Abstract
Considering the challenges associated with conventional chemotherapy, targeted and local delivery of chemotherapeutics via nanoparticle (NP) carriers to the lungs is an emerging area of interest. Recent studies and growing clinical application in cancer nanotechnology showed the huge potential of NPs as drug carriers in cancer therapy, including in lung carcinoma for diagnosis, imaging, and theranostics. Researchers have confirmed that nanotechnology-based inhalation chemotherapy is viable and more effective than conventional chemotherapy, with lesser side effects. Recently, many nanocarriers have been investigated, including liposomes, polymeric micelles, polymeric NPs, solid lipid NPs, and inorganic NPs for inhalation treatments of lung cancer. Yet, the toxicity of such nanomaterials to the lungs tissues and further distribution to other organs due to systemic absorption on inhalation delivery is a debatable concern. Here, prospect of NPs-based local lung cancer targeting through inhalation route as well as its associated challenges are discussed.
Introduction
At present, lung cancer causes 23% of total cancer-related mortality worldwide, surpassing the combined mortality caused by breast, colon, and prostate cancer.Citation1–Citation4 Moreover, it is also recognized as the cancer having most intense fatality, with minimal survival rate. Lung cancer is mainly categorized into two forms; namely non-small-cell lung carcinoma (NSCLC) (major and common form that comprises of 85% cases) and small-cell lung carcinoma (SCLC).Citation1,Citation2 NSCLC is further categorized as epidermoid, large cell, bronchoalveolar, adenocarcinoma, and squamous cell carcinoma.Citation1,Citation2,Citation5–Citation7 These NSCLC forms are histologically distinct from each other. It is responsive to the chemotherapy; however, their response differs against a specific therapy. On the other hand, SCLC rarely occurs but shows fast metastasis and aggressive growth, with average survival of merely 4 months if not treated.Citation5 Pathophysiology of SCLC indicates that it commences from neuroendocrine tumors, and therefore it contains neurosecretory vesicles and neurofilaments.Citation6 While, SCLC is very aggressive in nature, its treatment by radiation and chemotherapy has better responsiveness than NSCLC.Citation1,Citation2,Citation6 Irrespective of the different types of lung cancer, the major reason behind the poor survival rate, like most other cases of cancer, is failure of early detection and confirmation only made at the stage IV.Citation8 Long-term survival and improved quality of life for lung cancer patients under conventional cancer therapies is still not fulfilled.Citation9 Most of the chemotherapeutics are available as intravenous (iv) formulations. Moreover, some important chemotherapeutics used against lung cancers are very lipophilic in nature and required higher doses and/or surfactant-based solubilization to improve the systemic drug availability. In addition, oral administration of cancer chemotherapeutics is often limited due to first-pass metabolism.Citation10–Citation12 Systemic drug bioavailability is not the only concern here, as even at higher dose or systemic availability, only limited quantity of drugs is delivered to lung tumor. Due to their nontargeting nature, majority of chemotherapeutics act on normal tissues, leading to adverse effects.Citation12 Local targeting in lung cancer with the opportunity of minimal systemic exposure can play an important role in safer chemotherapy with better patient compliance. In this direction, drug delivery through inhalation route shows the enormous potential for lung cancer targeting. This offers some obvious advantages such as avoidance of first-pass metabolism, fewer systemic side effects, and a needle-free approach that offer better comfort to the subjects.Citation12–Citation14 The progress of pharmaceutical nanotechnology successfully deals with the challenges of drug delivery in cancer chemotherapeutics. Since, nanoparticles (NPs) have extraordinary characteristics like small particle size, large surface area, and the capability of changing their surface properties, they therefore have several advantages over other delivery system.Citation14 Additionally, it has been proven that NP-based drug delivery systems assure passive (size-based targeting due to their size up to ~100 nm) as well active targeting (surface functionalization by targeting ligand) and enhanced therapeutic efficacy of anticancer agents.Citation15 NPs can restrict the biodistribution profile and target the drug to the tumor regions, which increases therapeutic efficiency and reduces nonspecific toxicity of anticancer drugs. Moreover, NPs possess good biocompatibility; many of these materials are biodegradable and have the capability to protect nucleic acids from degradation and deliver the biotherapeutic to tumor cells in vivo.Citation16,Citation17 While significant work has been done in recent years to target lung cancer using different types of NPs, majority of them were administered iv, which remained with limited success and poor site-specific drug availability in lung cancer.Citation18,Citation19
Over the past decade, a new direction in nanotechnology has been raised to focus of targeting to the lungs diseases including cancer. The focus is to combine the nanotechnology-based therapeutic delivery with pulmonary/inhalational route of administration. This strategy has been encouraged due to the possible usefulness of lung as a portal for drug entrance, including peptides and proteins. The lungs are well-organized entrance for drugs to the bloodstream as they have large surface area for absorption (~100 m), with very thin absorption membrane (0.1–0.2 µm). Furthermore, the lungs show comparatively lesser local metabolic activity, and unlike the oral route of drug administration, pulmonary/inhalation route is not vulnerable to first-pass metabolism.Citation19,Citation20 Nanocarriers through inhalational route offer many advantages like; 1) they achieve uniform distribution of drug among the alveoli, 2) better solubilization of the drug, 3) sustained drug release which subsequently decreases dosing frequency, 4) better patient compliance, 5) lesser side effects, and 6) improved drug internalization to the cells.Citation21,Citation22 Therefore, targeted inhalational NP delivery to the lungs is a potential area of research in cancer nanotechnology that catches the attention of many formulation scientists, oncologists, and biomedical researchers. Here, we discuss the challenges in delivery of chemotherapeutics to lung cancer, the significance of applying inhalational NPs in lungs cancer drug targeting, and the concern of toxicity in using this approach.
