Abstract
Radiation therapy is an effective cancer treatment option in conjunction with chemotherapy and surgery. Emerging individualized internal and systemic radiation treatment promises significant improvement in efficacy and reduction of normal tissue damage; however, it requires cancer cell targeting platforms for efficient delivery of radiation sources. With recent advances in nanoscience and nanotechnology, there is great interest in developing nanomaterials as multifunctional carriers to deliver therapeutic radioisotopes for tumor targeted radiation therapy, to monitor their delivery and tumor response to the treatment. This paper provides an overview on developing nanoparticles for carrying and delivering therapeutic radioisotopes for systemic radiation treatment. Topics discussed in the review include: selecting nanoparticles and radiotherapy isotopes, strategies for targeting nanoparticles to cancers, together with challenges and potential solutions for the in vivo delivery of nanoparticles. Some examples of using nanoparticle platforms for the delivery of therapeutic radioisotopes in preclinical studies of cancer treatment are also presented.
Introduction
Cancer is one of the leading causes of death worldwide. In 2008, a total of 1,437,180 new cancer cases and 565,650 cancer deaths were estimated in the United States alone.Citation1 Despite advances in our understanding of tumor biology, cancer biomarkers, surgical procedures, radio- and chemotherapy, the overall survival rate from cancer has not improved significantly in the past two decades.Citation1 Early detection, pathological characterization, and individualized treatments are recognized as important aspects for improving the survival of cancer patients. Many novel approaches, such as imaging for the early detection of molecular events in tumors, comprehensive and personalized treatments, and targeted delivery of therapeutic agents to tumor sites, have been developed by various research groups; and some of these are already in clinical trials or applications for cancer patients. Radiation therapy, in conjunction with chemotherapy and surgery, is an effective cancer treatment option, especially for radiation-sensitive tumors. Radiation therapy utilizes high dose ionizing radiation to kill cancer cells and prevent progression and recurrence of the tumor. In current clinical oncology practices, half of all cancer patients will be treated with radiation therapy either alone or in combination with other treatments.
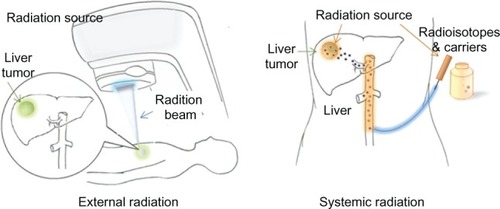
Traditionally, radiation therapies fall into one of three categories: external radiation, internal radiation and systemic radiation therapy. External radiation therapy delivers high-energy x-rays or electron or proton beams to a tumor from outside the body, often under imaging guidance. Internal radiation therapy (also called brachytherapy) places radiation sources within or near the tumor using minimally invasive procedures. Systemic radiation therapy delivers soluble radioactive substances, either by ingestion, catheter infusion, or intravenous administration of tumor-targeting carriers, such as antibodies or biocompatible materials, which carry selected radioisotopes. Although systemic radiation offers desirable advantages of improved efficacy as well as potentially reducing radiation dosage and side effects, in vivo delivery of radioisotopes with tumor targeted specificity needs to address many challenges that include: 1) the selection of radioisotopes with a proper halflife; 2) a delivery vehicle that can carry an optimal amount of radioisotopes and has favorable pharmacokinetics; 3) suitable tumor biomarkers that can be used to direct the delivery vehicle into cancer cells; and 4) specific tumor targeting ligands that are inexpensive to produce and can be readily conjugated to the delivery vehicles. In addition, a multifunctional carrier that not only delivers radioisotopes but also provides imaging capability for tracking and quantifying radioisotopes that have accumulated in the tumor is highly desirable.
Recent advances in nanotechnology have led to the development of novel nanomaterials and integrated nanodevices for cancer detection and screening, in vivo molecular and cellular imaging,Citation2 and the delivery of therapeutics such as cancer cell killing radio-isotopes.Citation3,Citation4 An increasing number of studies have shown that the selective delivery of therapeutic agents into a tumor mass using nanoparticle platforms may improve the bioavailability of cytotoxic agents and minimize toxicity to normal tissues.Citation5–Citation7 In this review, we attempt to provide an overview on developing nanoparticles for carrying and delivering therapeutic radioisotopes for cancer treatment. We will discuss the following topics: selecting nanoparticles and radiotherapy isotopes, strategies for targeting nanoparticles to cancers, and challenges and potential solutions for in vivo delivery of nanoparticles. Some examples of using nanoparticle platforms for the delivery of therapeutic radioisotopes in preclinical studies, detection, and monitoring of therapy will also be presented.
Cancer cell killing radioisotopes and radiation therapy
Radiation therapy utilizes radiation energy to induce cell death. By directly delivering external radiation beams to a tumor in the patient, external radiation therapy offers a relatively simple and practical approach to cause radiation damage in the tumor. Although the intensity, location and timing for external radiation can be well controlled and modulated, its main disadvantages include: 1) the destruction of normal tissue adjacent to tumors and in the path of the beam; 2) the need of high radiation doses for penetrating tissues with a large field or volume; 3) prolonged treatment with the requirement of daily hospital visits for 5–6 weeks; and 4) the use of only selected radiation sources due to the technical requirements and limitations of radiation devices and radiation sources (eg, high energy x-rays). Therefore, external radiation treatment may not be applicable to certain cancers and not effective in the improvement of clinical symptoms.Citation8 In contrast to external radiation treatment, systemic radiotherapy delivers radiation energy from the radioisotopes that are conjugated to a suitable delivery carrier, such as antibodies, liposome emulsions or nanoparticles with tumor targeting ligands, and transported to the tumor site as illustrated in . If the tumor can be accessed from major arteries, the conjugated radioisotopes can be directly infused through a catheter or by intravenous administration. Since tumor targeted and localized delivery of radiation enhances the treatment effect and reduces the toxicity to normal tissue, systemic radiotherapy is considered to be a promising approach for personalized oncology. Although systemic radiotherapy presents major challenges in the design and production of delivery vehicles, it offers great opportunities for the application of novel nanomaterials and nanotechnologies.
Radioisotopes for radiation therapy
Although many radioisotopes can be used as radiation sources, only a few have been developed and applied in preclinical and in vivo studies. When selecting a candidate for experimental and clinical studies, the advantages and disadvantages of radioisotopes should be evaluated based on their physical and chemical properties, patient and environmental safety, specific requirements for in vivo applications, and technical feasibility. summarizes the physical and radiation properties of therapeutic radioisotopes that have been used in previous studies. These radioisotopes can be categorized into three types, ie, α, β and auger particles.
