?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A spectrophotometer with special cuvette was developed for evaluating the photocatalytic activities of suspended fine particles. The spectrophotometer can continuously irradiate UV light using LED to the sample solution, and changes in the absorbance at 664 nm during photocatalytic degradation of methylene blue (MB) were monitored continuously. From the onset of MB degradation, the absorbance decreased and reached a steady value at the end of the reaction. This process was expressed by first order kinetics and the photocatalytic activities of various fine particles could be evaluated quantitatively based on the reaction rate constant (k). The effect of photocatalysis using various TiO2 fine particles on the physiological activities of Euglena gracilis was related with k value.
Introduction
The use of nano sized fine particles is of increasing importance because they are suitable for manifold applications in the fields of electronics,Citation1 sensing,Citation2 catalysis,Citation3 and medical,Citation4 etc. Some types of particles (for example TiO2) show photocatalytic activities. Many kinds of photocatalytic fine particles with various activities are commercially produced and widely used for various applications. They are used as photocatalysts in environmental decontamination of air, soil, and water,Citation5–Citation7 pigments,Citation8 and as cosmetic additives.Citation9 Large quantities of the fine particles are discharged to the environment intentionally or unintentionally in the course of their production, use, and disposal. These will inevitably lead to pollution of both the biotic and abiotic components of the environment. Reactive oxygen species (ROS) produced by photocatalysis cause inactivation of various organisms.Citation10–Citation13 Studies on the potential effects of these photocatalysts on human health and on the environment are therefore very important.Citation13,Citation14 In order to assess the potential risks of photocatalytic fine particles or to efficiently utilize them in various fields, it is very necessary to develop methods for quantitative evaluation of their photocatalytic activities. Many kinds of fine particles with various photocatalytic activities are commercially produced but there are few reports of their relative photocatalytic activities.Citation15
In this study, an apparatus was developed for quantitative monitoring of photocatalytic reaction of nano fine particles based on spectrophotometry. With this apparatus, it was possible to evaluate the photocatalytic activities of various fine particles on the basis of the reaction rate constant (k) in first order kinetic. The effect of photocatalysis using TiO2 fine particles, which measured k values, on the physiological activities of Euglena gracilis was also investigated.
Materials and methods
Chemicals
Titanium oxide anatase form [ANA] (100% anatase crystalline form, average particle size = 50 nm, Wako Pure Chemical Industries, Japan), titanium oxide rutile form [RU] (100% rutile crystalline form, average particle size = 100 nm, Wako) titanium oxide amorphous [AM] (average diameter = 40 nm, Wako); AP25 (20% rutile 80% anatase crystalline form, average particle size = 40 nm, Japan Aerosil Co, Tokyo, Japan), STS-01 (photocatalytic sol, average particle size = 7 nm, Ishihara Sangyo Kaisha, Ltd Osaka, Japan), and STS-02 (photocatalytic sol, average particle size = 7 nm, Ishihara Sangyo) were used in this study. Methylene blue (MB) and Daigo’s artificial seawater SP (in mg per liter: MgCl2·6H2O, 9, 474: CaCl2·2H2O, 1, 326: Na2SO4, 3, 505: KCl, 597: NaHCO3, 171: KBr, 85: Na2B4O7·10H2O, 34: SrCl2, 12: NaF, 3: LiCl, 1: KI, 0.07: CoCl2·6H2O, 0.0002: AlCl3·6H2O, 0.008: FeCl3·6H2O, 0.005: Na2WO4·2H2O, 0.002: (NH4)6Mo7O24·4H2O, 0.02: MnCl2·4H2O, 0.0008: NaCl, 20, 747) were purchased from Wako.