Challenges in inhalation nanochemotherapy
The recent decade has witnessed huge growth in the development of nanocomposite-based drug delivery systems. The current ongoing research is trying to develop safe, efficient, and feasible nano-based drug delivery for highly sensitive imaging action and better therapeutic applications. So far, nanotechnology-based research has been facing numerous challenges in transforming NPs to nanomedicines to clinical practice. During the synthesis of such carrier systems, complications are seen in the robust designing of an appropriate size particle to carry the drug/gene payload efficiently. Nonuniform size distribution, irregular structure/shape, biocompatibility compliance, undefined surface chemistry, and robust reproducibility are the key developmental concerns. Concerning the disease state, delivery of NPs in lung cancer has faced numerous challenges that include improper physiochemical properties of the particles and biological barriers due to lung anatomy and physiology. During clinical stage, nanomedicine-based systems develop striking hindrances such as immune reaction, higher clearance from circulation, and lower efficiency of targeting. Therefore, comprehensive knowledge about biological behavior of nano drug carriers is crucial to achieve the maximum efficient drug delivery. Optimization of physiochemical parameters is an utmost requisite in determining the particle–particle interaction within the biological atmosphere, agglomeration, adsorption of macromolecules onto NP surface, and their intracellular uptake. NPs (size <200 nm) with spherical shape easily move to tumor vasculature, and this process is being exploited to enhance permeation and retention (EPR) effect.Citation23 Like other cancerous tissues, the occurrence of fine blood vasculature in lungs and poor lymphatic flow facilitates the EPR effect, which enhances the entry of NPs into the tumors. However, NPs with a size less than 50 nm are less likely to be retained in the tumor tissues for extended period of time. Active targeting involves decorating the NP surface with specific ligands to facilitate the interaction with overexpressed receptor on tumor cells. Various methods are exploited for size optimization of NPs; however, they end up with distorted release of encapsulated drug. Therefore, it is a challenge to keep the size up to 200 nm in the production cycle. Surface charge plays a key role in determining the fate of NPs in vivo, and further, their particle interaction and agglomeration are mainly dependent upon the zeta potential of the nanocomposites. Cationic polymers like N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride, dioleoyl phosphatidylethanolamine, etc are sometimes used with engineered NPs for efficient delivery of drug to cancer cells; however, often they cause hemolytic action and raise the issue of safe delivery.Citation24 Another concern is instability of nanocarriers due to accumulation of NPs in physiological environment. Once the aggregates are formed, it is quite hard to separate or redisperse the NPs after forced application. It can also cause drug leaching and lower the drug loading and therapeutic efficacy. Nowadays different functional groups are attached to the NP surface for anchoring of different drugs/biomolecules, but they produce complex systems that are often hard to mimic. Considering the effect of drug and/or NPs on respiratory tract, including the effect on the lungs, toxicity is an important concern in its application. Direct application of some chemotherapeutics (for example: irinotecan, gemcitabine, paclitaxel, and its congeners) itself triggered the inflammatory reaction in excess exposure to pulmonary tract. The toxicity related issue is further discussed in detail in “Toxicity concern with inhalational nanomedicines” section. Local delivery of drug or drug-carrying particles to the lungs required inhalation devices such as pressurized metered dose inhalers, nebulizers, and dry powder inhalers. Often, the nano-sized dosage form is delivered as nebulization of colloidal dispersions or solid form using pressurized metered dose inhalers and dry powder inhalers. Physical instability such as agglomeration and poor dispensability remained a challenge. Moreover, chemical instability related to hydrolysis or decomposition of drug and carrier materials is an additional and equally important issue. Exhalation and rapid clearance related poor deposition of majority of the inhaled particles of size up to 1 µm in the lung is well established. To get rid of this problem, micron-sized powder carriers containing NPs or dispersible NP agglomerates were considered to improve the lungs deposition of inhalational NPs. Altogether, a fair understanding of aerodynamic properties for pulmonary delivery and respiratory tract anatomy/physiology is required, which requires a collaborative effort from pharmacologists, oncologists, and drug delivery scientists.
Inhalational nanomedicine for lung cancer: recent progress
Although the inhalational route of drug delivery is one of the primordial administration routes, it has received much attention in the recent decade only. Approval of human insulin powder of recombinant DNA for inhalational delivery, for example, Exubera® (later withdrawn by Pfizer citing the poor acceptance of patients and clinicians) and Afrezza® (manufactured by MannKind Corporation, Danbury, CT, USA and approved by US Food and Drug Administration in 2014), further encourage the researchers to exploit this route, particularly for local lung cancer chemotherapy using NPs as drug carriers. A number of particulate systems ranging in micro- and nanoscale have been investigated for selective delivery of chemotherapeutics to the lungs through the inhalational route. Here, we discuss these particulate systems (keeping in mind the scope of our study, we focus only on nanotechnology-based formulation) from the available researches. The summary of the investigated inhalational NPs carrying chemotherapeutics for local targeting of lung cancer are presented in .
Table 1 Examples of different nanoparticles investigated as targeted anticancer therapeutic carrier for inhalation treatment in lung cancer
Polymeric nanocarriers
Polymeric micelles
The polymeric micelles represent a potential nanocarrier for efficient delivery of anticancer agents.Citation25,Citation26 A polymeric micelle is principally formed when the hydrophobic part of the block copolymer is driven to the interior, which can encapsulate a poorly soluble drug, whereas the hydrophilic portion of the block copolymer faces outward to form the shell. Currently, numerous modified polymeric micelles carrying anticancer agents are in preclinical and clinical phases of development. Gill et alCitation27 produced polyethylene glycol (PEG)5000–distearoylphosphatidylethanolamine (PEG5000–DSPE) micelles bearing paclitaxel through solvent evaporation technique. Their observations showed that the PEG5000–DSPE micelles administered intratracheally were capable of maintaining maximum paclitaxel concentrations in lungs for long duration. Intratracheal-delivered polymeric micelles containing paclitaxel exhibited highest aggregation of paclitaxel in the lungs, with AUC0–12 (area under the plasma concentration–time curve from 0 to 12 hours) in lungs 45 times greater than iv-administered formulation and 3 times greater than intratracheally administered taxol. Concentration of paclitaxel in other tissues and plasma was found to be considerably low.Citation27 Furthermore, toxicity studies demonstrated that there was no considerable increase in degree of lung injury markers in PEG5000–DSPE-treated group in comparison to the saline-treated group.
Polymeric nanoparticles
Polymeric NPs are extensively explored nowadays for their remarkable potential as a drug delivery system for anticancer compounds. They are prepared either by encapsulation, dissolution, and entrapment of drug in biodegradable polymers or by embedding drug in polymeric matrix. Roa et alCitation9 investigated the therapeutic effect of inhaled doxorubicin (DOX)-loaded NPs in a cancer-bearing mouse model (BALB/c model). Spray and freeze drying methods were used to combine DOX-loaded NPs with inhalable effervescent and noneffervescent carrier particles. Cytotoxicity was determined in human NSCLC cell line NCL-H460. Their study suggested that inhalable DOX NP powders enhanced the survival time of cancer-bearing mice with lesser cardiotoxicity in comparison with iv administration of the same dose of drug. Likewise, Kalantarian et alCitation28 designed 5-fluorouracil (5-FU) NPs by supercritical antisolvent process that permits single-step production of 5-FU NPs. The aerodynamic behavior of the NPs was assessed in vitro and demonstrated that the respiratory fraction can increase up to 21% by the application of the mixture of coarse and fine lactose as a carrier. Furthermore, Tomoda et alCitation29 produced inhalable nanocomposite for the treatment of lung cancer.
