Abstract
Objective
The aim of this study was to use amplitude of low-frequency fluctuation (ALFF) to explore regional brain activities in healthy subjects after sleep deprivation (SD).
Materials and methods
A total of 16 healthy subjects (eight females, eight males) underwent the session twice: once was after normal sleep (NS), and the other was after SD. ALFF was used to assess local brain features. The mean ALFF-signal values of the different brain areas were evaluated to investigate relationships with clinical features and were analyzed with a receiver-operating characteristic curve.
Results
Compared with NS subjects, SD subjects showed a lower response-accuracy rate, longer response time, and higher lapse rate. Compared with NS subjects, SD subjects showed higher ALFF area in the right cuneus and lower ALFF area in the right lentiform nucleus, right claustrum, left dorsolateral prefrontal cortex, and left inferior parietal cortex. ALFF differences in regional brain areas showed high sensitivity and specificity. In the SD group, mean ALFF of the right claustrum showed a significant positive correlation with accuracy rate (r=0.687, P=0.013) and a negative correlation with lapse rate (r=−0.706, P=0.01). Mean ALFF of the dorsolateral prefrontal cortex showed a significant positive correlation with response time (r=0.675, P=0.016).
Conclusion
SD disturbed the regional brain activity of the default-mode network, its anticorrelated “task-positive” network, and the advanced cognitive function brain areas.
Introduction
Sleep is very important for humans to live a normal life. Sleep deprivation (SD), widespread in today’s society, is a sleep-loss status generally caused by personal or environmental reasons.Citation1 SD can lead to a series of changes in emotional balance, and has a detrimental effect on cognitive function, attention, learning, and working memory.Citation2–Citation5 Long-term SD duration is associated with exaggerated neural reactivity, multisystemic and multiorganic dysfunction, and negative metabolic, psychological, physiological, or even behavioral reactivity.Citation1,Citation6–Citation8
Many studies have been carried out in short-term SD (24 hours), and found that SD adversely affects brain function and cognitive domains.Citation9,Citation10 A previous study employing resting-state functional magnetic resonance imaging (rs-fMRI) to explore brain-activation patterns by acupuncture stimuli during SD status found that sanyinjiao (SP6) elicits greater and anatomically different activations in the salience network than those of sham stimuli.Citation11 rs-fMRI studies have found altered functional connectivity in the sleep-deprived brain.Citation12–Citation15 Furthermore, multiple observations of altered connectivity within and between various resting-state networks have been reported for SD.Citation12–Citation18 These findings suggest that SD disturbs resting-state brain-activity patterns for internal processing of information.Citation19 However, the neurologic mechanisms remain unclear.
The amplitude of low-frequency fluctuation (ALFF) can locate which brain region has abnormal regional spontaneous neuronal activity in blood oxygen level-dependent signals, and has been shown to have good-to-moderate test–retest reliability ranging from minimal to robust.Citation20–Citation22 Therefore, the simple calculation and reliable characterization of the ALFF measurement make it a potentially useful tool for rs-fMRI data analysis to investigate various functional brain changes.Citation15 Recently, the use of ALFF measurement has been successfully applied to SD,Citation15,Citation23 wakefulness and light sleep,Citation24 obstructive sleep apnea,Citation25 primary insomnia,Citation26 and primary angle-closure glaucoma.Citation27 In this study, we hypothesized that SD would result in aberrant regional brain activity. To test the hypothesis, we utilized ALFF as an index to investigate the potential neurologic mechanisms of SD.
Materials and methods
Subjects
Sixteen healthy university students (mean age 24.51±2.75 years, mean education duration 16.8±1.8 years) responding to a web-based questionnaire were recruited. All subjects met the following criteria, as in previous studies:Citation1,Citation15,Citation28 1) no symptoms associated with sleep disorders and no history of any psychiatric or neurologic disorders; 2) right-handed; 3) good sleeping habits; 4) good sleep onset and/or maintenance, and no history of swing shift, shift work, sleep complaints, or other sleep disorders; 5) regular dietary habits, and had not consumed any stimulants, alcohol, tea, cigarettes, medications, or caffeine for at least 3 months prior to the study; 6) no foreign implants in the body, and no inborn or other acquired diseases; 7) moderate body shape and weight; and 8) Pittsburgh Sleep Quality Index score <5.