Table 1 Characteristics of some therapeutic radioisotopes
α-emitters
More than 100 different radioisotopes emit α-particles; however, most of them decay too quickly to be considered for therapeutic use. Only a few α-emitters, including actinium-225 (225Ac), astatine-211 (211At), bismuth-213 (213Bi) and bismuth-212 (212Bi), have therapeutic potential and these have been investigated in animal models or humans. α-particles are positively charged helium nuclei with a higher energy (5,000–8,000 keV) and shorter range (50–80 μm) than β-particles. Correspondingly, α-particles have a higher linear energy transfer (LET), approximately 100 keV/μm, than β-particles. LET refers to the average radiation energy deposited in tissue per unit length of track (keV/μm). Cell death occurs only when α-particles traverse the cell nucleus rather than going directly to the cytoplasm. Owing to these features, α-particles are better suited to the treatment of microscopic and small-volume tumors, or residual tumors. In these instances, α-particles are more efficient and specific in causing tumor cell death than β-particles, as shown in many studies.Citation9–Citation11 Earlier studies using the single-cell model showed that one cell-surface decay of the α-emitter 211At resulted in the same degree of cell killing as approximately 1,000 cell-surface decays of the β-emitter 90Y.Citation12 The therapeutic effect of several α-emitters has been investigated for decades. Recently, α-emitter therapy has received renewed interest, especially for the treatment of microscopic, small tumors or minimal residual tumorsCitation13,Citation14 in a variety of cancer types, including leukemia, lymphoma, glioma, melanoma, and peritoneal carcinomatosis. However, poor radionuclide supply, complicated methodologies for calculating the radiation dosimetry and the need for relevant data relating to normal organ toxicity limit the applications of α-emitter radioisotopes and impede the development of targeted α-emitters.Citation15
β-emitters
Radioisotopes that function as β-emitters are widely used in radioimmunotherapy trials. Commonly used β-emitter radioisotopes are iodine-131 (131I), yttrium-90 (90Y); copper-67 (67Cu), rhenium-186 (186Re), lutetium-177 (177Lu), and copper-64 (64Cu). 131I and 90Y are the most popular candidates since these two isotopes are readily available and inexpensive.131I has a long half-life (8 days) and also provides γ-emissions that can be used in imaging for tracking and quantifying the radioisotope in vivo. Furthermore, relatively straight forward chemical modifications can be applied to crosslink 131I chelates to the proteins, allowing for the labeling of tumor targeting antibodies with radioisotopes.Citation16,Citation17 However, 131I has some drawbacks, such as rapid degradation and a short retention time in the tumor.Citation13 Additionally, the high energy γ-emission presents some safety concerns to patients and the environment. 90Y has fewer environmental radiation restrictions than 131I because of its pure β-emitter nature, higher energy and low-range (12 mm), and a longer residence time in the tumor, making it more suitable for the irradiation of large tumors that require a higher radiation dose and a stable link between radioisotopes and the tumor targeting antibody.Citation13 β-particles have lower LET and longer radiation ranges than α-particles. Because of their long radiation range (several millimeters), β-particles can destroy tumor cells through the “crossfire effect,” even though the radioimmunoconjugate is not directly bound to the cells. Therefore, they are particularly useful in overcoming treatment resistance. However, longrange β-particles can produce nonspecific cytotoxic effects by destroying surrounding normal cells. Thus, β-particles are considered to be most suitable for the treatment of bulky or large volume tumors.Citation18
Auger-emitters
An auger is a high LET (4–26 keV/um), low energy (≤1.6 keV), and short-range (≤150 nm) electron derived from inner-shell electron transitioning. During the decay of these radioisotopes, the vacancy formed in the K shell as a consequence of electron capture or internal conversion is rapidly filled by electrons dropping in from higher shells, resulting in a cascade of atomic electron transitions and emitting a characteristic X-ray photon or an auger. Auger emitters, such as gallium-67 (67Ga), iodine-123 (123I) and iodine-125 (125I), have also been used for cancer radiotherapy. Auger emitters deposit high LET over extremely short distances and are therefore most effective when the decay occurs in the nucleus and less so when the decay occurs in the cytoplasm. The dimensions of many mammalian cell nucleus components, such as 30-nm chromatin fiber, fall in the range of the auger emitter (< 150 nm); therefore, auger emitters are more damaging to those cellular structures. As a result, the use of auger emitters has been relatively restricted because of the extreme toxicity of such radioisotopes.Citation19
The selection of radioisotopes for cancer therapy should take into account the specific cancer types, characteristics of the tumor, toxicity and safety of radioisotopes, availability and production of radioisotopes, and the chemistry that is involved in assembling the radioisotopes to the delivery carriers. It has been shown that a combination of radioisotopes with different energies can be more beneficial than using a single radioisotope. For example, a previous study showed that the combination of a high energy and long tissue range radioisotope with a medium energy and shorter tissue range radioisotope is able to destroy both large-volume tumors and micrometastases.Citation13
Antibodies conjugated radioisotopes for tumor targeted radiation therapy
Most conventional anticancer agents do not greatly differentiate between cancerous and normal cells, leading to systemic toxicity and adverse effects, which limits the maximum allowable dose of the agent. The use of monoclonal antibodies (mAb) interacting with cancer cell surface markers is a simple tumor targeting strategy that can lead to a considerable enhancement of treatment efficacy.Citation20 Small, high affinity antibody fragments, such as single chain antibodies and affibodies, which are expressed as recombinant proteins in prokaryotic cells, have the additional advantage of cost-effective production of their targeting ligands.Citation21 mAbs or mAb fragments as ligands for the delivery of radioisotopes, as internal radiation sources, have been investigated extensively.Citation22–Citation24 The method of using mAbs conjugated with radioisotopes for internal or systemic radiation treatment is also known as radioimmunotherapy.
However, there are several limitations in using antibodyconjugated radioisotopes for the delivery of radiation therapy. Firstly, mAbs may bind to cell surface markers that are expressed at a low level in normal tissues, causing potential systemic toxicity. Secondly, mAbs have only a few sites available for conjugating radioisotopes. Therefore, delivery of a large dose of therapeutic radioisotopes may require a larger amount of antibodies. Thirdly, the use of mAbs presents potentially unwanted immune responses. Additionally, antibodies may be susceptible to protease degradation. These limitations may be addressed using nanomaterial based delivery systems.