They developed the poly(lactic-co-glycolic acid) (PLGA) NPs loaded with cancer chemotherapeutics 6-{[2-(dimethylamino)ethyl]amino}-3-hydroxyl-7H-indeno[2,1-c] quinolin-7-one dihydrochloride in the form of nanocomposite (size 200 nm) to effectively deposit the particles deep within the lung tissue. It was observed that the cytotoxicity of nanocomposite particles against A549 cells was greater than that of free drug. When the nanocomposite particles were administered in rats by inhalation, drug concentration in the lung was much higher than that in plasma. Furthermore, drug concentration in lungs administered by inhalation of NPs was much higher than that after iv administration of free drug. In another study, Godugu et alCitation30 developed NPs for inhalational delivery in lung cancer. The rationale behind their study was to assess the effect of telmisartan (Tel) and losartan (Los) on intratumoral distribution of NPs and anticancer effects in lung cancer. A549 and H1650 NSCLC cells were used to perform in vitro cytotoxic studies by delivering the drugs orthotopically and metastatically to Nu/nu mice. They observed that Tel and Los exhibited significant anticancer effects in orthotopic and metastatic lung tumor models. Inhalational delivery of Tel showed enhanced antifibrotic effect as compared to Los. Tel, by virtue of its dual pharmacophoric nature, could be an ideal candidate for combination therapy to improve the NP’s intratumoral distribution and anticancer effects. Tel-induced antifibrotic effects can be used to improve the efficiency of nanotherapeutics in different types of tumors. Tel, when administered inhalationally at a minimum dose of 1.12 mg/kg, showed significant fibrolysis and increased intratumoral distribution of NPs in lung tumors.Citation30 Poor lung deposition and fast exhalation is a major limitation against lung targeting by inhalational NPs. Recently, attempts have been made to overcome this issue and make use of NPs effectively in targeting lung cancer.Citation31 For example, the most common strategies are using a special aerosolizer that can generate particles as micron-sized liquid droplets, applying nanocomposite microparticles and NPs of size cutoff more than 500 nm. Choi et alCitation31 showed the application of special aerosolizer for better lung deposition of NPs. Most recently, the well-defined lung pharmacokinetics and antitumor efficacy in BALB/c nu/nu mice bearing H226 cell-induced metastatic tumors was ascribed for inhalable TRAIL (apoptosis-inducing ligand) adsorbed NPs of albumin (human serum albumin) coupled with DOX.Citation31 It was found that this inhalable NP showed synergistic cell killing and significantly improved the lung deposition at its center and spread throughout. It remained in the lungs for and gradually released the DOX over 3 days.Citation31 In another study by Azarmi et al,Citation32 they developed DOX-loaded NPs were incorporated as a colloidal drug delivery system into inhalable carrier particles by a spray–freeze–drying method. The cytotoxic effects of free DOX, blank NPs, or DOX-loaded NPs were evaluated on H460 and A549 lung cancer cells. They observed that the drug-loaded NPs carried as dry powders demonstrated a concentration-related increase in in vitro cytotoxicity. This study encourages the strategy of applying NPs in the local treatment of lung cancer as a drug delivery vector.
Polymer–drug conjugates
The hydrophilic polymers conjugated to proteins and anticancer drugs are one of the most extensively explored approaches for the drug delivery, which establishes polymer therapeutics as one of the first classes of anticancer nanomedicine. The prospects of using more sophisticated polymer-based vectors in chemotherapeutics are expanding day by day. Xie et alCitation33 produced inhalational cisplatin–hyaluronan (HA–Pt) combination to treat lung cancer and studied the comparison with conventional cisplatin (cis-diamminedichloroplatinum or CDDP) iv infusion. Cytotoxicity studies were carried out in human lung cancer cell line A549. Their results showed that as compared to conventional CDDP iv infusion, the HA–Pt lung instillation group had not only higher platinum accumulations in the lung tissues and the draining lung surrounding nodes, but also demonstrated a sustained release plasma profile with a reduced peak plasma concentration.
Lipidic nanocarriers
Solid lipid nanoparticles
Recently, solid lipid nanoparticles (SLNs) have gained huge notice for delivery of drugs, particularly poorly water soluble drug candidates. They offer improved properties by combining the benefits of liposomes, NPs, and nanoemulsions. They are usually prepared by high-pressure homogenization or microemulsification technique, where drug is efficiently entrapped in a lipid matrix.Citation34 SLNs overcome the general limitations of polymeric systems by exhibiting low toxicity due to the presence of biodegradable lipid, and an extremely small size which facilitates circumventing of reticuloendothelial system (RES). Videira et alCitation35 carried out the preclinical evaluation of a pulmonary delivered paclitaxel-loaded lipid nanocarrier for studying antitumor effect in lung cancer. They used MXT-B2 cell lines for cytotoxicity assay. It was found that inhalational treatment of paclitaxel-loaded SLNs was more efficient in reducing the number and size of lung metastases in comparison to iv delivery of same drug. Furthermore, Videira et alCitation36 in another study used glyceryl behenate to produce NPs by melt homogenization method, and it was radiolabelled with 99mTc using the lipophilic chelator d,l-hexamethylpropyleneamine oxime. Their results revealed an important role of the lymphatic pathway in the uptake of inhaled nanoparticulates. Their study suggested the possibility of pulmonary administration of radiolabelled SLN as a lymphoscintigraphic agent and direct administration of cytotoxic drugs to target lung cancer that may metastasize via lymphatic drainage. Similarly, Hu et alCitation37 developed an inhalational formulation of epirubicin (EPI)-loaded SLNs (EPI-SLNs) as an inhalable nanomedicine for lung cancer therapy. Cytotoxicity study was carried out in A549 alveolar epithelial cells, and blank SLNs were found to be nontoxic, while higher cytotoxicity of EPI-SLNs was observed in comparison to that of EPI solution. In vitro deposition assessment suggested that SLNs remained stable during nebulization with better respirable fraction in comparison to EPI solutions. In vivo pharmacokinetic analysis demonstrated that the drug concentration attained by inhalation of EPI-SLNs was found to be greater than the drug concentration in plasma. Furthermore, the drug concentration in lungs after inhalation of EPI-SLNs was much higher than that after administration of EPI solution.