Cultivation of Euglena gracilis
E. gracilis IAM E-6 (strain Z), obtained from the algal collection of the Institute of Applied Microbiology, University of Tokyo, Japan was used in this study as a model micro-algae. E. gracilis produces antioxidant such as alpha-tocopherol. Modified Hutner medium was used for the cultivation as described previouslyCitation16 The medium was sterilized by autoclaving at 121°C for 15 min. To prevent deactivation by heat, filter-sterilized vitamin B12 was added to the medium after sterilization. Cultivation was carried out by inoculating E. gracilis into a 500 mL Erlenmeyer flask containing 100 mL medium and the flask was then incubated under illumination by black light (National Electric, Tokyo) on rotary shaker (120 rpm) at 25°C. The distance between the flask and the black light was adjusted to give a light intensity of 1.0 mW/cm2 at the surface of the flask. The ANA, AP25, or RU (100 mg/L) was added to the E. gracilis cell culture.
Analytical methods
The alpha-tocopherol was extracted from the cell according to the methods.Citation17E. gracilis cells harvested by centrifugation at 1,000 × g for 5 min were washed with N2 saturated distilled water before the addition of 0.1 mL of 50 mM L-ascorbic acid and extracted with 3.75 mL of chloroform-methanol (1:2 v/v) by shaking vigorously for 20 min in N2. The mixture was centrifuged at 1,000 × g for 5 min to remove cell debris. The extraction was repeated two times. The cell debris was extracted with 4.75 mL of chloroform-methanol-water (1:2:0.8 v/v). An equal volume of distilled water was added to combined extracts and mixed gently. The chloroform layer was separated from the water-methanol layer after cooling on ice and centrifuged at 1,000 ×g for 5 min, and evaporated to dryness at room temperature under a stream of N2 gas to obtain the lipid fraction (the alpha-tocopherol content of the cells was determined from the fraction). Vitamin E homolog kit for biochemistry and analysis (Eisai, Tokyo, Japan) was used for the preparation of the calibration curve. The alpha-tocopherol was measured using high-performance liquid chromatography equipped with an Intelligent Spectrofluorometer 821-FP (Nihon Bunko, Tokyo, Japan) and packed silica gel column (150A-5, 4.6 × 250 mm, GL Science, Tokyo, Japan). The fluorescent lamp was used for the detection of alpha-tocopherol. The wavelengths were Ex = 295 nm and Em = 340 nm. A mixture of n-hexane, 1,4-dioxane, and ethanol (97.6:2.0:0.4 v/v) was used as the elute at a flow rate of 1.5 mL/min. The column temperature was 40°C. Dry cell weight was determined according to the method described previouslyCitation16 The total chlorophyll (a + b) concentration was determined according to the methodCitation18 using N, N′-dimethylformamide (DMF). Cells were extracted with DMF overnight. The cells were removed by centrifugation (1,000 × g for 5 min), and the absorbance of the supernatant was measured using a spectrophotometer (V-550, JASCO, Tokyo, Japan). Total chlorophyll (a + b) concentration was calculated as Chls a + b (mg/mL in DMF) = 17.67 A646.8 + 7.12 A663.8, where A646.8and A663.8 are the absorbance at wavelengths of 646.8 nm and 663.8 nm, respectively.
All the experiments were performed in triplicate. Although the results of representative single experiments are shown in , , and , they were confirmed to be highly reproducible (there was less than 2.5% deviation in the results).
Figure 1 Schematic diagram of an apparatus for evaluating the photocatalytic activity of suspended fine particles in the liquid.
