (IPC.L) (t=−3.32, P=0.001) (). No other region-to-region connectivities were found.
Figure 2 Correlation matrix of average time series of pair-wise subregions in DMN.
Abbreviations: PIs, patients with primary insomnia; GSs, good sleepers; DMN, default mode network; FC, functional connectivity; PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex; HF, hippocampus formation; IPC, inferior parietal cortices; MTL, medial temporal lobe; R, right; L, left.
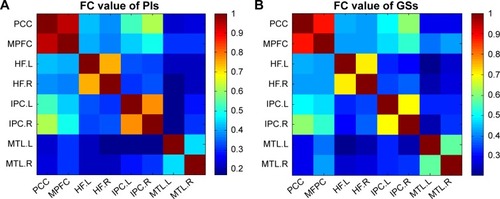
Figure 3 FC differences within the DMN between the PIs and the GSs.
Abbreviations: FC, functional connectivity; DMN, default mode network; PIs, patients with primary insomnia; GSs, good sleepers; PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex; HF, hippocampus formation; IPC, inferior parietal cortices; MTL, medial temporal lobe; R, right; L, left.
No correlation results
There were no significant correlations between the region-to-region FC strength within the DMN and the clinical questionnaires (P>0.05).
Discussion
To the best of our knowledge, this study was the first to use the region-to-region FC method to investigate the FC differences of the DMN subregions, so as to better understand the underlying neural mechanisms of the PIs. Fortunately, in the present study we found that the PIs showed decreased FC between the MPFC and the right MTL, and between the left MTL and the left IPC compared with the GSs. Although no significant correlations were found between the abnormal paired FC subregions and the clinical measurements, the PIs showed more negative emotions compared with the GSs.
Recent researches indicate that the prefrontal cortex is particularly sensitive to the sleep, and has a major role in mediating sleep physiology.Citation55–Citation57 Several neuropsychological studies have revealed that the PIs had impaired performance in tests involving the prefrontal cortex.Citation58,Citation59 The MPFC, as a core region of the DMN, associated with the social cognitive processes, is related to decision making and self-regulations.Citation60,Citation61 Koenigs et al found that the focal injuries to the left DMPFC are associated with the sleep initiation and maintenance.Citation62 Joo et al found that the PIs showed significant decreased gray matter volume in the MPFC compared with the GSs.Citation10 Our previous study found that male PIs showed lower regional homogeneity in the MPFC compared with male GSs.Citation2 These studies demonstrated that the PIs had a disturbed function and structure in the MPFC.
The MTL, which consists of several critical memory-related structures, including the hippocampus, amygdala, cingulate gyrus, and the surrounding hippocampal areas (such as the entorhinal, perirhinal, and parahippocampal cortices),Citation63 was associated with a variety of sensory information integration. The MTL is considered as a sensitive predictor for conversion to Alzheimer’s disease.Citation64–Citation66 Nofzinger et al reported that the PIs have a smaller reduction in relative metabolism from wakefulness to non-rapid eye movement sleep in the hippocampus, parahippocampal, and amygdaloid.Citation67 Our previous study found that the PIs showed lower regional homogeneity area in the MTL.Citation2 These studies demonstrated that the PIs had a functional disorder in the MTL. Furthermore, previous studies showed that the MTL (entorhinal cortex, parahippocampal cortex, and perirhinal cortex), connected via the medial dorsal nucleus of the thalamus to the medial, orbital, and lateral prefrontal cortex, which is essential to the declarative memory.Citation68–Citation70 Therefore, we speculated that the decreased FC between the MPFC and the right MTL in our study may be associated with the cognitive deficits in PIs.
