Abstract
Nanotechnology has virtually exploded in the last few years with seemingly limitless opportunity across all segments of our society. If gene and RNA therapy are to ever realize their full potential, there is a great need for nanomaterials that can bind, stabilize, and deliver these macromolecular nucleic acids into human cells and tissues. Many researchers have turned to gold nanomaterials, as gold is thought to be relatively well tolerated in humans and provides an inert material upon which nucleic acids can attach. Here, we review the various strategies for associating macromolecular nucleic acids to the surface of gold nanoparticles (GNPs), the characterization chemistries involved, and the potential advantages of GNPs in terms of stabilization and delivery.
Introduction
The ability of gold nanoparticles (GNPs) to interact with and enter cells has encouraged researchers to attach various compounds and biological macromolecules to gold in an effort to combine functionality with transport. Realization that GNPs can potentially stabilize and protect DNA, RNA, and other conjugates in solution proposes additional benefits over multistep methods that require separate approaches to delivery and stabilization. Excitement over the potential of GNPs has spurred research in many directions, which in addition to gene and RNA stabilization and delivery include a diversity of applications in materials science and sensor technologies as summarized in .
Figure 1 Graphical abstract designed to highlight various applications of gold nanoparticles with different kinds of macromolecule conjugates, such as thioalkylated DNA,Citation11 RNA,Citation90 proteins,Citation24 peptides,Citation45 carboxylic acids,Citation8 and poly(amidoamine) (PAMAM) dendrimers.Citation9
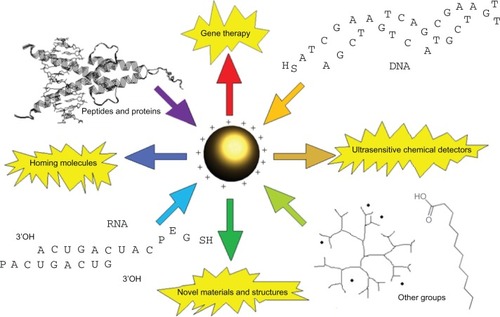
For example, it is now possible to manipulate GNPs to detect extremely small concentrations of analyte,Citation1 serve as scaffolds for 2- and 3-dimensional constructs,Citation2 and behave as real-time observable nodes for imaging studies.Citation3 In terms of biomedical applications for GNPs, nanotechnology is poised to finally deliver upon its promise to transform the face of molecular medicine, as evidenced by its existence in clinical trials on human subjects.Citation4
This review focuses on the recent literature for the use of GNPs as delivery agents and their association and advantages in combination with biomacromolecules, particularly proteins, DNA, and RNA for potential biomedical applications. The discussion begins with functionalization of GNPs and the chemical functional groups used to produce derivatives for the purpose of such biomolecular interactions. The topic continues with characterization chemistries, spectroscopic techniques, and methods to study the conjugates formed between the GNPs and the biomolecules. Finally, the applications of these conjugates, particularly their ability to stabilize and deliver DNA and more recently RNA, are discussed.
Functionalization strategies and chemistries
Functionalization of nanoparticles is necessary for their stability, functionality, and biocompatibility. The ultimate goal in functionalization is to preserve the properties of the GNP and the bound biological molecule. In other words, the biological molecule should be stable and able to retain its biorecognition properties and GNPs should be able to retain their unique properties such as strong plasmon absorption bands,Citation5 light scattering,Citation6 etc. For biomedical applications, surface-functionalization of GNPs is essential in order to target them to specific disease areas and allow them to selectively interact with cells or biological molecules. In general, functionalization of GNPs can be performed by either using chemical functional groups or biological molecules.