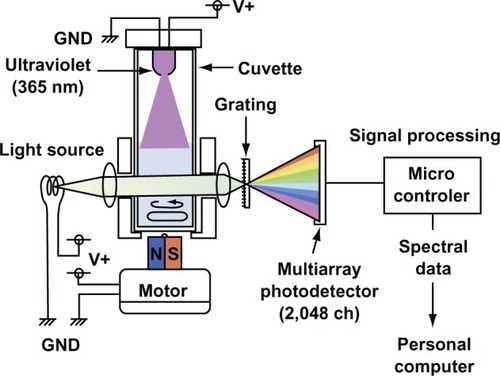
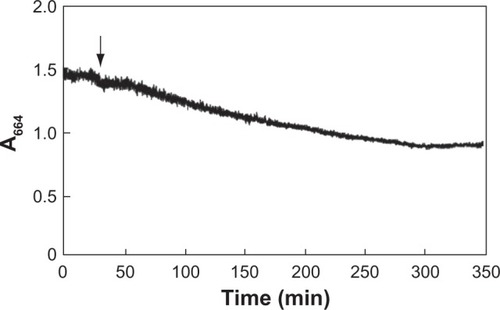
Figure 2 Changes in absorbance at 664 nm during photocatalytic reaction. ANA (TiO2 anatase form) 125 mg/L, methylene blue 5 mg/L in the distilled water at 25°C. The arrow indicates the start of UV (365 nm) irradiation.
Results
The spectrophotometer with special cuvette
An apparatus for evaluating the photocatalyst activity of suspended fine particles was developed. It comprised a spectrophotometer equipped with a special cuvette. shows a schematic diagram of the system. The characteristics of this apparatus are as follows; (a) UV light (365 nm) was irradiated continuously at 0.7 mW/cm2 in the surface of the sample by LED, which produces very low heat; (b) a magnetic stirrer is situated under the cuvette (diameter = 10 mm, length = 43 mm, working volume = 2.0 mL, the temperature was kept at 25°C) and the content can be mixed with a mini magnetic bar (diameter = 2 mm, length = 5 mm, the agitation speed = 600 rpm); (c) the absorbance and spectra (wavelength from 400 nm to 700 nm) can be monitored continuously by means of personal computer. This apparatus enables the samples in the cuvette to be mixed homogeneously, giving a stable absorbance over a long period of time. It is therefore possible to obtain stable absorbance readings for even heterogeneous fine particles with high specific gravity (it was confirmed in various TiO2 solutions without MB under UV irradiation or no irradiation [data not shown]). The degradation of MB was used to evaluate photoactivity of TiO2. Changes in the spectra (400–700 nm) of photocatalytic decomposition of MB were monitored. It was observed that the absorbance decreased with time at each wavelength (data not shown). A wavelength of 664 nm was selected as the optimal wavelength for monitoring MB decomposition (Otsuka-Yao-Matsuo et al used the same wavelength for the measurement of MB decomposition).Citation19 The absorbance at 664 nm of the sample solution (MB + TiO2) was not allowed to exceed to 1.9, which was the limit for possible quantitative evaluation.
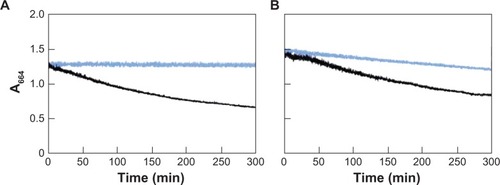
shows changes in the absorbance at 664 nm during photocatalytic decomposition of MB (5 mg/L) using ANA (125 mg/L). Before UV irradiation, the A664 did not change. After UV irradiation, the A664 decreased sharply due to photocatalytic decomposition of MB and then leveled off after about 300 min. The above results showed that the process of photocatalytic decomposition of MB by TiO2 could be monitored optically, making it possible to use changes in the A664 to measure the rate of reaction and to estimate the end of reaction.
Photocatalytic activities of TiO2 fine particles depend to a large extent on their crystalline form. In the case of the photoinactive RU (125 mg/L) or AM (125 mg/L) suspended in MB (5 mg/L) solution at 25°C, the A664 did not change under UV irradiation (data not shown). shows the changes in A664 during photocatalytic reaction with ANA and AP25. The photocatalytic activity of ANA in the distilled water is slightly higher than that of AP25. In artificial seawater, there was no photocatalytic activity of AP25, while the activity of ANA decreased significantly ().