The IPC, a large and heterogeneous region, is obligatorily or unintentionally engaged in the recall, consolidation, and retrieval of episodic memory information,Citation71–Citation73 as well as being implicated in diverse cognitive operationsCitation74 that include bodily awareness,Citation75 generating a sense of personal responsibility,Citation76 and moral decision making,Citation77 action, language, and mathematical problem solving.Citation78,Citation79 Chee and Chuah found that the sleep deprivation reduced IPL deactivation during a visual short-term memory task.Citation80 Horovitz et al revealed reductions in the IPC-MPFC FC during deep sleep and after partial sleep deprivation.Citation81,Citation82 De Havas et al also found that the IPC node of the DMN was consistently impaired and might represent an early marker for the effects of 24-hour sleep deprivation, as well as serving as an indicator of hitherto-unexplored behavioral impairments.Citation83 Gao et al found acute sleep deprivation showed decreased amplitude of low frequency fluctuation in the IPC.Citation84 A previous regional homogeneity study found little decreased spontaneous brain activity in the IPC in patients with obstructive sleep apnea.Citation85 Our previous sleep deprivation study found that a total of 72 hours sleep deprivation disturbed the spontaneous activity of the IPC area and its connectivity pattern with other DMN subregions.Citation30 These studies showed consistent evidence that the IPC had abnormal spontaneous activity and connectivity pattern in the sleep disorders.
Recent studies suggest a role of DMN parietal regions during retrieval of information, which in concert with the medial temporal structures.Citation86 Meanwhile, Tessitore et al revealed that the reduced FC between the MTL and the IPC may play a role in the development of cognitive decline in the Parkinson’s disease, and the functional abnormalities precede the structural abnormalities.Citation87 In this study, our findings demonstrated that the PIs compared with the GSs had decreased FC between the MPFC and the right MTL, and between the left MTL and the left IPC. These abnormal connectivities may be a potential functional basis of the emotional, memory, and cognitive decline in the PIs. Although no significant correlations were found between the abnormal paired FC subregions and the clinical measurements, these findings demonstrated the FC impairment of the intrinsic DMN subregions and help us know more about the underlying mechanism of the PIs.
Conclusion
Using the resting-state seed-based FC method, we found decreased region-to-region FC between the MPFC and the right MTL, and between the left MTL and the left IPC. These findings will help us insight into a deeper understanding of the neural mechanism of the PIs. However, there are several limitations that should be paid attention to. First, a larger sample size should be studied. Second, future studies should go beyond examining limited DMN to make a comprehensive analysis. Third, some PIs were selected without reference to the polysomnogram, although all PIs were asked to wear a Fitbit Flex tracker to monitor their sleep quality.
Acknowledgments
This work was supported by Jiangxi Provincial Department of Science and Technology Support Program (grant No 20132BBG70061, and No 20141BBG70026), Jiangxi Provincial Department of Natural Science Foundation Project (grant No 20132BAB205100), and National Natural Science Foundation of China (grant No 81560285).
Disclosure
The authors report no conflicts of interest in this work.