Biocompatible nanoparticles
The chemistry and physical properties of nanomaterials
Nanoparticles are typically colloidal materials that can be fabricated with a variety of compositions and morphologies using special techniques and chemistries. Nanomaterials currently used in biomedical applications include fluorescent CdSe nanoparticles known as quantum dots (QDs), photosensitive gold nanoparticles, magnetic nanoparticles, as well as polymer based polymeric nanoparticles and nanoscale liposomes. Nanoparticles, especially metallic and metal oxide nanoparticles, in the “mesoscopic” size range of 5–100 nm in diameter often exhibit unique chemical and physical properties that are not possessed by their bulk or molecular counterparts.Citation25 For example, QDs made from CdSe exhibit photoluminescence with a controllable wavelength ranging from the visible to near infrared depending on their size. Colloidal gold nanoparticles exhibit unique surface plasmon resonance (SPR) properties derived from the interaction of electromagnetic waves with the electrons in the conduction band.Citation26 Magnetic nanoparticles, such as iron III oxide (Fe3O4), are superparamagnetic and exhibit high magnetization and yet no residual magnetization in the absence of an externally applied magnetic field.Citation27 Both the chemical properties and reactivity of the nanoparticles are controlled by the surface chemistries offered by functionalized polymer coatings or blocks, which are also important to the stability and biocompatibility of the nanoparticles, interactions between particles, biomolecules and cells, in addition to tissue distributions of the nanoparticles.
Nanoparticles provide a large surface area and various types of functional groups that allow for chemical reactions taking place on the nanoparticle surface and to assemble or load bioactive ligands or small molecular agents. Diverse nanomaterials with unique properties have been found in various biomedical applications, including in vitro or in vivo imaging, separation and purification of cells or biomolecules, and delivery of therapeutic agents.
Biocompatibilities and functionalization of nanoparticles
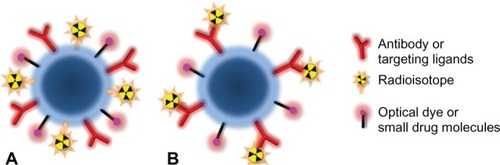
Well designed polymers can be used as coatings to stabilize metallic or metal oxide nanoparticles from aggregating and precipitating in physiological conditions while maintaining the desired physical properties. These polymers should also provide biocompatibilities that include minimizing toxicity from metallic nanoparticles, modulating interactions between nanoparticles and biomolecules, cells and tissues, together with altering the secretion and biodistribution of the nanoparticles. Coating polymers can be functionalized with reactive functional groups, such as –COOH, –NH2 and –SH, which are prepared for the conjugation of the tumor targeting ligands or other agents as illustrated in . For carrying and delivering therapeutic radioisotopes, coating polymers with reactive functional groups allows for covalent cross-linking or non-covalently incorporating chelates of radioisotopes.
Figure 2 Multifunctional nanoparticle platforms for tumor targeting, imaging, and delivery of drugs and/or radioisotopes.
There are a variety of polymers and their derivatives, such as dextran, polyethylene glycol (PEG), and dendrimer, developed for ensuring the biocompatibility and functionalization of nanoparticles.Citation28,Citation29 For instance, Zhang and co-workers modified the surface of iron oxide nanoparticles with trifluoroethyl ester-terminal-PEG-silane, which was then converted to an amine-terminated PEG.Citation30 The terminal amine groups were used for the conjugation of Cy5.5, a near infrared (NIR) optical probe, and chlorotoxin, a targeting peptide for glioma tumors. In vitro MRI and confocal fluorescence microscopy showed a strong preferential uptake of the multimodal nanoparticles by glioma cells compared to the control nanoparticles and noncancerous cells, indicating the cancer targeting abilities of the probes for gliomas. To reduce nonspecific uptake of nanoparticles by normal tissues and extend the blood circulation time of nanoparticles to allow particle accumulation at the target site, polymer coatings must be specifically designed to meet such applications. The physical characteristics of polymer coated nanoparticles affect their in vivo performance. Surface morphology, overall particle size and surface charge are all considered important factors that determine toxicity and biodistribution. The overall particle size must be small enough to evade uptake by reticuloendothelial system (RES) but large enough to avoid renal clearance, leaving a window of between 5.5 and 200 nm.Citation31 However, it has also been demonstrated that for particles smaller than 40 nm in diameter, both the biodistribution and the half-life of nanoparticles are determined by the coating material rather than the mean size.Citation32 Nanoparticles with different coating materials usually have different surface charges, which plays a critical role in blood half-lives. It has been demonstrated that both positively and strong negatively charged particles tend to nonspecifically stick to cells.Citation33,Citation34 Thus, it is generally considered that nanoparticles with a neutral surface charge are recommended to extend circulation times. Additionally, nanoparticles with neutral surfaces also show a high stability for resisting protein binding and provide steric hindrance for preventing aggregation after in vivo administration.Citation31 Recently, new coating materials from zwitterionic polymers have been developed to provide a biocompatible surface with both positive and negative charges, which exhibit high resistance to nonspecific protein adsorption.Citation35 In addition, new polymer coatings exhibit high resistance to nonspecific protein binding and uptake by macrophages in the normal liver and spleen.Citation36,Citation37 Combining PEG with a crosslinkable polysiloxane coating, Chen and colleagues developed an antibiofouling copolymer PEO-b-PγMPS for coating nanocrystals.Citation38 This new copolymer made hydrophobic iron oxide nanoparticles mono-disperse in physiological conditions with great stability. Furthermore, this amphiphilic blocked coating polymer can be functionalized with reactive amine groups on the particle surface, making it readily available for the conjugation of tumor targeting ligands and therapeutic agents such as radioisotope chelates. Furthermore, these composite nanoparticles showed a reduced nonspecific uptake.
Advantages of using nanoparticles to deliver therapeutic agents
One of the major obstacles in the delivery of therapeutic agents, especially small molecular agents such as radioisotope chelates, is rapid elimination of the agents and their widespread distributions into normal organs and tissues. One common solution to this problem is the administration of the agent in a large quantity, which is not cost-effective and often results in undesirable toxicity. Nanoparticles with proper biocompatible polymer coatings potentially provide better platforms for carrying therapeutic agents and subsequently delivering the agents to the tumor. There are several advantages of using nanoparticles to deliver therapeutic radioisotopes, for instance: 1) nanoparticles have prolonged blood retention time, ranging from 30 minutes to more than 24 hours, depending on the morphology and size of the particle, coating materials, and compositions of nanoparticle conjugates; 2) nanoparticle carriers targeting cancer cells are capable of enhancing local accumulation of the nanoparticles with therapeutic agents, leading to high tumor retention times and concentrations of the agents; 3) nanoparticles can load more drug molecules or radioisotopes on a single particle than conventional carriers, sometimes more than one type of therapeutic agent or radioisotope; 4) internalization of receptor targeted nanoparticles leads to the uptake of large amounts of radioisotopes into the target cells, resulting in effective killing of tumor cells with a relatively low level of receptor-expression; and 5) the unique chemical and physical properties of nanoparticles, such as magnetization and photosensitizing, provide additional capabilities and functions for improving delivery of the radioisotopes (for example the external application of a magnetic field) and monitoring the response to radiotherapy. With the controlled formulation of nanoparticles and carefully planned drug carrying strategies, nanoparticle platforms may offer the appropriate pharmacokinetics to enable optimal delivery of therapeutic radioisotopes for cancer treatment.