Chemical functional groups
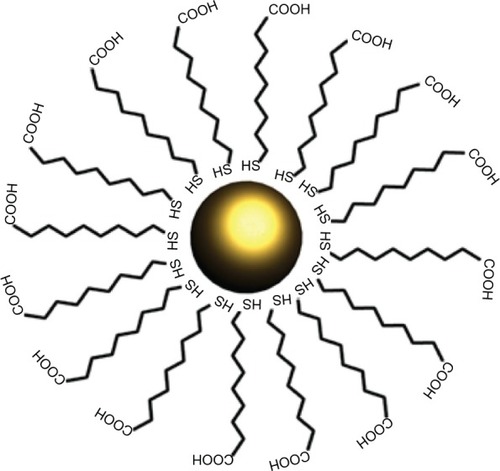
Colloidal GNPs are normally stabilized against aggregation by using long hydrocarbon ligand chains consisting of various functional groups. One end of these molecules is adsorbed on the gold surface, whereas the other end points towards the solution, as shown in for the adsorption of mercaptocarboxylic acid molecules on a GNP surface. In the case of water-soluble nanoparticles, these functional groups are often carboxylic acids which stabilize the nanoparticles by electrostatic repulsion and can be exploited for the conjugation of other molecules to the particles. The choice of ligand depends on the particle size and the solvent. These ligands can also be used as anchor points for further attachment of biological molecules which will be demonstrated in the following section. Mercaptocarboxylic acids are popular in the stabilization of GNPs due to the strong affinity of sulfur for gold.Citation7,Citation8
Polyethylene glycol (PEG) is also used as a ligand, as it provides colloidal stability because nanoparticles with PEG brushes on their surfaces repel each other for steric reasons.Citation9 The use of PEG as a ligand enables the fabrication of unusually robust, water-soluble GNPs that do not aggregate even under extreme pH and ionic strength conditions or in the presence of proteins. In some cases, it may be necessary to change the ligand. Ligand exchange is motivated by several aspects: (1) the transfer of GNPs from an aqueous to an organic phase (and vice versa) and (2) by exchanging hydrophilic surfactants with hydrophobic surfactants (and vice versa).Citation10 Doing so enables further tuning of surface properties of the nanoparticles.
Modification with biological molecules
Biological molecules can be exploited for the functionalization of nanoparticles. GNP conjugates bring together the unique properties and functionalities of both materials (eg, strong plasmon absorption bands, light scattering of the nanoparticles, and the ability of biological molecules to achieve high specific binding by molecular recognition).Citation8 Several ways have been utilized to attach biological molecules to nanoparticles. Among these, using the electrostatic interactions between the GNPs and the biological molecules is one of the easiest ways to functionalize and stabilize GNP bioconjugates. For example, when GNPs are positively charged, they bind by stable ionic interactions to negatively charged and nucleophilic moieties. Specifically, GNPs may interact with the phosphate ester backbone of nucleic acids,Citation11 electron-dense regions of poly(amidoamine) dendrimers,Citation12 or negatively-charged carboxylate groups.Citation13 The molecular mass of the biological molecule and the overall charge of its functional groups are important in this regard. Biological molecules containing simultaneous acidic and basic groups (for example, proteins) are efficient in stabilizing GNPs.Citation14 In general, it is commonly accepted that stabilization of GNPs by biological molecules occurs through passive adsorption of the biological molecule onto the nanoparticle surface by electrostatic and hydrophobic interactions. A strong negative charge on the citrate-stabilized GNP surface provides opportunity for Coulombic interaction with NH2 groups of lysine residues of proteins adsorbed on the nanoparticle surface.
Thiolated biomolecules can directly be bound on GNP surface via thiol–gold affinity interactions. DNA-GNP aptamers have been created for recognition of thrombin,Citation15 platelet-derived growth factors,Citation16 and cancer-associated epitopes.Citation17 In addition, biological molecules that have a functional group which can bind to the gold surface (like thiols or specific peptide sequences) can replace some of the original stabilizer molecules when they are added directly to the particle solution. In this way, molecules like oligonucleotides, peptides, or PEG can be readily linked to GNPs.Citation18–Citation20
The advantage of physical adsorption is that the nanoparticles have a minimum effect on the structure and function of the biological molecule. With native structure intact, the activity, selectivity, and specificity of the biological molecule towards a particular target are largely unaffected. However, the drawback of this approach is the possibility of downstream desorption of the biological molecules from the GNP surface. Therefore, characterization of synthesized conjugates is necessary, in particular to rule out aggregation effects or unspecific binding during the conjugation process.