Nanostructured lipid carriers
Nanostructured lipid carriers (NLCs) have gained expanding scientific and commercial attention in the last few years as alternate carriers for pharmaceutical delivery, particularly cancer chemotherapeutics. NLCs provide the opportunity to overcome the limitations associated with cancer chemotherapeutics, like poor solubility, specificity and steadiness, normal tissue toxicity, as well as targeting of drug against drug-resistant cases. Recently, NLCs of 9-bromo-noscapine (9-Br-Nos-NLCs) (9-Br-Nos; known to have alteration activity of microtubulin polymerization), a poorly water soluble molecule, was developed as a prospect for NPs-based inhalation treatments against NSCLC.Citation38 The work ascribed the detailed study on cytotoxicity in lung cancer cells (A549), lung pharmacokinetics, and biodistribution. For better aerodynamic/lungs deposition, 9-Br-Nos-NLCs was mixed with spray dried lactose.Citation38 The nanocarrier illustrated enhanced cytotoxicity, apoptosis, and cellular uptake of 9-Br-Nos. Active endocytosis and passive diffusion are considered as main mechanism for improved cellular uptake.Citation38 It has been found that the drug exposure was improved with significant enhancement in half-life of the drug in lungs (~3.75 times higher T1/2 than 9-Br-Nos powder) after inhalational application.Citation38 Taratula et al,Citation39 in their investigation, synthesized multifunctional NLCs and evaluated them for effective delivery of an anticancer drug and siRNA straight into the lungs by inhalation. It demonstrated an increase in antitumor activity compared to iv treatment. It has been found that NLCs accumulated predominately in the lungs and, more specifically, in lung cancer cells, leaving healthy organs and nontumorous lung cells intact. It was also observed that it increases the antitumor effectiveness as compared to free drug or similar systems delivered iv. Similarly, Patlolla et alCitation40 designed inhalable celecoxib-encapsulated NLC (Cxb-NLC) to treat lung cancer. It was found that Cxb-NLC exhibited dose- and time-dependent cytotoxicity against A549 cells. Nebulization of Cxb-NLC demonstrated a 4-fold higher AUCt/D in lung tissues compared to the Cxb-Soln in BALB/c mice.Citation40 The formulation was capable of releasing the Cxb in controlled manner for extended duration of time, and the aerodynamic diameter was found to be within the nebulization limits. Aerosolization of Cxb-NLC improved the Cxb pulmonary bioavailability in comparison to solution, and it may perhaps be a good nanomedicine for lung cancer having better patient compliance with minimal dosing intervals.
Dendrimers
Dendrimers represent a nanodrug carrier that link molecular chemistry to polymer science.Citation41 They have different structures depicted by a core, an inner dendritic constitution of extremely branched polymers, and an exterior of multivalent functional groups. The functional group on their surface gets incorporated with charged polar compounds by electrostatic interaction, whereas the uncharged, nonpolar compounds get embedded in the hydrophobic interior. Since they possess both hydrophobic and hydrophilic parts in their structures, different types of drug molecules could be productively entrapped within dendrimers based on their solubility. Kaminskas et alCitation42 produced DOX- conjugated dendrimer for pulmonary administration for the treatment of lung cancer. They explored the utility of a 56 kDa PEGylated polylysine dendrimer, conjugated to DOX, to promote the controlled and prolonged exposure of cytotoxic drug (DOX) to the lung cancer. The anticancer efficacy of inhaled DOX-conjugated dendrimer (D-DOX) for lung cancer was evaluated in the syngeneic MAT 13762 IIIB rat model of lung metastatic cancer. Intratracheal delivery of the D-DOX twice a week led to a more than 95% decrease in lung tumor burden after 2 weeks, while iv delivery of DOX solution decreased lung tumor burden by only 30%–50%.Citation42
Inorganic nanocarriers
Magnetic nanoparticles
The magnetic NPs (MNPs) are prepared by either entrapping drug into magnetic micro/nanosphere or embedding as a magnetically-active disc. In magnetic targeting, the liberation of drug in blood circulation is controlled by applying strong magnetic field. Different magnetic materials with a variety of magnetic properties are available, such as magnetite, iron, nickel, cobalt, neodymium-iron-boron, and samarium-cobalt. Furthermore, some liquids can also be strongly magnetized in magnetic field known as ferrofluids. Ferrofluids are principally colloidal suspensions of nanodimension ferromagnetic particles. Currently, commonly used MNPs are made of iron oxide due to its biodegradability, biocompatibility, superparamagnetic effects, and capability to act as a contrast agent in magnetic resonance imaging. These NPs on dissociation convert into ferritin and/or hemosiderin following their internalization within the lysosomes of RES cells.Citation43 As theranostics in lung cancer therapy. (Fe3O4) NPs used for the localized hyperthermia induced cancer cells, killing, diagnosis, and targeted delivery of chemotherapeutics to the cancer region under the influence of magnetic field.Citation44 Verma et alCitation45 produced magnetic core–shell NPs by surface coating of Fe3O4 MNPs, with a polymer PLGA intended for nebulized drug delivery for the treatment of lung cancer. The polymeric shell of these framed NPs contains a potential anticancer drug quercetin. A549 lung carcinoma cells were utilized for cytotoxicity study. They observed that the mean particle size of the developed MNPs and PLGA-MNPs was 9.6 and 53.2 nm, respectively. There was no cytotoxicity found when ~100 µg/mL PLGA-MNP was administered to the cultured human lung epithelial cells. Therefore, the MNP-based nanocarrier system was considered as biocompatible. Moreover, the drug-loaded PLGA-MNPs considerably decreased the number of viable A549 cells.