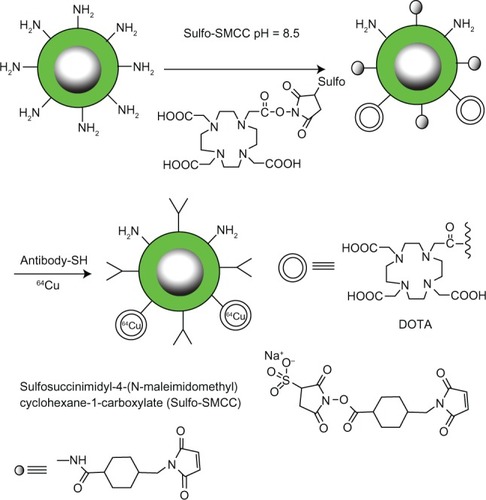
Biodegradable nanoparticles can be employed to gradually release drugs. For example, therapeutic agents can be interspersed within a poly(DL-lactide-co-glycolide) (PLGA) matrix. As the biocompatible polymer is degraded, the therapeutic agents are released in a sustained manner.Citation39 Additionally, particles such as micelles are useful in drug delivery since their hydrophobic centers allow for drug encapsulation while their hydrophilic surfaces allow for ease in transport.Citation40 Alternatively, the particle surface allows for modifications since it provides functional groups the opportunity to conjugate small molecular agents. Radioisotopes are typically loaded onto the nanoparticles using the conjugation strategy shown in .
Figure 3 A scheme for conjugating radioisotope chelates to surface functionalized nanoparticles coated with amphiphilic polysiloxane block polymers containing active –NH2 groups.
Nanoparticle delivery platforms often provide a multi-functional capacity that enables loading multiple moieties, such as targeting ligands and therapeutic agents, using multiple reaction steps. This is particularly important to the tumor targeted delivery of radioisotopes in vivo. It has been reported that nanoparticles consisting of streptavidin that linked three biotinylated components: the antiHer2 antibody trastuzumab, the tat peptide, and the 111In-labeled antiRIa messenger RNA antisense morpholino (MORF) oligomer, produce significant radiation-induced antisensemediated cytotoxicity of tumor cells in vitro.Citation41
Strategies for targeting nanoparticles to cancers
Two mechanisms, passive and active targeting, are typically used in targeting nanoparticles to tumors. In passive targeting, nanoparticles and payloads reach the tumor through rich, chaotic, and highly permeable tumor vasculature and are accumulated and subsequently remain in the tumor due to its lack of lymphatic drainage. In active targeting, nanoparticles are engineered to target specific biomarker molecules that are unique and over populated in a tumor or cancer cell surface. Extensive reviews and discussions on the mechanisms of targeting nanoparticles to tumors are beyond the scope of this review and can be found elsewhere.Citation3,Citation42–Citation44 Active targeting nanoparticles carrying therapeutic agents, such as radioisotopes, to tumors are the current research focus and the subject of intensive investigations. Differences in the expression of cellular receptors between normal and tumor cells represent a great opportunity for targeting nanoparticles to cancer cells. For engineering tumor targeted nanoparticles, different ligands, such as antibodies, peptides, and small molecules targeting the related receptors that are highly expressed in tumor cells, are usually conjugated to the surface coating polymer of nanoparticles. There have been some reports of in vivo tumor targeting with various targeting ligand/nanoparticle combinations, such as folic acid-modified dendrimers (≈9% ID g–1; ID g–1 = injected dose per gram tissue),Citation45 synthetic small-molecule-modified iron oxide (IO) nanoparticles (≈4% ID g–1),Citation46 polyethylene glycol-coated (PEGylated) arginine – glycine – aspartic acid (RGD) peptide-modified carbon nanotubes (10%–15% ID g–1),Citation47 and PEGylated single-chain variable fragment (ScFv) antibody-modified gold nanoparticles (≈8% ID g–1).Citation26 Tumor-targeted nanoparticles as potential carrier vectors of therapeutic agents are believed to be the next promising platform for nanobiotechnology
Using antibodies as tumor targeting ligands for magnetic or photosensitive nanoparticles has been extensively studied in vitro and in vivo in the area of cancer imaging. Conjugates composed of nanoparticles and antibodies were found to maintain the properties of both the antibody and the nanoparticle. Conjugation of magnetic iron oxide nanoparticles with Herceptin, a well-known antibody against the HER2/neu receptor which is over-expressed in breast cancer cells, showed in vivo cancer targeting and imaging of HER2/neu with a high sensitivity, which enables the magnetic resonance imaging (MRI) detection of tumors as small as 50 mg. The smallest parts of the antibody, the variable regions or so called ScFv, are among the most frequently used ligands. Because antibody fragments lack the Fc domain that binds to Fc receptors on phagocytic cells, particulates derived with mAb fragments have increased circulation times in the blood compared to particulates derived with whole mAbs.Citation48
In contrast to whole mAbs and antibody fragments, small molecule ligands typically can be readily obtained from chemical syntheses in a large quantity, which may be an important factor in translating novel methods into clinical practices. Small peptide ligands, such as tumor integrin αv β3 targeted high-affinity Arg-Gly-Asp (RGD) ligand which has a higher binding affinity in its conformationally constrained cyclic form than in its linear form, have been extensively investigated for their in vitro and in vivo applications of delivering tumor targeted nanoparticles carrying imaging and therapeutic agents. The RGD peptide, which has a higher binding affinity in cyclic conformation than its linear form, is able to bind to αvβ3 or αvβ5 integrins that are highly expressed in angiogenic tumor endothelial cells and subpopulations of tumor cells. It is likely that RGD-targeted nanoparticles can act on tumor endothelial cells and produce anti-angiogenesis effect.Citation49,Citation50 The folate receptor (FR) is an attractive molecular target for tumor targeting because it is over expressed by several types of tumor cells (eg, ovarian, colorectal, breast, nasopharyngeal carcinomas), however, it has limited expression in most normal tissues.Citation51–Citation52 FR-mediated tumor delivery of various agents, such as therapeutic drugs and gene products as well as imaging agents with radionuclides or nanoparticles for imaging, have been reported.Citation53–Citation55 Folic acids targeting cancer cells over expressing folate receptors have been covalently conjugated to 66 nm liposomes via spacers of various lengths to target the liposomes to kB cells with a high level of folate receptor expression. The binding of folate-PEG liposomes to kB cells can be competitively inhibited by excess free folate or by antiserum against the folate receptor, demonstrating that the interaction is mediated by the cell surface folate-binding protein. These folate-PEG liposomes show potential for delivering large quantities of low molecular weight compounds into folate receptor-bearing cells.