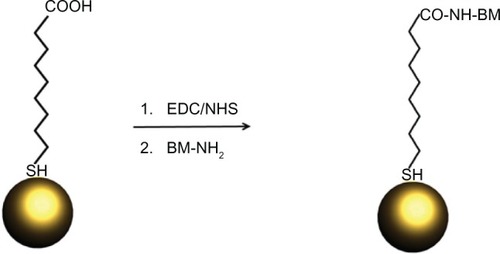
Alternatively, biological molecules can also be attached to the shell of stabilizer molecules around the GNPs by bioconjugate chemistries. The most common protocol is the covalent linkage of amino groups on the biological molecules with carboxyl groups at the free ends of stabilizer molecules by using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide HCl-mediated reaction.Citation21 Using this protocol, almost all kinds of biological molecules can be attached to the nanoparticle surface (). This so called “click chemistry” has been shown to give GNPs molecule-specific binding sites, such as for biotin–streptavidin (SA) linkingCitation22 and azide-linking.Citation23 For proteins, azide linkages have created GNPs with fully functional lipases from Thermomyces lanuginosus.Citation24
Figure 3 Reaction scheme for the EDC-mediated functionalization of gold nanoparticles.
A variety of macrostructures can form when various gold nanomaterials assemble with biomolecules. Different methods for preparing various GNPs provide scientists with a “bottom-up” approach to materials synthesis where the scientists engineer the building blocks and the macrostructures build themselves.Citation2 Although this approach is not yet in widespread usage for nucleic acids, protein-functionalized GNPs can self-arrange into monolayers and provide a scaffold onto which arrays of enzymes attach. Controlling interparticle proximity by changing the length of DNA linkers allows for elaborate monolayer construction such as sheets with evenly spaced wells.Citation25 These monolayers can also be stacked and arranged in alternating molecular patterns through layer-by-layer deposition.Citation26–Citation28
Another means that may be amenable for attaching macromolecular DNA or RNA is by coating the gold surface with alternating layers of cationic and anionic polyelectrolytes.Citation29 Synthesizing DNA or peptide linkers between GNPs confer these building blocks with programmable spatial coordinates. This can give an exquisite level of control, as the polymerase chain reaction can be performed directly on the surface of GNPs for this kind of organization.Citation18 Any or all of these approaches may potentially be modified for gene or RNA stabilization and delivery as well, though remain to be tested. The possibilities for functionalization upon GNPs are vast. These are just a portion of the reported functionalization methods and types of groups that have been attached to GNPs. New ways of functionalization and different types of group attachment are subjects of an intensely growing area of nanotechnology research.