Targeting inhalational nanomedicines to the lung cancer
The pulmonary delivery of nanomedicines possesses the potential to enhance and maintain local drug concentration to treat lung cancer. If the site of action is targeted, the required dose is decreased, which eventually decreases the systemic side effects. Furthermore, variability in oral dosing due to alteration in gastric conditions can be avoided. Generally, it carries several forms of molecular recognition, which facilitates a drug or drug formulation to interact specifically with a disease-causing molecular phenomenon, and/or notably recognizes and binds to target tissues. It targets receptors by utilizing target-specific natural or artificial receptor ligands or target-specific antibodies attached with drugs or drug formulations. One of the examples of targeted drugs is tumor-specific antibody Herceptin® (a clinically approved monoclonal antibody manufactured by Genentech, Inc., San Francisco, CA, USA), which is used for the treatment of breast cancer. Passive targeting refers to the preferred accumulation of drug or drug formulation in a specific tissue depending on its biophysical properties. For instance, the differential accumulation of particles or aerosol droplets in various lung regions is based on their sizes, ie, EPR effect. Drugs can be formulated as solutes or particles in aerosol droplets of appropriate size and used in drug delivery.Citation46 The term active targeting is frequently used in place of molecular or biological targeting; however, it can precisely be defined as any active process applied on a formulation which will cause localized drug effect.Citation47 Thus, it also includes methods of physical targeting where physical force is applied on drug formulations and/or target cells to mediate localized delivery. Examples include electroporation, sonoporation, and magnetic targeting. Aerosol delivery of drugs by inhalation is the easiest way to target cancerous tissues of lung.Citation48 Taratula et alCitation49 produced mesoporous silica nanoparticles-based drug delivery system to treat lung cancer by inhalation method. This system was effective in delivering anticancer drugs in cancer cells. Anticancer drugs were conjugated with two kinds of siRNA targeted to MRP1 and BCL2 mRNA for inhibition of pump and nonpump cellular resistance in NSCLC, respectively. A549 human lung adenocarcinoma epithelial cells (A549 cell line) were used for in vivo body distribution study. They observed that this system was capable of delivering various anticancer drugs into lung cancer cells in conjugation with siRNA for concurrent initiation of cell death and inhibition of pump and nonpump cellular drug resistance. The drugs and siRNA transported by the targeted mesoporous silica nanoparticle-based drug delivery system preserve their specific activity. Moreover, simultaneous induction of cell death by anticancer drugs and suppression of pump and nonpump resistance by siRNA substantially enhances the drugs’ cytotoxicity. Similarly, Okamoto et alCitation50 produced chitosan–interferon-β gene complex powder to treat lung metastasis in mice by inhalation method. Chitosan was used as a nonviral carrier and mannitol, as a dry powder carrier. CT26 cells were used as the cell line for their study. The expression of a luciferase expression plasmid driven by the cytomegalovirus promoter (pCMV-Luc) and plasmid DNA, encoding farnesylated enhanced green fluorescent protein (pEGFP-F), suggested that the genes expressed in both normal and tumorous tissues and the intratracheal powder resulted in higher expression than the iv or intratracheal solution. The average survival time was prolonged by the intratracheal powder of pCMV–murine interferon-β (Muβ) administered at a dose of 1 µg compared to the untreated control. Furthermore, Tseng et alCitation51 produced inhalational gelatin NPs by combination of biotinylated epithelial growth factor (bEGF) for lung cancer treatment. Gelatin nanoparticles (GPs) were used as native carriers, which were implanted with NeutrAvidinFITC on the particle’s surface (GP–Av). Subsequently, the bEGF molecules were combined with NeutrAvidinFITC, configuring a core–shell-like structure (GP–Av–bEGF). Since A549 have large quantities of EGF receptor, it was therefore found in in vitro cell culture test that GP–Av–bEGF showed greater entrance in adenocarcinoma cells (A549) than that in normal lung cells (HFL1) and lung squamous cells (H520). GP–Av–bEGF NPs were administered by inhalation to the lung of SCID (severe combined immunodeficiency) mice and showed that cancerous lung had greater fluoresced agglomeration. This study demonstrated that uptake of GP–Av–bEGF by A549 cells is time and dose dependent. In his another study, Tseng et alCitation12 modified GPs with NeutrAvid-inFITC-bEGF and designed EGF receptor-seeking NPs (GP–Av–bEGF). Aerosol droplets of the GP–Av–bEGF were administered by nebulizer to a mouse model (BALB/cAnN. Cg-Foxn1nu/CrlNarl) for lung cancer. The cell line used was A549. They observed that the GP–Av–bEGF was effectively bound to the EGFR-overexpressing cells in the lung cancer mouse model. Furthermore, aerosol droplets having appropriate mass median aerodynamic diameter produced by these NPs were accumulated in the lower respiratory tract, and jet nebulization does not alter the targeting functions of EGF. It was found that the GP–Av–bEGF was mainly deposited in the cancerous lung tissue. They also demonstrated that the GPs delivered by inhalation do not cause lung inflammation and are thus safe for use. Thus, it may prove to be a valuable drug carrier when delivered by simple aerosol method to treat lung cancer. Tseng et alCitation52 further developed biotinylated-EGF-modified gelatin NPs carrier to enhance cisplatin accumulation in cancerous lungs through inhalation. They used GPs as carriers of cisplatin (CDDP), with anticipated improved therapeutic effect and reduced side effects. CDDP integrated with GPs (GP–Pt) were combined with bEGF to produce GP–Pt–bEGF having anticancer activity. An in vitro anticancer study showed that the GP–Pt–bEGF was more effective than free CDDP or GP–Pt, owing to its quick onset of action on the cell cycle and a lower half maximal inhibitory concentration (IC50) for the inhibition of A549 cell growth. The in vivo anticancer study demonstrated that GP–Pt–bEGF had better antitumor activity and less toxicity as compared to free CDDP and GP–Pt in a subcutaneous model. Inhalational formulations of GP–Pt–bEGF administered to mice with lung cancer through aerosol delivery showed that it can target the EGFR-overexpressing cells to get high dosage in the cancerous lungs and decreases the nephrotoxicity of cisplatin. The novel approach of nano-magnetic drug targeting is made feasible with aerosols that composed of magnetically responsive NPs along with anticancer drug to specific regions of the lung. This strategy is quite encouraging in accumulating effective doses of drugs in cancerous sites of lungs and thus decreasing the toxic effects. Dames et alCitation53 called their compositions as “Nano-magnetosols”, for magnetic drug targeting through the airways. These may be easily produced with state-of-the-art nebulizers which are used clinically and have an appropriate amount of iron oxide NPs. These make them susceptible to magnetic field control. This newer strategy is interesting as it overcomes the normal accumulation process of inhaled aerosol droplets in the lungs, which only permits targeting of the central airways or lung periphery but not local regions of the lungs. Sadhukha et al,Citation54 in their investigation, produced MNPs for targeted hyperthermia in lung cancer via inhalation therapy. They assessed the efficiency of tumor-targeted super paramagnetic iron oxide NPs (SPIONs) for magnetic hyperthermia in lung cancer. EGFR-targeted, inhalable SPIONs were synthesized and characterized for targeting lung tumor cells as well as for magnetic hyperthermia- mediated antitumor efficacy in a mouse orthotopic model of NSCLC. For this study, cell lines used were A549 and A549-luc (luciferase-transfected A549). Their results show that EGFR targeting enhances tumor retention of SPIONs. Moreover, it was found that the treatment of hyperthermia by targeted SPIONs causes considerable inhibition in lung tumor growth in vivo. Their work demonstrated the potential for developing an effective anticancer treatment modality for the treatment of NSCLC based on targeted magnetic hyperthermia. Similarly, Shim et alCitation55 produced ethylphosphocholine-based nanolipoplexes for inhalational administration of anticancer siRNA for the treatment of lung cancer. Cationic dioleoyl-sn-glycero- 3-ethylphosphocholine and cholesterol (ECL)-based nanoliposomes illustrated the maximum pulmonary cellular delivery in vivo and minimum cytotoxicity in vitro. The delivery efficacy of fluorescent siRNA in ECL nanoliposomes was 26.2 times greater than that of naked siRNA in vivo. When treated with Mcl1 (myeloid cell leukemia sequence 1)-specific siRNA (siMcl1) by means of ECL nanolipoplexes, it decreased the target expression in B16F10 cell lines, while the control luciferase-specific siGL2 in ECL nanolipoplexes did not. It was found that intratracheal delivery of siMcl1 in ECL nanolipoplexes silenced Mcl1 mRNA and protein levels in lung cells in metastatic lung cancer mouse models induced by B16F10 or Lewis lung carcinoma cells. Furthermore, reduced formation of melanoma tumor nodules was observed in the lung.