Delivery of tumor targeted therapeutic radioisotopes with nanoparticles
With surface functionalized nanoparticles and a range of surface chemistries for the conjugation of peptide ligands and antibody moieties, tumor targeting antibodies cross-linked with therapeutic radioisotopes or radioisotope chelates are readily conjugated to nanoparticles. Integrin targeted nanoparticle carrying radioisotopes have demonstrated its effect on tumor vasculatures. In the study by Li and colleagues, integrin antagonist (IA) 4-[2-(3,4,5,6-tetrahydro pyrimidin-2-ylamino) ethoxy]-benzoyl-2-(5)-aminoethylsulfonylamino-β-alanine, which binds to the integrin αvβ3, and a monoclonal antibody against murine Flk-1, were used to target nanoparticles radiolabeled with 90Y.Citation22 A single treatment with IA-nanopartcile-90Y caused significant tumor growth delay in murine tumor models K1735-M2 (melanoma) and CT-26 (colon adenocarcinoma), compared to untreated tumors, as well as tumors treated with anti-Flk-1 mAb, anti-Flk-1 mAb-NP, and conventional radioimmunotherapy with 90Y-labeled anti-Flk mAb.
However, selection of nanoparticle carriers and radioisotopes should be based on which nanoparticle carrier can improve the pharmacokinetics and enhance the delivery of therapeutic agents, their therapeutic effects and the additional functionalities offered by nanoparticle carriers as demonstrated in several previous studies are discussed here.
Slow clearance and prolonged blood circulation with nanoparticle carriers
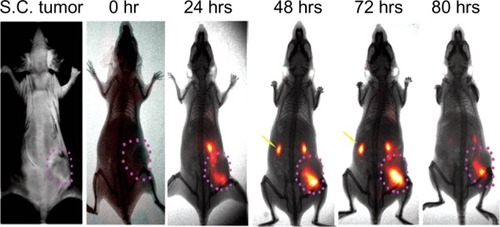
One major benefit of using nanoparticles to deliver therapeutic radioisotopes for radiation treatment is that the large size and high molecular weight of nanoparticle carriers cause the nanoparticle-radioisotope conjugates to be cleared from the body slowly. Nanoparticle-radioisotope conjugates may have longer blood circulation time than radioisotopes alone or mAb-radioisotope conjugates. The slow clearance and long blood retention time of nanoparticle carriers can be demonstrated by our study on the delivery time courses of optically sensitive ATF-Cy5.5 conjugates and ATF-Cy5.5-iron oxide nanoparticle conjugates in mice bearing orthotopic pancreatic xenography tumors (). ATF-Cy5.5 conjugates were found to accumulate in the tumor in less than two hours and subsequently be secreted through the kidney in 7 hours after intravenous administration of the agent. However, ATF-Cy5.5-iron oxide nanoparticle conjugates started to accumulate in the tumor after 4 hours and the accumulation continued, peaking at 48 hours. Slow clearance and long blood retention time are critically important to the active targeting of tumors because cell surface marker targeted agents need time to reach inside the tumor and high and effective concentration of agents in the blood facilitates delivery of the agents. The unique pharmacokinetics of nanoparticle carriers has strong implications in optimizing internal radiation sources with proper consideration of the half time of the radioisotopes, radiation dosages, and treatment regime.
Figure 4 Time dependent accumulation of urokinase plasminogen activator receptor (uPAR) targeted-ATF-nanoparticle-NIR conjugates using amino terminal fragment (ATF) peptide of uPA as uPAR targeting ligands.
Rossin and colleagues developed 64Cu-radiolabeled folate-conjugated shell cross-linked nanoparticles as candidate agents to shuttle radionuclides and drugs into tumors over expressing the folate receptor.Citation56 They reported that 64Cu-radiolabeled folate-conjugated shell cross-linked nanoparticles exhibited a long circulation time in blood and were able to passively accumulate in tumors. Because 64Cu is also a radioisotope capable of positron emission tomography (PET), 64Cu-radiolabeled folate-conjugated shell cross-linked nanoparticles are a class of promising radioisotope agents for imaging and treating early-stage solid tumors.
Improve local delivery and tumor retention time of radioisotopes with magnetic nanoparticles
Systemic radiation therapy consists of a focused radiation source that remains at the tumor site over a period time. Magnetic nanoparticles, such as biocompatible superparamagnetic iron oxide nanoparticles with core sizes of 10–50 nm, suit this purpose well. Applying an external magnetic field to magnetic nanoparticle-based radioisotopes delivered to tumors may enhance their therapeutic effect and decrease the adverse effect of systemic radiotherapy.Citation57 A static magnetic field can be applied after the radioisotopes have localized in the tumors, constraining these magnetic nanoparticles to helical paths, which would result in substantially confining the emitted particles within the tumor’s boundaries, thus increasing the radiation dose to the tumor.Citation58 Chen and colleagues Citation24 developed a radioimmunoconjugate-131I – anti-vascular endothelial growth factor (VEGF) monoclonal antibody (Sc-7269) cross-linked to dextran coated magnetic iron oxide nanoparticles and investigated their therapeutic effect and safety in nude mice bearing human liver cancer. While 131I – anti-VEGF monoclonal antibody (Sc-7269) provides tumor targeting and a radiation source, the use of magnetic nanoparticles was intended to improve the delivery of the radioisotopes to the tumor and tumor retention time of the radiation source under the force of an external magnetic field. Their results suggested that the radioimmunotherapy of intratumoral injection of 131I – Sc-7269 – nanoparticles is safe and efficient for the treatment of liver cancer.
Radiation treatment combined with hyperthermia induced by nanoparticles
Local hyperthermia at the site of the tumor has been shown to be an effective cancer treatment because increasing the temperature in living tissues to 42–46°C leads to cell death.Citation59 Heat can be induced in cancer cells through the external application of an alternating magnetic field (AMF) and tumor targeted nanoparticles. Nanoparticle-based hyperthermia has been demonstrated to be a potential method for treating cancer.Citation60 Furthermore, application of the external AMF can activate nanoparticles carrying radioisotopes to preferentially be retained in the microenvironment of the tumor tissue at higher concentrations than in the surrounding normal tissue. In an earlier study by DeNardo and colleagues, investigators evaluated the pharmacokinetics, tumor uptake, and the therapeutic effect of 111In-chimeric L6 (ChL6) monoclonal antibody (mAb)-linked iron oxide nanoparticles in a thymic mice bearing human breast cancer (HBT 3477) xenografts when inductively heating these 111In-ChL6 mAb linked iron oxide nanoparticles with an externally applied AMF.Citation23 They found significant therapeutic responses from AMF/bioprobe therapy with up to an eight times longer mean time to quintuple tumor volume with the therapy compared with no treatment. Their data suggested that mAb-conjugated nanoparticles, when given intravenously, escaped into the extravascular space and bonded to cancer cell membrane antigens, so that 111In-ChL6 mAb linked iron oxide nanoparticles could be used in concert with an externally applied AMF to deliver thermoablative cancer therapy.