Characterization
Functionalized GNPs behave in a variety of fashions depending on their size, charge, shape, concentration, and differences in bound ligands.Citation30–Citation32 Methods for quantifying these properties include the detection of electrical, optical, and photothermal emission and absorption, mobility shift, and size differences. GNPs can be characterized by their localized surface plasmon resonance (LSPR), whereby free electrons oscillate in response to light stimulation. This resonance can manifest as light scattering, which is detectable by spectroscopy. At certain wavelengths, light absorption produces heat instead of scattering. Field coupling between adjacent GNPs produces distance-dependent disturbances in their LSPR which allows for characterization of interparticle proximity.Citation33 The addition of groups to GNPs can result in the formation of heterogeneous products. Distinctive bands corresponding to the number of oligonucleotides bound to GNPs have been shown by gel electrophoresis.Citation34,Citation35
The distribution of GNPs with varying levels of conjugation can be quantified by high-performance liquid chromatography (HPLC). Theoretical models have been devised to predict the approximate heterogeneity of a given GNP-ligand solution.Citation36 Since the melting temperature (Tm) of GNP-DNA conjugates is size-dependent, they can be separated based on size from a heterogenous solution via serialized centrifugation at successively lower temperatures.Citation37 GNPs can also be characterized by size according to their electron dynamics in response to photoexcitation where absorption of light can cause the “bleaching” of surface plasmon interband transitions.Citation38 Adsorption of molecules to gold nanorods (GNRs) can be confirmed by surface-enhanced Raman scattering, Fourier transform infrared spectroscopy, X-ray photoemission spectroscopy, nuclear magnetic resonance (NMR), dynamic light scattering, and fluorescence spectroscopy.Citation5,Citation6,Citation39 Since GNPs less than 2 nm in size have different electron distributions than larger nanoparticles, the application of agents that bind to the surface of those particles will follow different collisional models for quenching. This allows for characterizing the transition of nanoparticles to free atoms in suspension and confirming their binding to ligands.Citation40 Quartz crystal microbalance (QCM) is yet another technique successfully used to compare the binding affinity of GNPs to different ligands.Citation41
Stabilization
The literature relates stabilization properties to functionalized GNPs that demonstrate the ability to prevent molecular aggregation and/or degradation. Our interests are more focused on the ability of the nanomaterial to prevent nucleic acids and other conjugates from degradation. DNA-GNP complexes exhibit unique properties and increase stabilization in the presence of DNA hydrolyzing enzymes in physiological conditions.Citation42 Current research illustrates various factors that prove to have significant relevance to the degree of stabilization, as well as techniques in which stabilization may be achieved.
In terms of physical stabilization, GNRs can be coated by cetyltrimethylammonium bromide to form stable lattices of different shapes such as honeycombs and square tiles.Citation43 Zeta-potential measurements of ligands bound to GNPs can be used to confirm stabilization as this is a mode of detecting the magnitude of repulsion between particles and thus their propensity to aggregate.Citation44 Peptide chemistries, in addition to assembly advantage, may also provide stabilization.Citation45 SA protein displays relatively superior biomolecular-colloidal stability for the purpose of antisense oligonucleotide delivery involving cellular penetration by way of signaling peptide sequences from viruses.Citation46 Evidence suggests that increased density in an oligonucleotide conjugated to a GNP correlates to increased stabilization, likely as a result of interference of high surface charge densities on foreign nucleic acid recognition enzymes that are involved in the immune response.Citation47 Similar to the effects of oligonucleotide density, multidentate peptide ligands have shown positive potential.Citation45 Stability of DNA on GNPs has been reported to be sequence-specific with different nucleosides having different binding affinities to the assemblage.Citation42 Immobilization of DNA onto gold has also been reported to enhance its thermal stability.Citation48
Polyvalent and PEGylation approaches are also reported to stabilize DNA in conjunction with gold.Citation49,Citation50 For example, PEG, possessing a pentaethylenehexamine at one end of each polymer chain (N6-PEG), acts as a surface-modifier in cooperation with the cations of salts in physiological conditions to reinforce the formation of the GNPs. In addition, immobilization of the PEG-derived substances on the modified surfaces results in the high-level stabilization of the GNPs.Citation51 Amphiphile DNA micelles, and/or electrostatic assembly and peptide capping, also have a bearing on particle and DNA stabilization.Citation52–Citation54 The stabilizing effects of protein folding, which are dependent upon length, hydrophobic properties, and electrostatic charge, imply applications for peptide capping ligands for DNA/GNP-complex stabilization.Citation54