Toxicity concern with inhalational nanomedicines
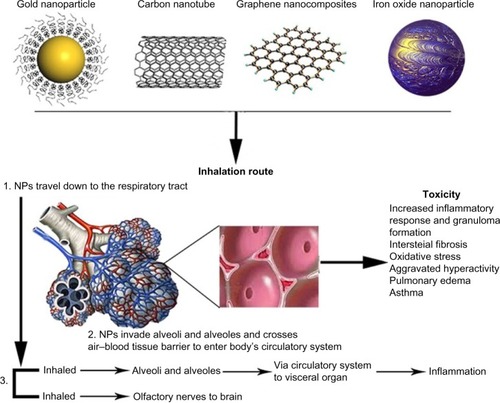
In view of the particulate drug carriers (nano or micro sized) as inhaled dispersed system, local response as increased macrophages usually happen due to the deposition of poor or insoluble nature of the carrier. It is often considered as a nonalarming physiological response. However, prolonged exposure of such particles enhances the deposition (which is an additive if the carriers are nonbiodegradable) that may lead to further intense inflammatory responses such as irritation, cellular injury, edema, phagocytosis impairment, and breakdown in defense mechanisms. Oxidative stress and inflammation-mediated functional disturbances are major toxicity caused by inhalational NPs. Different in vivo studies demonstrated inflammatory effects of NPs as temporary reaction and evaluated whether the degree of the inflammatory reaction is linked to the exposure dose of NPs or not. Actually, the inflammation in lungs was induced by inhalationCitation56,Citation57 or instillation exposuresCitation58–Citation60 of different kinds of nanomaterials. These investigations exposed local conquest of leukocytes, enhanced numbers of inflammatory cells in bronchoalveolar lavage fluid, liberated of LDH, and enhanced cytokine production. Furthermore, characteristic granulomatous responses were observed following pulmonary exposures of carbon nanotubes.Citation61–Citation63 Inhalation NPs accumulate in all parts of the respiratory tract, although bigger particles might be extracted out in the upper airways, while smaller ones spread to the distal airways.Citation64–Citation66 Considerable quantities of specific particle size ranges can accumulate in each area, for instance, 90% of NPs of 1 nm diameter accumulate in the nasopharyngeal area, while only 10% of these NPs accumulate in the tracheobronchial area, and nearly nothing reaches the alveolar area. On the contrary, 15% of NPs of 20 nm diameter accumulate in the nasopharyngeal area, 15% in the tracheobronchial area, and approximately 50% in the alveolar area.Citation66 The deposition pattern of the particulate matter in lung is affected by disease state. Any kind of particles, including NPs, deposited significantly more in the asthmatic lungs compared to the normal one. Higher deposition may trigger local inflammation and synergistically intensify the preexisting inflammation of disease in case of asthma and chronic obstructive pulmonary disease. In addition to these, irrespective of biocompatibility, the insoluble nondegradable/slow degradable material-based particles may lead to pulmonary inflammation to some extent on alveoli deposition. It is now established that the use of chemicals which are endogenous to pulmonary area (such as DPPC) in the formulation significantly reduced the toxicities. Migration of inhaled NPs to the systemic circulation and diffusion to other organs is an important concern of the toxicity (exemplified in ). Once the NPs are absorbed through the lung epithelium, they can enter the blood and lymph and spread to the cells in the bone marrow, lymph nodes, spleen, heart, and to the central nervous system and ganglia after translocation ().Citation65–Citation69 Ultrafine particles are labeled as more toxic compared to larger particles with similar chemical composition, owing to their huge surface area, which causes cytotoxicity, allergic reaction, or inflammation.Citation67,Citation70–Citation72 Apart from epidemiological and controlled clinical studies, the outcomes of NPs in the respiratory tract are also investigated through inhalation in rodents and in vitro cell culture systems.Citation67 Ultrafine particles cause gentle pulmonary inflammatory reactions in rodents and have consequences on extrapulmonary organs.Citation64,Citation67,Citation70 Shape and structure of NPs can also influence inhalational toxicity.Citation67,Citation70,Citation73 For instance, carbon nanotubes have different pulmonary effects than carbon black and graphite that are bigger structures of same chemical composition.Citation74,Citation75 There are fewer epidemiological studies on human exposures and therefore lung cancer threat cannot directly be linked with engineered nanomaterials. The methods causing NP carcinogenicity are unidentified and may include primary genotoxic affront or secondary genotoxic reaction owing to particle-induced inflammation.Citation76 This discussion enlightens the need of extensive studies in the nanotoxicity to the lungs to identify the optimal particle characteristics complimenting the pulmonary tract, systemic/nonpulmonary biodistribution and its effect on vital organs, robust technique to analysis, and quantifying the particles concentration in the pulmonary compartments.