Natarajan and colleagues developed a new class of radioconjugated nanoparticles (111In-DOTA-di-scFv-NP) for the imaging and therapy of anti-MUC-1-expressing cancers.Citation61 They showed the binding of radioconjugated nanoparticles to cancer cells was time dependent with increased binding over time. They also compared the ability of 20 nm, 30 nm, and 100 nm nanoparticles to conjugate 111In-mAb and found that 100 nm nanoparticles could be conjugated to 111In-mAb so that the resulting radioimmunonanoparticles (RINP) had characteristics suitable for AMF therapy. Although 100 nm RINP targeted tumors less efficiently than 20 nm RINP, their heating capacity is typically 6 times greater, suggesting the 100 nm RINP could still deliver better therapy with AMF.Citation62
Improving boron neutron capture therapy with nanoparticles
Boron neutron capture therapy (BNCT) is based on a nuclear reaction in which a neutron of appropriate energy reacts with the stable 10B isotope, and a high-energy α-particle is released in addition to a recoiling 7Li nucleus. The energy of the released α-particle provides the main therapeutic effect and is deposited in the tissue within 10 µm of the site of neutron capture. This localized therapy is designed to minimize toxicities. Because the recoil range of the emitted lithium and helium particles in tissue is 5 and 7 μm, respectively, which is comparable to the size of a single cell, the cellular internalization and subcellular localization of the boron carrier with respect to the cell nucleus is a major determinant of the therapeutic efficacy for this approach.Citation63 BNCT has been effectively used in treating prostate and breast cancers in clinical oncology. Using monoclonal antibodies serving both as the targeting ligand and the delivery carrier is challenging for this therapy. Recently, various nanoparticle approaches, such as dendrimers, liposomes, cationic acrylamide copolymersCitation64 and single-walled carbon nanotubes,Citation65 have been developed and tested to deliver boron isotopes that target tumors. For example, boron carbide nanoparticles are studied as a system for T cell-guided boron neutron capture therapy.Citation66 Nanoparticles were produced by a method of ball milling in various atmospheres using commercially available boron carbide. In vitro thermal neutron irradiation of B16 melanoma cells incubated with sub-100 nm nanoparticles (381.5 mg/g 10B) induced complete cell death, while the nanoparticles alone induced no toxicity.
Challenges and future directions of nanoparticle delivery of therapeutic radioisotopes
Improving systemic delivery of nanoparticles
One of the major challenges in the application of nanoparticles in vivo is the nonspecific uptake of nanoparticles by the liver and spleen in the RES when using systemic delivery. For tumor targeted delivery of therapeutic agents, such as radioisotopes, the trapping followed by a rapid clearance of nanoparticles by the RES effectively reduces the tumor targeting efficiency of nanoparticles and potentially causes undesirable organ and normal tissue damage after agents accumulate in the liver and spleen. Therefore, minimizing and evading RES uptake and improving blood retention time of nanoparticles are active areas of research and development in the field. Several strategies, which are mainly focused on optimizing surface coating polymers, have been investigated. PEGylation and reducing surface charge of nanoparticle coatings may significantly reduce the RES uptake and improve the tumor targeting of nanoparticles as demonstrated by many groups.Citation67–Citation69 Antibiofouling and increased renal excretion rather than RES clearance were observed in nanoparticles with surface modified silica containing coating polymers.Citation70,Citation71
Multifunctional nanoparticles
In recent years, there has been a tremendous interest in developing multifunctional nanomaterials for cancer detection, treatment, treatment monitoring and image-guided interventions.Citation4,Citation48,Citation70 For example, theranostic nanoparticles are capable of simultaneous imaging and therapy. Nanoparticles carrying cancer cell killing radioisotopes represent an ideal theranostic system with the radioisotope providing both therapeutic and imaging modalities or combining its therapeutic capability with the imaging capability of nanoparticle carriers. In particular, radioisotopes 111In or 64Cu, which emit gamma rays during their decay, have been most frequently used as the imaging probes for PET. Other intrinsic properties of nanoparticles may offer additional functions to further improve tumor specific radiation treatment with the combination of other therapeutics; for example, the ability to induce hyperthermia and increase tumor retention time with magnetic iron oxide or photosensitizing and photoacoustics from gold nanoparticles. Trimodal imaging probes have also been designed by adding another imaging modality to dual MRI and optical imaging probes. The representative multimodal imaging probe is iron oxide nanoparticles coupled with fluorescent organic dyes. The iron oxide nanoparticles chemically crosslinked with dextran have been widely used for in vivo as well as in vitro MRI because of their biocompatibility, small hydrodynamic size, and stable hydrophilic polymer coating. Nahrendorf et alCitation72 developed multimodal imaging probes composed of magnetic iron oxide nanoparticles conjugated with arginyl peptides, near infrared (NIR) fluorescent indocyanine dye, Cy5.5, and DTPA to chelate PET tracer 64Cu, resulting in a PET, MRI, and optically detectable imaging agent. In another example, RGD peptide was used for the targeted delivery of nanoparticles into integrin αvβ3-positive tumors. Both small-animal PET and T2-weighted MRI showed integrin-specific delivery of conjugated RGD-polyaspartic acid-iron oxide nanoparticles and prominent RES uptake.Citation70 The bifunctional imaging approach may allow for earlier tumor detection with a high degree of accuracy and provide further insight into the molecular mechanisms of cancer.
Improving detection and follow-up of therapy effects
In addition to its therapy function, nanoconstructs that are capable of reporting responses to therapeutics, especially in the real time monitoring of tumor changes at the molecular level post-treatment, are highly desirable. Imaging techniques, especially clinically feasible modalities in MRI, single photon emission computed tomography (SPECT) and PET, play important roles in the evaluation of the therapeutic effects of radiation therapy. For example, in a recent study by Jacene and colleagues fluorine-18 fluorodeoxyglucose (18F-FDG) PET/CT was used for monitoring the response of non-Hodgkin’s lymphoma to radioimmunotherapy.Citation73 They found 18F-FDG uptake in tumors typically drops significantly after radioimmunotherapy in non-Hodgkin’s lymphoma. A continued decline in tumor maximum standardized uptake values between 12 and 24 weeks without additional therapy can occur, suggesting a need for delayed-response assessment. In patients who progress after radioimmunotherapy, new sites of disease, rather than recurrence or progression at previous disease sites, is commonly detected. Large declines in 18F-FDG uptake tend to be seen in those with the longest progression-free survival. Another recent study investigated the efficacy and side-effects of 90Y ibritumomab tiuxetan (Zevalin®) in patients with earlystage extranodal indolent lymphoma of the ocular adnexa with 111In ibritumomab tiuxetan imaging.Citation74 All patients had 111In ibritumomab tiuxetan imaging to confirm expected biodistribution of 90Y-Zevalin during the radiation therapy. Investigators concluded rituximab followed by 90Y ibritumomab tiuxetan was an effective and safe frontline treatment for early-stage extranodal indolent B-cell lymphoma of the ocular adnexa.