References
- RiemannDSpiegelhalderKFeigeBThe hyperarousal model of insomnia: a review of the concept and its evidenceSleep Med Rev2010141193119481481
- DaiXJPengDCGongHHAltered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI studyNeuropsychiatr Dis Treat2014102163217525484585
- O’ByrneJNBerman RosaMGouinJPNeuroimaging findings in primary insomniaPathol Biol201462526226925129873
- RiemannDVoderholzerUSpiegelhalderKChronic insomnia and MRI-measured hippocampal volumes: a pilot studySleep200730895595817702263
- WinkelmanJWBensonKLBuxtonOMLack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 TeslaSleep Med201011657658220466585
- WinkelmanJWPlanteDTSchoerningLIncreased rostral anterior cingulate cortex volume in chronic primary insomniaSleep201336799199823814335
- NohHJJooEYKimSTThe relationship between hippocampal volume and cognition in patients with chronic primary insomniaJ Clin Neurol20128213013822787497
- SpiegelhalderKRegenWBaglioniCInsomnia does not appear to be associated with substantial structural brain changesSleep201336573173723633756
- AltenaEVrenkenHVan Der WerfYDvan den HeuvelOAVan SomerenEJReduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric studyBiol Psychiatry201067218218519782344
- JooEYNohHJKimJSBrain gray matter deficits in patients with chronic primary insomniaSleep2013367999100723814336
- AltenaEVan Der WerfYDSanz-ArigitaEJPrefrontal hypoactivation and recovery in insomniaSleep20083191271127618788652
- DrummondSPWalkerMAlmklovECamposMAndersonDEStrausLDNeural correlates of working memory performance in primary insomniaSleep20133691307131623997363
- ChenMCChangCGloverGHIncreased insula coactivation with salience networks in insomniaBiol Psychol2014971824412227
- FristonKJFunctional and effective connectivity: a reviewBrain Connect201111133622432952
- Van DijkKRHeddenTVenkataramanAEvansKCLazarSWBucknerRLIntrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimizationJ Neurophysiol2010103129722119889849
- StoffersDAltenaEvan der WerfYDThe caudate: a key node in the neuronal network imbalance of insomnia?Brain2014137Pt261062024285642
- LiYWangEZhangHFunctional connectivity changes between parietal and prefrontal cortices in primary insomnia patients: evidence from resting-state fMRIEur J Med Res2014193224915847
- HuangZLiangPJiaXAbnormal amygdala connectivity in patients with primary insomnia: evidence from resting state fMRIEur J Radiol20128161288129521458943
- BucknerRLAndrews-HannaJRSchacterDLThe brain’s default network anatomy, function, and relevance to diseaseAnn NY Acad Sci200811241113818400922
- FoxMDRaichleMESpontaneous fluctuations in brain activity observed with functional magnetic resonance imagingNat Rev Neurosci20078970071117704812
- GusnardDARaichleMERaichleMESearching for a baseline: functional imaging and the resting human brainNat Rev Neurosci200121068569411584306
- GusnardDAAkbudakEShulmanGLRaichleMEMedial prefrontal cortex and self-referential mental activity: relation to a default mode of brain functionProc Natl Acad Sci20019874259426411259662
- CabezaRDolcosFGrahamRNybergLSimilarities and differences in the neural correlates of episodic memory retrieval and working memoryNeuroimage200216231733012030819
- SimpsonJRJrDrevetsWCSnyderAZGusnardDARaichleMEEmotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxietyProc Natl Acad Sci200198268869311209066
- SimpsonJRJrSnyderAZGusnardDARaichleMEEmotion-induced changes in human medial prefrontal cortex: I. During cognitive task performanceProc Natl Acad Sci200198268368711209065
- MasonMFNortonMIVan HornJDWegnerDMGraftonSTMacraeCNWandering minds: the default network and stimulus-independent thoughtScience2007315581039339517234951
- FoxMDSnyderAZVincentJLThe human brain is intrinsically organized into dynamic, anticorrelated functional networksProc Natl Acad Sci2005102279673967815976020
- RaichleMEThe restless brainBrain Connect20111131222432951
- UddinLQKellyAMBiswalBBCastellanosFXMilhamMPFunctional connectivity of default mode network components: correlation, anticorrelation, and causalityHum Brain Mapp200930262563718219617