Research design and procedures
To evaluate sleep status, all subjects were monitored for 1 week by wearing a Fitbit Flex tracker (http://help.fitbit.com).Citation28 Each of the subjects underwent the session twice randomly. Once was after normal sleep (NS), and the other was after a total of 24 hours’ SD. Each subject underwent an MRI scan at each session, before which all subjects underwent an attention network test.Citation15,Citation29,Citation30 A simple questionnaire was administered immediately after the scans to ascertain whether the subjects were awake during the scans. SD was conducted in a specialized room, and started from 7 pm and ended at 7 pm the following day.
All volunteers were informed of the purposes, methods, and potential risks. This study was approved by the human-research ethics committee of Sir Run Run Shaw Hospital of Zhejiang University. All volunteers participated voluntarily, and all signed an informed con sent form.
MRI parameters
MRI scans were performed on a 3 T MR scanner (Trio; Siemens, Munich, Germany). High-resolution T1-weighted images were acquired with a three-dimensional spoiled gradient-recalled sequence in a sagittal orientation: 176 images (repetition time 1,900 ms, echo time 2.26 ms, thickness 1 mm, gap 0.5 mm, acquisition matrix 256×256, field of view 250×250 mm, flip angle 9°) were obtained. Finally, rs-fMRI scan was obtained with eyes opened. A total of 240 functional images (repetition time 3,000 ms, echo time 30 ms, thickness 4 mm, gap 1.2 mm, acquisition matrix 64×64, flip angle 90°, field of view 220×220 mm; 36 axial slices with gradient-recalled echo-planar imaging pulse sequence) covering the whole brain were obtained.
fMRI data analysis
Based on the MatLab 2012a (MathWorks, Natick, MA, USA), data preprocessing was performed by Data Processing Assistant for Resting-State fMRI (http://rfmri.org/DPARSF) software, including Digital Imaging and Communication in Medicine form transformation, slice timing, head-motion correction, spatial normalization, and smooth. The first ten time points of the functional images were discarded, due to the possible instability of the initial MRI signal and the participants’ adaptation to the scanning environment. Participants who had more than 1.5 mm maximum translation in x, y, or z and 1.5° motion rotation were rejected. Friston six head-motion parameters were used to regress out the head-motion effects based on recent work showing that the higher-order models were more effective in removing head-motion effects.Citation31,Citation32 After head-motion correction, the fMRI images were spatially normalized to Montreal Neurological Institute space and resampled at a resolution of 3×3×3 mm. Smoothening was performed with a Gaussian kernel of 6×6×6 mm3 full width at half maximum. After preprocessing, the time series for each voxel was temporally band-pass filtered (0.01–0.08 Hz) and linearly detrended to reduce low-frequency drift and physiological high-frequency respiratory and cardiac noise. The details of the ALFF calculation have been reported in previous studies.Citation15,Citation27,Citation33 To reduce the global effects of variability across the participants, the mean ALFF value of each voxel was divided by the global mean ALFF value for each participant.
Receiver-operating characteristic curve
Discrimination results are considered excellent for areas under the curve between 0.9 and 1, good between 0.8 and 0.9, fair between 0.7 and 0.8, poor between 0.6 and 0.7, and failed between 0.5 and 0.6.Citation34 Since different ALFF areas might be utilized as markers to separate the SD group from the NS group, the mean signal values of the different areas were extracted and used for receiver-operating characteristic (ROC)-curve analysis to investigate whether these specific ALFF differences had the sensitivity and specificity to distinguish the SD group from the NS group.
Brain–behavior correlation analysis
Based on the ALFF findings, the mean ALFF-signal values of the different brain areas were extracted and their correlations calculated with the behavioral performances using the IBM Statistical Package for the Social Sciences (SPSS, version 21.0) software, with the statistical threshold set at P<0.05.
Statistical analysis
A two-paired t-test was used to assess the differences in brain activity between the two groups. A corrected significance level of individual voxels of P<0.001 and contiguous cluster volume ≥351 mm3, using an AlphaSim corrected threshold of cluster P<0.05, were used to determine statistical significance.