Figure 1 Organs exposure and toxicity associated with inhalational NPs.
Abbreviation: NP, nanoparticle.
Conclusion
Chemotherapy using nanotechnology-based carriers through the inhalational route of administration is worthy and embodies a novel upcoming targeted drug delivery “inhalational nanomedicine”. It offers an effective and safer mean of lung cancer theranostics. To further strengthen this novel strategy, extensive studies at the preclinical level are required to develop a NP design with tumor specified targeting ability in lung after local delivery that has minimal effect on healthy lungs tissues and nonsignificant systemic/other organ toxicity. However, in our opinion, lung cancer being terminal, increase of life span by 1–2 years would be highly beneficial. Particulate matter-associated fibrosis might be a result of long (10 years) exposure. Hence, the risk–benefit ratio of the nanoparticles is undisputed, if they are cancer therapeutics.
Disclosure
The authors report no conflicts of interest in this work.
References
- RamalingamSSOwonikokoTKKhuriFRLung cancer: new biological insights and recent therapeutic advancesCA Cancer J Clin20116129111221303969
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- American Lung AssociationTrends in Lung Cancer Morbidity and Mortality. Epidemiology and Statistics Unit, Research and Scientific Affairs Available from: http://www.lung.org/lung-disease/lung-cancer/learning-more-about-lung-cancer/understanding-lung-cancer/Accessed February 28, 2013
- FerlayJSoerjomataramIErvikMGLOBOCAN 2012 v1.0. Cancer Incidence and Mortality Worldwide: IARC Cancer Base Number 11 [Internet]Lyon, FranceInternational Agency for Research on Cancer2013 Available from: http://globocan.iarc.frAccessed December 13, 2013
- Cochrane Database of systematic reviewsChemotherapy can improve survival rates for nonsmall cell lung cancer Available from: http://summaries.cochrane.org/CD002139/chemotherapy-can-improve-survival-rates-for-non-small-cell-lung-cancerAccessed February 28, 2013
- LongoDLApproach to the patient with cancerFauciASBraunwaldEIssel-bacherKJHarrison’s Principles of Internal Medicine18th edNew York, NYMcGraw Hill2011493499
- KomakiRCoxJDMoss Radiation Oncology: Rationale, Technique, ResultsSt Louis, MOMosby-Year Book1994
- National Canscer InstituteSEER Stat Fact Sheet: Lung and Bronchus 2002–2008 Available from: http://seer.cancer.gov/statfacts/html/lungb.htmlAccessed December 19, 2012
- RoaWHAzarmiSAl-HallakMHInhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse modelJ Control Release2011150495521059378
- LuJLiongMZinkJITamanoiFMesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugsSmall2007381341134617566138
- KumarASahooSKPadheeKReview on solubility enhancement techniques for hydrophobic drugsInt J Comprehensive Pharmacy20113317
- TsengCLWuSYWangWHTargeting efficiency and biodistribution of biotinylated-EGF-conjugated gelatin nanoparticles administered via aerosol delivery in nude mice with lung cancerBiomaterials2008293014302218436301
- SungJCPulliamBLEdwardsDANanoparticles for drug delivery to the lungsTrends Biotechnol20072556357017997181
- AzarmiSRoaWHLobenbergRTargeted delivery of nanoparticles for the treatment of lung diseasesAdv Drug Deliv Rev20086086387518308418
- AkhterSAminSAhmadJNanotechnology to combat multidrug resistance in cancerResistance to Targeted ABC Transporters in CancerSwitzerlandSpringer International Publishing2015245272
- AkhterSAhmadIAhmadMZNanomedicines as cancer therapeutics: current statusCurr Cancer Drug Targets201313436237823517593
- RameshRNanoparticle-mediated gene delivery to the lungMethods Mol Biol200843430133118679632
- YuanDLvYYaoYEfficacy and safety of Abraxane in treatment of progressive and recurrent non-small cell lung cancer patients: a retrospective clinical studyThorac Cancer201234341347
- PilcerGAmighiKFormulation strategy and use of excipients in pulmonary drug deliveryInt J Pharm201039211920223286
- ZhangLJXingBWuJXuBFangXLBiodistribution in mice and severity of damage in rat lungs following pulmonary delivery of 9-nitrocamptothecin liposomesPulm Pharmacol Ther20082123924617561423
- MansourHMRheeYSWuXNanomedicine in pulmonary deliveryInt J Nanomedicine2009429931920054434
- AlipourSMontaseriHTafaghodiMPreparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary deliveryColloids Surf B Biointerfaces20108152152920732796
- MaedaHThe enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targetingAdv Enzyme Regul20014118920711384745
- SchreierHGagneLBockTPhysicochemical properties and in vitro toxicity of cationic liposome cDNA complexesPharm Acta Helv19977242152239372644
- KataokaKMatsumotoTYokoyamaMDoxorubicin-loaded poly(ethylene glycol)-poly(beta-benzyl-l-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significanceJ Control Release20006414315310640653
- KwonGNaitoMYokoyamaMOkanoTSakuraiYKataokaKBlock copolymer micelles for drug delivery: loading and release of doxorubicinJ Control Release199748195201
- GillKKNazzalSKaddoumiAPaclitaxel loaded PEG5000–DSPE micelles as pulmonary delivery platform: formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluationEur J Pharm Biopharm20117927628421575719
- KalantarianPNajafabadiARHaririanIPreparation of 5-fluorouracil nanoparticles by supercritical antisolvents for pulmonary deliveryInt J Nanomedicine2010576377021042422
- TomodaKOhkoshiaTHirotaaKPreparation and properties of inhalable nanocomposite particles for treatment of lung cancerColloids Surf B Biointerfaces20097117718219264458
- GoduguCPatelARDoddapaneniRMarepallySJacksonTSinghMInhalation delivery of Telmisartan enhances intratumoral distribution of nanoparticles in lung cancer modelsJ Control Release2013172869523838154
- ChoiSHByeonHJChoiJSInhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancerJ Control Release201519719920725445703
- AzarmiSTaoXChenHFormulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particlesInt J Pharm200631915516116713150
- XieYAillonKLCaiSPulmonary delivery of cisplatin–hyaluronan conjugates via endotracheal instillation for the treatment of lung cancerInt J Pharm201039215616320363303