Evaluation of the photocatalytic activities of various fine particles based on reaction rate constant
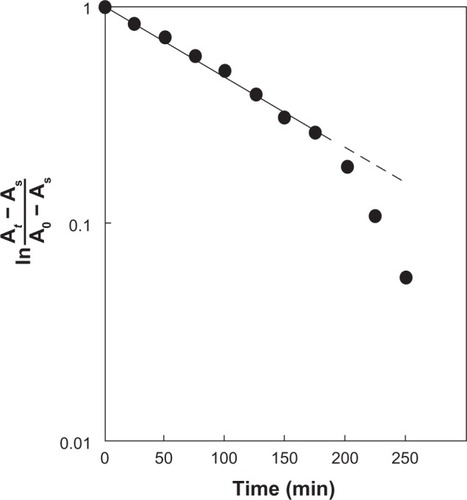
The degradation of MB by photocatalysis is a first order reaction and can be expressed by EquationEquation 1(1) :
where t = time (min), k = a constant (min−1), A0 = the initial absorbance, At = the absorbance at time t, and As = absorbance at the end of the process. The value of ln (At − As)/(A0 − As) was plotted against time during the degradation of MB by photocatalysis (). The major part of the reaction (about 70%) can be described by simple first order kinetics. The rate constant (k) was calculated from the initial straight line. shows a comparison of the k values for various photocatalysts. The k values varied depending on the suspending liquid and on the type of photocatalysts. The photocatalytic activity of ANA in artificial seawater decreased to one third of that in ultrapure water. The k value of STS-01 is 3.5 times larger than that of ANA or AP25. By using the values of k, it was possible to quantitatively evaluate and compare the photocatalytic activities of various fine particles.
Table 1 k values for various photocatalysts
Effect of photocatalysis using various TiO2 fine particles on the physiological activities of E. gracilis
The effect of photocatalysis using various TiO2 fine particles on the physiological activities of E. gracilis was investigated (). The k values of ANA (), AP25 (), and RU () in the E. gracilis cell culture medium were determined on the basis of MB degradation by using the apparatus described earlier. The k values of ANA, AP25, and RU in the medium were 7.1 ± 0.09 × 10−3 [min−1], 5.1 ± 0.07 × 10−3 [min−1], and 0 [min−1], respectively. For the distilled water, the k value for ANA was 7.65 ± 0.15 × 10−3 [min−1], for AP25, 8.05 ± 0.12 × 10−3 [min−1], while for RU, 0 [min−1] as shown in . The k values decreased due to the salts in the medium.
Figure 5 Effect of various photocatalysts on the physiological activities of E. gracilis at 72 h of cultivation. A) Inoculum at 0 h, B) no addition, C) 0.01% (w/v) ANA, D) 0.01% (w/v) AP25, E) 0.01% (w/v) RU. The data are expressed as mean ± SD (n = 3).
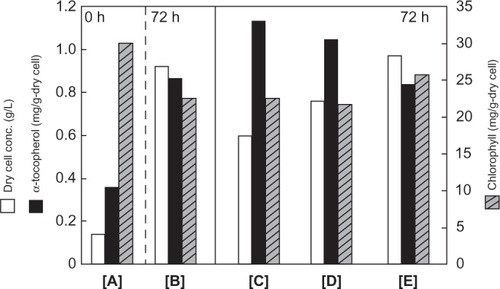
E. gracilis was cultivated for 72 hours in a medium containing RU (). The cell growth, chlorophyll content, and alpha-tocopherol content were the same as those obtained in a medium without RU (). On the other hand when ANA () or AP25 () was added to the medium, the cell growth was suppressed chlorophyll content was the same, while alpha-tocopherol contents of E. gracilis were 1.3 and 1.2 times higher than that of the control, respectively.