Nanoparticles developed into DNA triple helices require the presence of stabilizing triplex DNA binding molecules to assemble, due to the thermal instability of the triple helix at room temperature.Citation55 The common laboratory technique of centrifugation has recently been explored as a means to polarize GNP clusters, which facilitates the attraction and electrical conductivity between the molecules and results in more stable linear assemblages.Citation56 Laser irradiation, accompanied by ionization and pH shifts, is a technique which may stabilize GNPs that are functionalized with a single layer of thiobarbituric acid (TBA) through dimensional reduction, as well as the inherent reinforcing properties of TBA.Citation57
DNA delivery
The application of functionalized GNP-DNA conjugates in therapeutics is reliant upon efficient and successful delivery. Surface-functionalization here also affords controlled release and exquisite control of their activity and uptake into cells. From a physiochemical standpoint, the GNP primarily serves as the delivery agent, not the effector, as the ability to add noncovalent stable groups to GNPs makes it easier to dissociate the two inside the cell. GNPs have been shown to dissociate from conjugated groups by laser-induced, ultrafast thermal changes,Citation58 potentially allowing for in vivo controlled release without micromanaging local disturbances in the molecular environment. Although, once again, much of the work has focused on DNA, controlled release has been accomplished via optical or electrochemical methods.Citation59–Citation62
Remotely controlled GNPs with near-infrared illumination have demonstrated their potential as optical switches for gene delivery by use in releasing gene-interfering DNA oligonucleotides.Citation54 Another method for optical activation uses UV light as a means for illumination, activating the release of a biologically inhibiting caging group from a functional amino acid sequence covalently attached to a nanoparticle and thus permitting the nanocomplex to bind to a cell.Citation61 Nanoparticle bioconjugates confined to patterned gold electrodes by thiol bonds have shown both the means to control the electrochemical release of nanoparticle conjugates as well as the ability to restore the electrodes used by the same conjugation mechanism by way of the reductive secretion of endogenously constructed monolayers which allow for regulated release and regeneration of materials.Citation62
To date, there has been much more focus on publications discussing the chemistry and functionalization of GNPs for diagnostic applications than for DNA delivery. For example, much of the work on GNPs and DNA is aimed at chemical detectors and monitors, or so-called GNPs as chemical “antennas”, for DNA. This includes work looking at particle radiusCitation63 for the purpose of hybridizing DNA linkers onto the gold surface,Citation64 aggregation and disaggregation, or selective desorption.Citation65 Fluorescence-based methods,Citation66 or others specific for DNA sequences,Citation67 can allow for detection at very low concentrations with enhanced sensitivity.Citation1 GNPs possess different optical properties based on their distance from each other. Mirkin and coworkers have prepared and verified the length of oligonucleotides by attaching them to GNPs and correlating the absorbance of the resulting mixture with the length of the DNA between them. This assay is particularly sensitive and capable of detecting DNA at the femtomolar level.Citation1
In recent years, investigators have begun applying their knowledge of GNP chemistry and functionalization to the difficult challenge of DNA delivery. One seminal paper by Chithrani et alCitation68 determined size and shape dependence of GNP uptake into human cells. Another critical factor in delivery is the loading efficiency of the nucleic acid, which has been optimized for DNA oligonucleotides onto GNPs.Citation69 An alternative approach has been to load genes onto a GNP surface functionalized with amino acids or dendrimeric derivatization.Citation70 Another approach that we and others have taken is to deliver the nanoparticles with the gene as passenger complexes bound to gold microparticles.Citation71,Citation72 We have shown this to achieve bioactive delivery of a nanoparticle DNA vaccine. A variety of surface functionalizations of GNPs have been attempted to improve uptakeCitation72,Citation73 as well as intracellular distribution,Citation74 including derivatization with sugar,Citation75,Citation76 light-triggered chemistries,Citation77 and others.Citation78 Finally, one recent and exciting direction is the functionalization of gold for the purpose of both targeting and tissue specificity in an attempt to induce robust bioactivities, such as immune responses.Citation79 Such general approaches to nanoparticle–cell interaction and intracellular delivery are illustrated in .