Conclusion
With the advances in nanoscience and nanotechnology, many nanomaterials including nanoparticles made of metal oxide, dendrimers, liposomes, polymers, and micelles can be used as carriers to deliver chemical, pharmaceutical agents and/or therapeutic radioisotopes into tumors. Conjugating these nanoparticles with therapeutic isotopes and targeted antibodies can enhance therapeutic efficacy and decrease the toxicity and destruction of surrounding normal tissue by radioisotopes. In experimental and clinical studies, proper radioisotope selection that is specific to a particular tumor type and delivery mechanism should be considered when planning in vivo nanoparticle delivery. For example, α-particles should be used when micrometastatic diseases or minimal residual tumors are treated, while β-particle isotopes are recommended for bulky tumors. The half-life of radioisotopes should be considered when using an intravenous system of delivering nanoparticle-radioisotope conjugates to the tumor, given the different pharmacokinetics in nanoparticles. The nanoparticles carrying therapeutic radioisotopes should be a platform capable of not only delivering radioisotopes into the targeted tumors it should be capable of real-time imaging for the monitoring and follow-up of the treatment. In addition, the external magnetic field and other functions associated with nanoparticles can be used to enhance the delivery of radiation dosages and reduce normal tissue damage. The further development of nanoparticle-based carriers will offer a range of approaches for the targeted delivery of radioisotopes for use in the internal and systemic radiation therapy of cancers.
Acknowledgments
Authors are grateful to Miss Tianning Fan for her assistance in illustrations and to the National Cancer Institute for their grant support of the Center of Cancer Nanotechnology Excellence (CCNE, U54 CA119338-01) and in vivo Cellular and Molecular Imaging Center (ICMIC, P50CA128301-01A10003).
Disclosure
The authors report no conflicts of interest relevant to this research.
References
- JemalASiegelRWardE2008 Cancer statisticsCA Cancer J Clin200858719618287387
- JarzynaPASkajaaTGianellaAIron oxide core oil-in-water emulsions as a multifunctional nanoparticle platform for tumor targeting and imagingBiomaterials2009306947695419783295
- PengXQianXMaoHTargeted magnetic iron oxide nanoparticles for tumor imaging and therapyInt J Nanomed20083311321
- YangLPengXWangAReceptor-targeted nanoparticles for in vivo imaging of breast cancerClin Cancer Res2009154722473219584158
- ShenoyDLittleSLangerRAmijiMPoly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluationsMol Pharm2005235736616196488
- BaeYDieziTAZhaoAKwonGSMixed polymeric micelles for combination cancer chemotherapy through the concurrent delivery of multiple chemotherapeutic agentsJ Control Release200712232433017669540
- LeeHLeeEKimKJangNKJeongYYJonSAntibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imagingJ Am Chem Soc20061287383738916734494
- KorbLRadiotherapy for the palliation of prostate cancerSemin Surg Oncol200018757910617898
- HartmannFHorakEMGarmestaniKRadioimmunotherapy of nude mice bearing a human interleukin 2 receptor alpha-expressing lymphoma utilizing the alpha-emitting radionuclide-conjugated monoclonal antibody 212Bi-anti-TacCancer Res199454436243708044783
- KennelSJStabinMRoeskeJCRadiotoxicity of bismuth-213 bound to membranes of monolayer and spheroid cultures of tumor cellsRadiat Res199915124425610073661
- BallangrudAMYangWHCharltonDEResponse of LNCaP spheroids after treatment with an alpha-particle emitter (213Bi)-labeled anti-prostate-specific membrane antigen antibody (J591)Cancer Res2001612008201411280760
- HummJLA microdosimetric model of astatine-211 labeled antibodies for radioimmunotherapyInt J Radiat Oncol Biol Phys198713176717733667382
- GoldenbergDMTargeted therapy of cancer with radiolabeled antibodiesJ Nucl Med20024369371311994535
- SalvatoriMIndovina1LMansiLTargeted α-particle therapy: a clinical overviewCurrent Radiopharmaceuticals20081251253
- ZalutskyMRTargeted a-particle therapy of microscopic disease: providing a further rationale for clinical investigationJ Nucl Med2006471238124016882999
- MühlhausenUSchirrmacherRPielMSynthesis of 131I-labeled glucose-conjugated inhibitors of O6-methylguanine-DNA methyltransferase (MGMT) and comparison with nonconjugated inhibitors as potential tools for in vivo MGMT imagingJ Med Chem20064926327216392811
- SchipperMLRieseCGSeitzSEfficacy of 99mTc pertechnetate and 131I radioisotope therapy in sodium/iodide symporter (NIS)-expressing neuroendocrine tumors in vivoEur J Nucl Med Mol Imaging20073463865017160413
- MulfordDAScheinbergDAJurcicJGThe promise of targeted a-particle therapyJ Nucl Med200546199S204S15653670
- MitraANanALineBRNanocarriers for nuclear imaging and radiotherapy of cancerCurrent Pharmaceutical Design2006124729474917168775
- SaravanakumarGKimKParkJHCurrent status of nanoparticle-based imaging agents for early diagnosis of cancer and atherosclerosisJ Biomed Nanotechno200952035
- MarcucciFLefoulonFActive targeting with particulate drug carriers in tumor therapy: fundamentals and recent progressDrug Discovery Today2004921922814980540
- LiLWartchowCADanthiSNA novel antiangiogenesis therapy using an integrin or anti-FLK-1 antibody cotated 90Y-labeled nanoparticlesInt J Radiation Oncology Biol Phys20045812151227
- DeNardoSJDeNardoGLMiersLADevelopment of tumor targeting bioprobes (111In-chimeric L6 monoclonal antibody nanoparticles) for alternating magnetic field cancer therapyClin Cancer Res2005117087S7092S16203807
- ChenJWuHHanDUsing anti-VEGF McAb and magnetic nanoparticles as double-targeting vector for the radioimmunotherapy of liver cancerCancer Letters200623116917516399221
- NieSXingYKimGJSimonsJWNanotechnology applications in cancerAnnu Rev Biomed Eng2007925728817439359
- QianXPengXHAnsariDOIn vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tagsNat Biotechnol200826839018157119
- GuptaAKGuptaMSynthesis and surface engineering of iron oxide nanoparticles for biomedical applicationsBiomaterials2005263995402115626447
- VeisehOSunCGunnJOptical and MRI multifunctional nanoprobe for targeting gliomasNano Letters200551003100815943433
- XieJXuCKohlerYControlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced non-specific uptake by macrophage cellsAdv Mater20071931633166