Discussion
A spectrophotometer with special cuvette described here makes it possible to monitor continuously and the photocatalytic decomposition of MB by suspended fine particles using small amount of sample solution (2.0 mL) without sampling procedure. It was also possible to use changes in the absorbance to estimate the end of the reaction. In the case of sample containing heterogeneous fine particles in terms of size and specific gravity, it is difficult to evaluate the photocatalytic activity accurately (the results lack reproducibly). The method described here solves the problems, and it is excellent for measuring the photocatalytic activities of various fine particles accurately, reproducibly, and conveniently.
The changes in the absorbance varied depending on the type of photocatalysts and the suspending liquid. AP25 lost its photocatalytic activity, while that of ANA decreased significantly in artificial seawater (). With this apparatus, it was possible to highlight the difference in the effects of seawater on ANA and AP25. It is clear that if ANA and AP25 are released into the sea, the environmental impact of ANA is more than that of AP25. It is known that the presence of inorganic anions such as chloride in water reduces the photocatalytic activity. This can be explained by the competition with dissolved organic carbons for adsorption or reaction.Citation20 It was reported that metal ions, (sodium and magnesium) resulted in enhanced photocatalytic activity at concentrations similar to those in seawater.Citation21 The rate of photooxidative dye decolorization in seawater was slower than that in the NaCl solution even although sodium cation and chlorine anion are the major ions in seawater.Citation21 The reasons for the differences observed between ANA and AP25 in artificial seawater is currently being investigated. By using the values of k (reaction rate constant), it was possible to quantitatively evaluate and compare the photocatalytic activities of various fine particles under various conditions (). In the case of ANA, the photocatalytic activity in artificial seawater decreased to one third of that in distilled water. The photocatalytic activities of TiO2 fine particles differed greatly depending on such factors as the crystalline form, composition, particle size, and surface area.Citation8,Citation13
When ANA and AP25 were added to the medium under illumination by black light, the growth of E. gracilis was suppressed but their alpha-tocopherol contents increased (). The effects of ANA on E. gracilis were more pronounced than those of AP25. This may be due to the fact that the k value of ANA in the E. gracilis cell culture medium was higher than that of AP25. The modified Hutner medium contains many kinds of salts and this resulted in decrease in the k values of ANA and AP25. This is consistent with the results obtained when they were suspended in artificial seawater (). It is well known that various kinds of ROS are produced on TiO2 surface exposed to light rays of wavelengths below 410 nm. Among these, hydroxyl radical is dominant and is known to be responsible for the oxidative stress on living cells.Citation10 The ROS produced by photocatalytic reaction enhanced the alpha-tocopherol production by E. gracilis. This prevents oxidative decomposition of chlorophyll, lipid membranes, and proteins by ROS.Citation22 through scavenging ROS released during oxidative stress.Citation23 It was reported that the antioxidant alpha-tocopherol synthesis was activated to prevent damage by ROS.Citation24 It has also been reported that antioxidant carotenoid biosynthesis is regulated by ROS in some microalgae.Citation25 The increase in alpha-tocopherol content of E. gracilis exposed to TiO2 suggests that the TiO2 fine particle promoted production of alpha-tocopherol and/or selected E. gracilis cells with high content of antioxidant. Thus, TiO2 may be used to enhance production of useful antioxidants.
Quantitative evaluation of photocatalytic activities of fine particles using the apparatus developed in this study is very important, considering the ecotoxicity and environmental impacts of these particles. By knowing the potential risks of these photocatalytic particles, it is possible to make a risk–benefit analysis.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Exploratory Research (nr 20651006) from the Japan Society for the Promotion of Science (JSPS).
Disclosure
The authors report no conflicts of interest in relation to this paper.