Results
Behavioral results
Compared with the NS subjects, the SD subjects showed a lower response-accuracy rate (SD: 92%±1.95%, NS: 97.25%±2.42%, t=−5.852; P<0.001), a longer response time (SD: 631.61±86.67 ms, NS: 527.49±39.06 ms, t=3.794; P=0.002), and a higher lapse rate (SD: 7.11%±9.59%, NS: 0.09%±0.25%, t=2.534; P=0.028).
ALFF differences
Compared with the NS subjects, the SD subjects showed higher ALFF area in the right cuneus (Brodmann’s area [BA] 17, BA 18), and lower ALFF area in the right lentiform nucleus, right claustrum, left middle frontal gyrus (BA 46), and left inferior parietal cortex (IPC; BA 39). Details are presented in and .
Figure 1 ALFF differences between the SD and NS groups.
Abbreviations: ALFF, amplitude of low-frequency fluctuation; SD, sleep deprivation; NS, normal sleep.
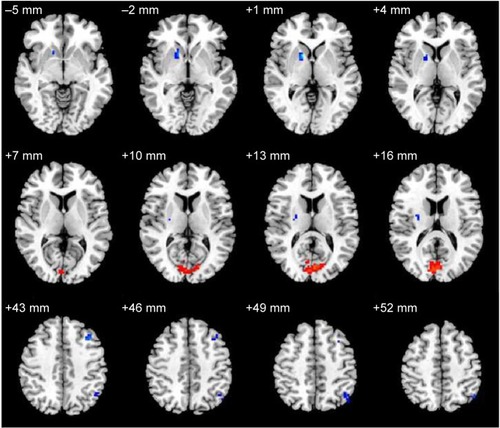
Table 1 ALFF differences between SD and NS conditions
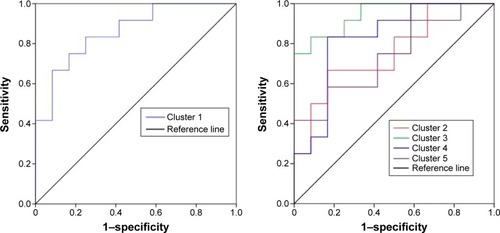
ALFF analysis shows high sensitivity and specificity
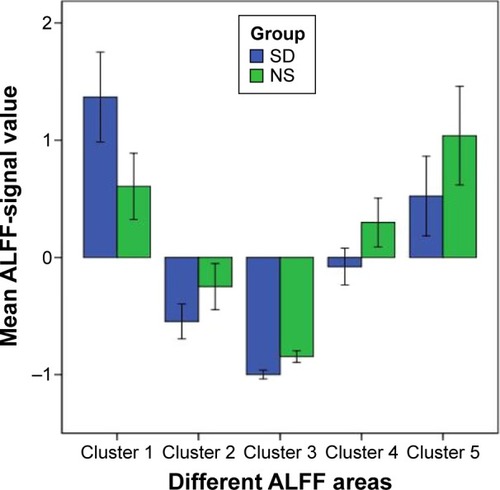
shows the mean ALFF-signal values of the altered regional brain areas. These different values were used for ROC-curve analysis to investigate whether these specific ALFF differences had the sensitivity and specificity to distinguish the SD group from the NS group. ROC-curve analysis showed that the area under the curve of the five different brain areas (from cluster 1 to cluster 5) was 0.861 with a cutoff point of 0.996 (mean ALFF-signal value), 0.757 with a cutoff point of −0.538, 0.944 with a cutoff point of −0.93, 0.826 with a cutoff point of 0.084, and 0.736 with a cutoff point of 0.471. Further diagnostic analysis showed that the different brain areas alone discriminated SD status from NS status with high sensitivity and specificity: corresponding values from clusters 1–5 were 0.667 and 0.917, 0.667 and 0.833, 0.833 and 0.917, 0.833 and 0.833, and 0.583 and 0.833, respectively. Details are presented in .
Figure 2 Mean ALFF-signal values of the altered regional brain areas.
Abbreviations: ALFF, amplitude of low-frequency fluctuation; NS, normal sleep; SD, sleep deprivation; IPC, inferior parietal cortex.
Brain–behavior correlation results
In the SD group, the mean ALFF value of the right claustrum showed a significant positive correlation with accuracy rate (r=0.687, P=0.013) and a negative correlation with lapse rate (r=−0.706, P=0.01). The mean ALFF value of the middle frontal gyrus showed a significant positive correlation with response time (r=0.675, P=0.016).