- DaiXJLiuCLGongHHLong-term sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI studyNeuropsychiatr Dis Treat20151176177225834451
- GujarNYooSSHuPWalkerMPThe unrested resting brain: sleep deprivation alters activity within the default-mode networkJ Cogn Neurosci20102281637164819702469
- YanHZhangYChenHWangYLiuYAltered effective connectivity of the default mode network in resting-state amnestic type mild cognitive impairmentJ Int Neuropsychol Soc201319440040923425569
- MurdaughDLShinkarevaSVDeshpandeHRWangJPennickMRKanaRKDifferential deactivation during mentalizing and classification of autism based on default mode network connectivityPLoS One2012711e5006423185536
- EdingerJDBonnetMHBootzinRRDerivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work GroupSleep20042781567159615683149
- American Psychiatric Association (APA)Diagnostic and Statistical Manual of Mental Disorders, 4th edn–text revision (DSM-IV-TR)Washington, DCAmerican Psychiatric Association2000
- BuysseDJReynoldsCF3rdMonkTHBermanSRKupferDJThe Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and researchPsychiatry Res19892821932132748771
- BastienCHVallièresAMorinCMValidation of the insomnia severity index as an outcome measure for insomnia researchSleep Med20012429730711438246
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry1960231566214399272
- HamiltonMThe assessment of anxiety states by ratingBr J Med Psychol1959321505513638508
- ZungWWA self-rating depression scaleArch Gen Psychiatry1965121637014221692
- ZungWWA rating instrument for anxiety disordersPsychosomatics19711263713795172928
- McNairDMLorrMDropplemanLMManual for the Profile of Mood StatesSan Diego, CAEducational and Industrial Testing Services1971
- McNairDMLorrMDropplemanLFEdITS Manual for the Profile of Mood StatesSan Diego, CAEdITS Educational and Industrial Testing Service1992
- SatterthwaiteTDElliottMAGerratyRTAn improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity dataNeuroimage20136424025622926292
- YanCGCheungBKellyCA Comprehensive assessment of regional variation in the impact of head micromovements on functional connectomicsNeuroimage20137618320123499792
- LiHJDaiXJGongHHAberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRINeuropsychiatr Dis Treat20151120721425653530
- HuangXZhongYLZengXJDisturbed spontaneous brain activity pattern in patients with primary angle-closure glaucoma using amplitude of low-frequency fluctuation: a fMRI studyNeuropsychiatr Dis Treat2015111877188326251603
- SaadZSGottsSJMurphyKTrouble at rest: how correlation patterns and group differences become distorted after global signal regressionBrain Connect201221253222432927
- DaiXJGongHHWangYXGender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI studySleep Med201213672072722503940
- QiRFZhangLJXuQAbnormal functional connectivity within the default mode network in patients with HBV-related cirrhosis without hepatic encephalopathy revealed by resting-state functional MRIBrain Res20141576738024907446
- GorgesMMullerHPLuleDLudolphACPinkhardtEHKassubekJFunctional connectivity within the default mode network is associated with saccadic accuracy in Parkinson’s disease: a resting-state FMRI and videooculographic studyBrain Connect2013326527223627641
- ZhangDRaichleMEDisease and the brain’s dark energyNat Rev Neurol201061152820057496
- FornitoAYoonJZaleskyABullmoreETCarterCSGeneral and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performanceBiol Psychiatry201170647221514570
- JaoTVertesPEAlexander-BlochAFVolitional eyes opening perturbs brain dynamics and functional connectivity regardless of light inputNeuroimage201369213423266698
- Dang-VuTTSchabusMDesseillesMFunctional neuroimaging insights into the physiology of human sleepSleep201033121589160321120121
- MuzurAPace-SchottEFHobsonJAThe prefrontal cortex in sleepTrends Cogn Sci200261147548112457899
- PerrierJClochonPBertranFSpecific EEG sleep pattern in the prefrontal cortex in primary insomniaPLoS One2015101e011686425611059
- Fortier-BrochuEBeaulieu-BonneauSIversHMorinCMInsomnia and daytime cognitive performance: a meta-analysisSleep Med Rev2012161839421636297