References
- NagasawaHIchimuraAIsodaSCarboxylate-passivated silver nanoparticles and their application to sintered interconnection: a replacement for high temperature lead-rich soldersJ Electron Mater20103912331240
- LiuSQTangZYNanoparticle assemblies for biological and chemical sensingJ Mater Chem2010202435
- NarayananRRecent advances in noble metal nanocatalysts for Suzuki and Heck cross-coupling reactionsMolecules2010152124213820428032
- OguraHMaruyamaMMatsubayashiRNanoscale particle therapies for wounds and ulcersNanomedicine2010564165620528458
- MillisALepreAElliottNBhopalSParkinIO’NeillSCharacterisation of the photocatalyst Pilkington Active™: a reference film photocatalyst?J Photochem Photobiol A Chem2004160213224
- EsterkinCRNegroACAlfanoOMCassanoAEAir pollution remediation in a fixed bed photocatalytic reactor coated with TiO2AIChE J20055122982310
- ChoiHStathatosEDionysiouDDSol-gel preparation of mesoporous photocatalytic TiO2films and TiO2/Al2O3composite membranes for environmental applicationsAppl Catal B Environ2006636067
- AdamsLKLyonDYAlvarezPJJComparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensionWater Res2006403527353217011015
- KaidaTKobayashiKAdachiMSuzukiFOptical characteristics of titanium oxide interference film and the film laminated with oxides and their applications for cosmeticsJ Cosmet Sci20045521922015190897
- CaiRKubotaYShuinTInduction of cytotoxicity by photoexcited TiO2particlesCancer Res19925223452348
- OtakiMHirataTOhgakiSAqueous microorganisms inactivation by photocatalytic reactionWater Sci Technol200043115118
- SunadaKWatanabeTHashimotoKStudies on photokilling of bacteria TiO2thin filmJ Photochem Photobiol A Chem2003156227233
- Hund-RinkeKSimonMEcotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnidsEnviron Sci Pollut Res200613225232
- OberdorsterGOberdorsterEOberdorsterJNanotoxicology: an emerging discipline evolving from studies of ultrafine particlesEnviron Health Perspect200511382383916002369
- XuNPShiZFFanYQDongJHShiJHuMZCEffects of particle size of TiO2on photocatalytic degradation of methylene blue in aqueous suspensionsInd Eng Chem Res199938373379
- OgbonnaJCTanakaHCyclic autotrophic/heterotrophic cultivation of photosynthetic cells: a method of achieving continuous cell growth under light/dark cyclesBioresour Technol1998656572
- ShigeokaSOnishiTNakanoYKitaokaSThe contents and subcellular distribution of tocopherols in Euglena gracilisAgric Biol Chem19865010631065
- PorraRJThompsonWAKriedemannPEDetermination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopyBiochim Biophys Acta1989975384394
- Otsuka-Yao-MatsuoSOmataTYoshimuraMPhotocatalytic behavior of cerium titanates, CeTiO4and CeTi2O6and their composite powders with SrTiO3J Alloys Compd2004376262267
- ChenHYZahraaOBouchyMInhibition of the adsorption and photocatalytic degradation of an organic contaminant in an aqueous suspension of TiO2by inorganic ionsJ Photochem Photobiol A Chem19971083744
- MakitaMHarataAPhotocatalytic decolorization of rhodamine B dye as a model of dissolved organic compounds: Influence of dissolved inorganic chloride salts in seawater of the sea of JapanChem Eng Process200847859863
- TaniYTsumuraHScreening for tocopherol-producing microorganisms and alpha-tocopherol production by Euglena gracilis ZAgric Biol Chem198953305312
- Munne-BoschSAlegreLThe function of tocopherols and tocotrienols in plantsCRC Crit Rev Plant Sci2002213157
- FujitaTOgbonnaJCTanakaHAoyagiHEffects of reactive oxygen species on alpha-tocopherol production in mitochondria and chloroplasts of Euglena gracilisJ Appl Phycol200921185191
- KobayashiMKakizonoTNagaiSEnhanced carotenoid biosynthesis by oxidative stress in acetate induced cyst cells of a green unicellular alga, Haematococcus pluvialisAppl Microbiol Biotech199359867873