Discussion
In this study, our results confirmed functional alterations in resting-state networks after SD. Compared with the NS subjects, the SD subjects showed higher ALFF areas in the right visual network and lower ALFF areas in the right lentiform nucleus, right claustrum, left dorsolateral prefrontal cortex (DLPFC), and left IPC. ALFF differences in regional brain areas showed high sensitivity and specificity. The reliability of the ALFF differences revealed that the different ALFF areas could serve as markers to distinguish the SD condition from the NS condition. Our findings may suggest that SD is associated with the model of excitation–inhibition imbalance in the central nervous system.
Previous studies have demonstrated that hyperarousal reactivation in the occipital gyrus was found in SD subjects and patients with insomnia.Citation1,Citation28 These findings supported our results that the SD group showed higher ALFF area in the right cuneus compared with the NS group. The vision-related regions were not only activated by real vision but also by visual mental imagery,Citation35,Citation36 since the visual cortex is relevant to emotional activities and emotional changes can lead to higher blood oxygen level-dependent signal regions in the visual cortex.Citation37,Citation38 The higher activity in the visual cortex may represent a compensatory reaction to recover the emotional changes.
Among the spontaneously synchronized neuronal networks, the “task-negative” network and its “task-positive” anticorrelated network (ACN) have received the most attention.Citation19 The task-negative network was the default-mode network (DMN). The IPC region, one key region of the DMN, is obligatorily or unintentionally engaged in the recall, consolidation, and retrieval of episodic memory information,Citation39–Citation41 as well as being implicated in diverse cognitive operationsCitation42 that include bodily awareness,Citation43 generating a sense of personal responsibilityCitation44 and moral decision making.Citation45 Chee and Chuah found that SD reduced the IPC deactivation during a visual short-term memory task.Citation46 Horovitz et al revealed a decreased functional connectivity between the IPC and the medial PFC during deep sleep and after partial SD.Citation47,Citation48 Nie et al found that patients with chronic insomnia demonstrated significantly decreased functional connectivity between the left medial temporal lobe and the left IPC compared with good sleepers.Citation49 De Havas et al also found that the IPC node of the DMN was consistently impaired and might represent an early marker for the effects of 24-hour SD, as well as serving as an indicator of hitherto-unexplored behavioral impairments.Citation50 Our results were in accordance with these findings. The DLPFC, one region of the ACN, recruits working-memory tasks, and has been implicated to be responsible for failure in working memory for the sleep-deprived brain.Citation51–Citation54 It is well known that sleep plays an important role in the formation and consolidation of memories.Citation55 Patients with insomnia show significant reduction in gray-matter concentrations and lower regional homogeneity in the DLPFC.Citation28,Citation56 One recent SD study reported a reduced anticorrelation between the DMN and ACN nodes at both task-related and resting states.Citation50 In support of these findings, in the present study we found that the SD subjects showed lower ALFF in the DMN and ACN nodes compared with NS subjects; furthermore, the mean ALFF value of the ACN node showed a significant positive correlation with response time (r=0.675, P=0.016). These findings may represent a decline in attention and memory processes by SD.
The lentiform nucleus and claustrum are associated with advanced cognitive function and involved in aberrant regional brain activity in patients with obstructive sleep apnea.Citation57 In the SD group, the mean ALFF value of the right claustrum showed a significant positive correlation with accuracy rate (r=0.687, P=0.013) and a negative correlation with lapse rate (r=−0.706, P=0.01). SD decreased the regional brain activity in several brain areas thus needs to attempt to recruit more specific advanced cognitive function brain areas to sustain alertness and accomplish cognitive performance. In this study, SD showed a continuing decline in the DMN–ACN activity, therefore, the lentiform nucleus and claustrum were recruited to sustain alertness and accomplish cognitive performance.
Conclusion
In this study, our results confirmed that SD demonstrated a compensatory reaction in the visual network and disturbed spontaneous neuronal activity in advanced cognitive function brain areas and the DMN–ACN compared with NS. The mean ALFF-signal values of the different brain areas showed high sensitivity and specificity. Our findings provide insights into the pathophysiological mechanism of SD, and may be helpful for understanding the pathophysiology of SD.
Disclosure
The authors report no conflicts of interest in this work.