- VeisehOSunCFangCSpecific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the bloodbrain barrierCancer Res2009696200620719638572
- SunCLeeJSZhangMMagnetic nanoparticles in MR imaging and drug deliveryAdv Drug Deliv Rev2008601252126518558452
- Briley-SaeboKBjørnerudAGrantDHepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: implications for magnetic resonance imagingCell Tissue Res200431631532315103550
- FujitaTNishikawaMOhtsuboYControl of in-vivo fate of albumin derivatives utilizing combined chemical modificationJ Drug Target199421571658069594
- KairdolfBAManciniMCSmithAMMinimizing nonspecific cellular binding of quantum dots with hydroxyl-derivatizied surface coatingsAnal Chem2008803029303418324840
- LaddJZhangZChenSZwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasmaBiomacromolecules200891357136118376858
- BreusVVHeyesCDTronKZwitterionic Biocompatible Quantum Dots for Wide pH Stability and Weak Nonspecific Binding to CellsACS Nano200932573258019719085
- YangWZhangLWangSFunctionalizable and ultra stable nanoparticles coated with zwitterionic poly(carboxybetaine) in undiluted blood serumBiomaterials2009305617562119595457
- ChenHWuXDuanHBiocompatible polysiloxane-containing diblock copolymer PEO-b-P γMPS for coating magnetic nanoparticlesACS Applied Materials and Interfaces200912134214020161520
- PanyamJZhouWZPrabhaSRapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene deliveryFASEB J2002161217122612153989
- NasongklaNBeyERenJMultifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systemsNano Letters200662427243017090068
- LiuXWangYNakamuraKAuger radiation – induced, antisensemediated cytotoxicity of tumor cells using a 3-component streptavidindelivery nanoparticle with 111InJ Nucl Med20095058259019289423
- WangMDShinDMSimonJWNanotechnology for targeted cancer therapyExpert Rev Anticancer Ther2007783383717555393
- Brannon-PeppasLBlanchetteJONanoparticle and targeted systems for cancer therapyAdvanced Drug Delivery Reviews2004561649165915350294
- van VlerkenLEVyasTKAmijiMMPoly (ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular deliveryPharmaceutical Research2007241405141417393074
- Kukowska-LatalloJFCandidoKACaoZNanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancerCancer Res2005655317532415958579
- WeisslederRKellyKSunEYCell-specific targeting of nanoparticles by multivalent attachment of small moleculesNat Biotechnol2005231418142316244656
- LiuZCaiWHeLIn vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in miceNat Nanotechnol20072475218654207
- YangLMaoHWangYYSingle chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imagingSmall2009523524319089838
- ZhangCFJugoldMWoenneECSpecific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scannerCancer Res2007671555156217308094
- CaiWBChenXYPreparation of peptide-conjugated quantum dots for tumor vasculature-targeted imagingNature Protocols200838996
- AntonyACFolate receptorsAnnu Rev Nutr1996165015128839936
- KeCYMathiasCJGreenMAFolate-receptor-targeted radionuclide imaging agentsAdv Drug Deliv Rev2004561143116015094212
- DasMMishraDDhakPBiofunctionalized, phosphonategrafted, ultrasmall iron oxide nanoparticles for combined targeted cancer therapy and multimodal imagingSmall200952883289319856326
- ThomasMKularatneSAQiLLigand-targeted delivery of small interfering RNAs to malignant cells and tissuesAnn N Y Acad Sci20091175323919796075
- SetuaSMenonDAsokAFolate receptor targeted, rare-earth oxide nanocrystals for bi-modal fluorescence and magnetic imaging of cancer cellsBiomaterials20103171472919822364
- RossinRPanDQiK64Cu-labeled folate-conjugated shell cross-linked nanoparticles for tumor imaging and radiotherapy: synthesis, radiolabeling, and biological evaluationJ Nucl Med2005461210121816000291
- AlexiouHJurgonsRSchmidRJMagnetic drug targetingbiodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatmentJ Drug Targeting200311139149
- RaylmanRRWahlRLMagnetically enhanced radionuclide therapyJ Nucl Med1994351571638271038
- StrefferCThe biological basis for tumor therapy by hyperthermia and radiationStrefferJHyperthermia and The Therapy of Malignant TumorsBerlinSpringer1987
- MorozPJonesSKGrayBNMagnetically mediated hyperthermia: Current status and future directionsInt J Hyperthermia20038267284
- NatarajanAXiongCGruettnerCDevelopment of multivalent radioimmunonanoparticles for cancer imaging and therapyCancer Biotherapy Radiopharmaceuticals200823829118298332
- NatarajanAGruettnerCIvkovRNanoferrite particle based radioimmunonanoparticles: binding affinity and in vivo pharmacokineticsBioconjugate Chem20081912111218
- SofouSRadionuclide carriers for targeting of cancerInt J Nanomed20083181199
- AzabAKSrebnikMDovinerVTargeting normal and neoplastic tissues in the rat jejunum and colon with boronated, cationic acrylamide copolymersJ Control Release2005106142516005094
- YinghuaiZPengATCarpenterKSubstituted carboraneappended water-soluble single-wall carbon nanotubes: new approach to boron neutron capture therapy drug deliveryJ Am Chem Soc20051279875988015998093
- MortensenaMWSørensenbPGBjorkdahlOPreparation and characterization of Boron carbide nanoparticles for use as a novel agent in T cell-guided boron neutron capture therapyApplied Radiation Isotopes20066431532416290943
- LiSDHuangLNanoparticles evading the reticuloendothelial system: role of the supported bilayerBiochim Biophys Acta200917882259226619595666
- FangCShiBPeiYYHongMHWuJChenHZIn vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle sizeEur J Pharm Sci200627273616150582
- WilhelmCBilloteyCRogerJPonsJNBacriJCGazeauFIntracellular uptake of anionic superparamagnetic nanoparticles as a function of their surface coatingBiomaterials2003241001101112504522
- LeeHYLiZChenKPET/MRI dual-modality tumor imaging using arginine-gycine-aspartic (RGD) – conjugated radiolabeled iron oxide nanoparticlesJ Nucl Med2008491371137918632815
- HeXNieHWangKTanWWuXZhangPIn vivo study of biodistribution and urinary excretion of surface-modified silica nanoparticlesAnal Chem2008809597960319007246
- NahrendorfMZhangHHembradorSNanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosisCirculation200811737938718158358
- JaceneHAFiliceRKasecampW18F-FDG PET/CT for monitoring the response of lymphoma to radioimmunotherapyJ Nucl Med20095081719091903
- EsmaeliBMcLaughlinPProBProspective trial of targeted radioimmunotherapy with Y-90 ibritumomab tiuxetan (Zevalin) for front-line treatment of early-stage extranodal indolent ocular adnexal lymphomaAnn Oncol20092070971419150940