- WilckensKAEricksonKIWheelerMEAge-related decline in controlled retrieval: the role of the PFC and sleepNeural Plast2012e624795
- BeerJSLombardoMVBhanjiJPRoles of medial prefrontal cortex and orbitofrontal cortex in self-evaluationJ Cogn Neurosci20102292108211919925187
- BecharaADamasioHDamasioAREmotion, decision making and the orbitofrontal cortexCereb Cortex200010329530710731224
- KoenigsMHollidayJSolomonJGrafmanJJLeft dorsomedial frontal brain damage is associated with insomniaNeuroscience20103047160411604321106842
- ZhouSYTongLSongFSelective medial temporal volume reduction in the hippocampus of patients with idiopathic generalized tonic-clonic seizuresEpilepsy Res2015110394825616454
- BarkhofFPolvikoskiTMvan StraatenECThe significance of medial temporal lobe atrophy: a postmortem MRI study in the very oldNeurology200769151521152717923614
- de LeonMJMosconiLBlennowKImaging and CSF studies in the preclinical diagnosis of Alzheimer’s diseaseAnn NY Acad Sci2007109711414517413016
- VisserPJVerheyFRHofmanPAScheltensPJollesJMedial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairmentJ Neurol Neurosurg Psychiatry200272449149711909909
- NofzingerEABuysseDJGermainAPriceJCMiewaldJMKupferDJFunctional neuroimaging evidence for hyperarousal in insomniaAm J Psychiatry2004161112126212815514418
- AggletonJPDumontJRWarburtonECUnraveling the contributions of the diencephalon to recognition memory: a reviewLearn Mem201118638440021597044
- AggletonJBrownMWInterleaving brain systems for episodic and recognition memoryTrends Cogn Sci2006101045546316935547
- AggletonJPBrownMWEpisodic memory, amnesia, and the hippocampal-anterior thalamic axisBehav Brain Sci199922342544411301518
- GusnardDARaichleMERaichleMESearching for a baseline: functional imaging and the resting human brainNat Rev Neurosci200121068569411584306
- FosterDJWilsonMAReverse replay of behavioural sequences in hippocampal place cells during the awake stateNature2006440708468068316474382
- WigGSGraftonSTDemosKEWolfordGLPetersenSEKelleyWMMedial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adultsProc Natl Acad Sci200810547185551856019001272
- LairdAREickhoffSBLiKRobinDAGlahnDCFoxPTInvestigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modelingJ Neurosci20092946144961450519923283
- FelicianOAntonJLNazarianBRothMRollJPRomaiguèrePWhere is your shoulder? Neural correlates of localizing others’ body artsNeuropsychologia2009478–91909191619428423
- Schaich BorgJHynesCVan HornJGraftonSSinnott-ArmstrongWConsequences, action, and intention as factors in moral judgments: an fMRI investigationJ Cogn Neurosci200618580381716768379
- RaineAYangYNeural foundations to moral reasoning and antisocial behaviorSoc Cogn Affect Neurosci20061320321318985107
- SchilbachLBzdokDTimmermansBIntrospective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social and unconstrained cognitionPLoS One201272e3092022319593
- SeghierMLThe angular gyrus: multiple functions and multiple subdivisionsNeuroscientist2013191436122547530
- CheeMWChuahYMFunctional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivationProc Natl Acad Sci2007104229487949217517619
- HorovitzSGBraunARCarrWSDecoupling of the brain’s default mode network during deep sleepProc Natl Acad Sci200910627113761138119549821
- SämannPGTullyCSpoormakerVIIncreased sleep pressure reduces resting state functional connectivityMAGMA2010235–637538920473549
- De HavasJAParimalSSoonCSCheeMWSleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performanceNeuroimage20125921745175121872664
- GaoLBaiLZhangYDaiXJNetraRFrequency-dependent changes of local resting oscillations in sleep-deprived brainPLoS One2015103e012032325798918
- PengDCDaiXJGongHHAltered intrinsic regional brain activity in male patients with severe obstructive sleep apnoea: a resting-state fMRI studyNeuropsychiatr Dis Treat2014101819182625278755
- SestieriCCorbettaMRomaniGLShulmanGLEpisodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analysesJ Neurosci201131124407442021430142
- TessitoreAEspositoFVitaleCDefault-mode network connectivity in cognitively unimpaired patients with Parkinson diseaseNeurology201279232226223223100395