References
- DaiXJGongHHWangYXGender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI studySleep Med20121372072722503940
- DrummondSPBrownGGThe effects of total sleep deprivation on cerebral responses to cognitive performanceNeuropsychopharmacology200125S68S7311682277
- JacksonMLHuqhesMECroftRJThe effect of sleep deprivation on BOLD activity elicited by a divided attention taskBrain Imaging Behav201159710821271311
- LuberBStanfordADBulowPRemediation of sleep-deprivation-induced working memory impairment with fMRI-guided transcranial magnetic stimulationCereb Cortex2008182077208518203694
- NilssonJPSöderströmMKarlssonAULess effective executive functioning after one night’s sleep deprivationJ Sleep Res2005141615743327
- OhayonMMSmolenskyMHRothTConsequences of shiftworking on sleep duration, sleepiness, and sleep attacksChronobiol Int20102757558920524802
- TsigosCChrousosGPHypothalamic-pituitary-adrenal axis, neuroendocrine factors and stressJ Psychosom Res20025386587112377295
- WalkerMPStickgoldRSleep, memory and plasticityAnnu Rev Psychol20065713916616318592
- BasnerMRaoHGoelNDingesDFSleep deprivation and neurobehavioral dynamicsCurr Opin Neurobiol20132385486323523374
- GoelNRaoHDurmerJSDingesDFNeurocognitive consequences of sleep deprivationSemin Neurol20092932033919742409
- GaoLZhangMGongHDifferential activation patterns of FMRI in sleep-deprived brain: restoring effects of acupunctureEvid Based Complement Alternat Med2014201446576025024729
- De HavasJAParimalSSoonCSCheeMWSleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performanceNeuroimage2012591745175121872664
- BoschOGRihmJSScheideggerMSleep deprivation increases dorsal nexus connectivity to the dorsolateral prefrontal cortex in humansProc Natl Acad Sci U S A2013110195971960224218598
- ShaoYWangLYeEDecreased thalamocortical functional connectivity after 36 hours of total sleep deprivation: evidence from resting state FMRIPLoS One20138e7883024205327
- DaiXJLiuCLGongHHLong-term sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI studyNeuropsychiatr Dis Treat20151176177225834451
- PiantoniGCheungBLVan VeenBDDisrupted directed connectivity along the cingulate cortex determines vigilance after sleep deprivationNeuroimage20137921322223643925
- YooSSGujarNHuPThe human emotional brain without sleep – a prefrontal amygdala disconnectCurr Biol200717R877R87817956744
- PicchioniDDuynJHHorovitzSGSleep and the functional connectomeNeuroimage20138038739623707592
- GaoLBaiLZhangYFrequency-dependent changes of local resting oscillations in sleep-deprived brainPLoS One201510e012032325798918
- SimpsonJRJrSnyderAZGusnardDARaichleMEEmotion-induced changes in human medial prefrontal cortex: I. During cognitive task performanceProc Natl Acad Sci U S A20019868368711209065
- MasonMFNortonMIVan HornJDWegnerDMGraftonSTMacraeCNWandering minds: the default network and stimulus-independent thoughtScience200731539339517234951
- YanHZhangYChenHWangYLiuYAltered effective connectivity of the default mode network in resting-state amnestic type mild cognitive impairmentJ Int Neuropsychol Soc20131940040923425569
- DaiXJMinYJGongHHEvaluation of the post-effect of acupuncture at sanyinjiao (SP 6) under sleep deprivation by resting-state amplitude of low-frequency fluctuation: a fMRI studyZhongguo Zhen Jiu2012324752 Chinese22295826
- HorovitzSGFukunagaMde ZwartJALow frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI studyHum Brain Mapp20082967168217598166
- LiHJDaiXJGongHHNieXZhangWPengDCAberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRINeuropsychiatr Dis Treat20151120721425653530
- DaiXJNieXLiuXMGender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI studyJ Clin Sleep Med201612336337426715399
- HuangXZhongYLZengXJDisturbed spontaneous brain activity pattern in patients with primary angle-closure glaucoma using amplitude of low-frequency fluctuation: a fMRI studyNeuropsychiatr Dis Treat2015111877188326251603
- DaiXJPengDCGongHHAltered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI studyNeuropsychiatr Dis Treat2014102163217525484585