- SubediRKKangKWChoiHKPreparation and characterization of solid lipid nanoparticles loaded with doxorubicinEur J Pharm Sci20093750851319406231
- VideiraMAlmeidaAJFabraAPreclinical evaluation of a pulmonary delivered paclitaxel loaded lipid nanocarrier antitumor effectNanomedicine201281208121522206945
- VideiraMABotelhoMFSantosACGouveiaLFDeLimaJJAlmeidaAJLymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticlesJ Drug Target20021060761312683665
- HuLDJiaYDingWPreparation and characterization of solid lipid nanoparticles loaded with epirubicin for pulmonary deliveryPharmazie20106558558720824958
- JyotiKKaurKPandeyRSJainUKChandraRMadanJInhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent: in vitro and in vivo studiesJ Colloid Interface Sci201544521923025622047
- TaratulaOKuzmovAShahMGarbuzenkoOBMinkoTNanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNAJ Control Release2013171334935723648833
- PatlollaRRChouguleMPatelARJacksonTTataPNSinghMFormulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriersJ Control Release201014423324120153385
- KhanOFZaiaEWJhunjhunwalaSDendrimer-inspired nano-materials for the in vivo delivery of siRNA to lung vasculatureNano Lett20151553008301625789998
- KaminskasLMMcLeodVMRyanGMPulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapyJ Control Release2014183182624637466
- PankhurstQAConnollyJJonesSKApplications of magnetic nanoparticles in biomedicineJ Phys D Appl Phys200336R167
- AkhterSAhmadZSinghMCancer targeted metallic nanoparticle: targeting overview, recent advancement and toxicity concernCurr Pharm Des2011171834185021568874
- VermaNKStauntonKCSattiAMagnetic core-shell nanoparticles for drug delivery by nebulizationJ Nanobiotechnology201311123343139
- HeyderJDeposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug deliveryProc Am Thorac Soc2004131532016113452
- PlankCSchererFRudolphCLocalized nucleic acid delivery: a discussion of selected methodsSchleefMDNA PharmaceuticalsWeinheim, GermanyWiley-VCH Verlag GmbH & Co KGaA200555116
- PattonJSFishburnCSWeersJGThe lungs as a portal of entry for systemic drug deliveryProc Am Thorac Soc20041433834416113455
- TaratulaOGarbuzenkoOBChenAMMinkoTInnovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNAJ Drug Target2011191090091421981718
- OkamotoHShirakiKYasudaRDanjoKWatanabeYChitosan–interferon-β gene complex powder for inhalation treatment of lung metastasis in miceJ Control Release201115018719521185340
- TsengCLWangTWDongGCDevelopment of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targetingBiomaterials2007283996400517570484
- TsengCLSuWYYenKCYangKC,LinFHThe use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalationBiomaterials2009303476348519345990
- DamesPGleichBFlemmerATargeted delivery of magnetic aerosol droplets to the lungNat Nanotechnol20072849549918654347
- SadhukhaTWiedmannTSPanyamJInhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapyBiomaterials201334215163517123591395
- ShimGChoiHWLeeSEnhanced intrapulmonary delivery of anticancer siRNA for lung cancer therapy using cationic ethylphosphocholine-based nanolipoplexesMol Ther201321481682423380818
- BermudezEMangumJBWongBAPulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particlesToxicol Sci200477234735714600271
- ElderAGeleinRFinkelsteinJNDriscollKEHarkemaJOberdorsterGEffects of subchronically inhaled carbon black in three species. I. Retention kinetics, lung inflammation, and histopathologyToxicol Sci200588261462916177241
- WarheitDBWebbTRReedKLPulmonary toxicity screening studies in male rats with TiO2 particulates substantially encapsulated with pyrogenically deposited, amorphous silicaPart Fibre Toxicol20063316438714
- WarheitDBWebbTRReedKLFrerichsSSayesCMPulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface propertiesToxicology200723019010417196727
- WarheitDBWebbTRSayesCMColvinVLReedKLPulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface areaToxicol Sci200691122723616495353
- LamCWJamesJTMcCluskeyRHunterRLPulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillationToxicol Sci200477112613414514958
- MercerRRScabilloniJWangLAlteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse modelAm J Physiol Lung Cell Mol Physiol200829418797
- WarheitDBLaurenceBRReedKLRoachDHReynoldsGAWebbTRComparative pulmonary toxicity assessment of single-wall carbon nanotubes in ratsToxicol Sci200477111712514514968
- CurtisJGreenbergMKesterJPhillipsSKriegerGNanotechnology and nanotoxicology: a primer for cliniciansToxicol Sci2006254245260
- HagensWIOomenAGde JongWHCasseeFRSipsAJWhat do we (need to) know about the kinetic properties of nanoparticles in the body?Regul Toxicol Pharmacol200749321722917868963
- MoghimiSMHunterACMurrayJCNanomedicine: current status and future prospectsFASEB J200519331133015746175
- OberdorsterGOberdorsterEOberdorsterJNanotoxicology: an emerging discipline evolving from studies of ultrafine particlesEnviron Health Perspect2005113782383916002369
- MedinaCSantos-MartinezMJRadomskiACorriganOIRadomskiMWNanoparticles: pharmacological and toxicological significanceBr J Pharmacol2007150555255817245366
- OberdorsterGSharpZAtudoreiVTranslocation of inhaled ultrafine particles to the brainInhal Toxicol2004166–743744515204759
- LanoneSBoczkowskiJBiomedical applications and potential health risks of nanomaterials: molecular mechanismsCurr Mol Med20066665166317022735
- BadeaIWettigSVerrallRFoldvariMTopical non-invasive gene delivery using gemini nanoparticles in interferon-gamma-deficient miceEur J Pharm Biopharm200765341442217292593
- FernandezLSuaLFCamargoRBasanteMGutierrezOMunozJEvaluation of the pulmonary inflammatory response in murine biomodels exposed to modified titanium oxide (tio2-Modified)Biomedical Research2015123
- GarnettMCKallinteriPNanomedicines and nanotoxicology: some physiological principlesOccup Med (Lond)200656530731116868128
- LacerdaLBiancoAPratoMKostarelosKCarbon nanotubes as nanomedicines: from toxicology to pharmacologyAdv Drug Deliv Rev200658141460147017113677
- WitzmannFAMonteiro-RiviereNAMulti-walled carbon nanotube exposure alters protein expression in human keratinocytesNanomedicine20062315816817292138
- SchinsRPKnaapenAMGenotoxicity of poorly soluble particlesInhal Toxicol200719118919817886067