- FanJMcCandlissBDFossellaJFlombaumJIPosnerMIThe activation of attentional networksNeuroimage20052647147915907304
- FanJMcCandlissBDSommerTRazAPosnerMITesting the efficiency and independence of attentional networksJ Cogn Neurosci20021434034711970796
- SatterthwaiteTDElliottMAGerratyRTAn improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity dataNeuroimage20136424025622926292
- YanCGCheungBKellyCA comprehensive assessment of regional variation in the impact of head micromovements on functional connectomicsNeuroimage20137618320123499792
- ZangYFHeYZhuCZAltered baseline brain activity in children with ADHD revealed by resting-state functional MRIBrain Dev200729839116919409
- El KhouliRHMacuraKJBarkerPBHabbaMRJacobsMABluemkeDARelationship of temporal resolution to diagnostic performance for dynamic contrast enhanced MRI of the breastJ Magn Reson Imaging200930999100419856413
- ThompsonWLKosslynSMSukelKEAlpertNMMental imagery of high- and low-resolution gratings activates area 17Neuroimage20011445446411467918
- IshaiAHaxbyJVUngerleiderLGVisual imagery of famous faces: effects of memory and attention revealed by fMRINeuroimage2002171729174112498747
- WangWLiKCShanBCStudy of acupuncture point Liv 3 with functional MRIZhonghua Fang She Xue Za Zhi2006402935 Chinese
- LangPJBradleyMMFitzsimmonsJREmotional arousal and activation of the visual cortex: an fMRI analysisPsychophysiology1998351992109529946
- GusnardDARaichleMERaichleMESearching for a baseline: functional imaging and the resting human brainNat Rev Neurosci2001268569411584306
- FosterDJWilsonMAReverse replay of behavioural sequences in hippocampal place cells during the awake stateNature200644068068316474382
- WigGSGraftonSTDemosKEWolfordGLPetersenSEKelleyWMMedial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adultsProc Natl Acad Sci U S A2008105185551856019001272
- LairdAREickhoffSBLiKRobinDAGlahnDCFoxPTInvestigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modelingJ Neurosci200929144961450519923283
- FelicianOAntonJLNazarianBRothMRollJPRomaiguèrePWhere is your shoulder? Neural correlates of localizing others’ body partsNeuropsychologia2009471909191619428423
- Schaich BorgJHynesCVan HornJConsequences, action, and intention as factors in moral judgments:an fMRI investigationJ Cogn Neurosci200618580381716768379
- RaineAYangYNeural foundations to moral reasoning and antisocial behaviorSoc Cogn Affect Neurosci2006120321318985107
- CheeMWChuahYMFunctional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivationProc Natl Acad Sci U S A20071049487949217517619
- HorovitzSGBraunARCarrWSDecoupling of the brain’s default mode network during deep sleepProc Natl Acad Sci U S A2009106113761138119549821
- SämannPGTullyCSpoormakerVIIncreased sleep pressure reduces resting state functional connectivityMAGMA20102337538920473549
- NieXLiHJWanALFunctional connectivity of paired default mode network subregions in primary insomniaNeuropsychiatr Dis Treat2015113085309326719693
- De HavasJAParimalSSoonCSCheeMWSleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performanceNeuroimage2012591745175121872664
- ChuahYMVenkatramanVDingesDFCheeMWThe neural basis of interindividual variability in inhibitory efficiency after sleep deprivationJ Neurosci2006267156716216822972
- DrummondSPBischoff-GretheADingesDFAyalonLMednickSCMeloyMJThe neural basis of the psychomotor vigilance taskSleep2005281059106816268374
- LawrenceNSRossTJHoffmannRGaravanHSteinEAMultiple neuronal networks mediate sustained attentionJ Cogn Neurosci2003151028103814614813
- YamasakiHLaBarKSMcCarthyGDissociable prefrontal brain systems for attention and emotionProc Natl Acad Sci U S A200299114471145112177452
- BackhausJJunghannsKBornJHohausKFaaschFHohagenFImpaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol releaseBiol Psychiatry2006601324133016876140
- JooEYNohHJKimJSBrain gray matter deficits in patients with chronic primary insomniaSleep201336999100723814336
- PengDCDaiXJGongHHLiHJNieXZhangWAltered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: a resting-state fMRI studyNeuropsychiatr Dis Treat2014101819182625278755