Figure 4 Gene delivery into a cell by A) remote-controlled laser, B) viral membrane, C) biolistics, D) reverse assemblage, E) micelle, and F) nanoparticle bound to gold microparticle. [Not to scale]
![Figure 4 Gene delivery into a cell by A) remote-controlled laser, B) viral membrane, C) biolistics, D) reverse assemblage, E) micelle, and F) nanoparticle bound to gold microparticle. [Not to scale]](/cms/asset/fdcf0555-32af-43d1-868b-d964d17e1ed0/dnsa_a_8984_f0004_c.jpg)
Nanoconjugations capable of reversing their assemblage have potential for drug delivery. The programmed disassembly is accomplished by DNA linkers containing mismatches at specified locations and yield efficient attachment and release from the surface of GNPs in a counterion-screened electric field.Citation62 As mentioned in the stabilization section, amino acids show efficacy in DNA binding and delivery. Two specific biomolecular complexes utilizing lysine and lysine dendron functionality display sensitivity to glutathione levels within cells and, therefore, provide selective delivery and subsequent expression of DNA.Citation70 A novel species of DNA block copolymer amphiphiles of typical micelle uniformity have been reported as potential mobilization agents for nanovesicles.Citation52
Viruses, such as the Moloney murine leukemia virus, have been utilized as tools for delivery of nanoparticles into the cell cytosol. By extrusion, GNPs may be coated with virus-derived membranes, thereby allowing them to bind and invade cells bearing particular viral receptor sites.Citation79 Complexation of DNA to cationic glycopolymer-stabilized GNPs shows potential as a solution to the problems of endosomal withdrawal and nuclear uptake in gene delivery with their demonstrated reluctance to aggregate in and ability to associate well in physiological conditions.Citation75 Transfection efficiencies of cationic glyconanoparticles display a size-dependency in their efficiency as gene delivery agents.Citation76
A biolistic method for the delivery of nucleic acids via cationic microparticles and nanoparticles has been declared to be less complex to carry out than the common calcium/spermidine-based strategy, and purportedly displays a remarkably efficient nucleic acid binding ability and functioning dependent upon concentration.Citation71 Correlating with factors of stabilization, the oligonucleotide density on the surface of the particles is directly proportional to uptake efficiency, thereby inferring that the more proteins stabilizing the nanoparticle, the greater the probability of successful uptake.Citation46 Also similar to stabilization characteristics is the importance of dimensional and electrical properties of nanoparticles in the delivery of therapeutic substances such as DNA or RNA. As shown in delivery to the gastrointestinal tract, larger-sized particles provided a greater surface area for functionalization with polymer chains which, through the subsequently enhanced stabilization, allowed for greater cellular uptake in the tested epithelial cells.
The polarity of the particles also contributed to the delivery efficiency, with positively-charged GNPs displaying highest deliverance potential and the negatively-charged GNPs showing greater cellular uptake than neutral GNPs, which was likely due to their affinity for membrane proteins.Citation76 Specifically, for DNA binding and cell uptake, biocompatible GNPs have been touted, so-called positively-charged colloidal gold nanoparticles (PGN). PGNs were shown to deliver interleukin-2 plasmid DNA and to elicit significant expression in mice.Citation80 GNPs grafted with polyethyenimine showed enhanced delivery of plasmid and importantly these retained activity in the presence of serum which is usually a great limitation for liposomal or polymeric delivery.Citation81 Importantly for cell uptake, coating the surface of GNP with PEG impacted endocytosis and intracellular trafficking pattern altering it relative to traditional citratestabilized GNP. In this work, the authors found that PEG-GNP was found in both cytosol and smaller intracellular vesicles, unlike citrate-stabilized GNP.Citation82 The plasma membrane is the first surface that the GNPs encounter and recently it has been shown that the surface charge of the GNP which was modulated by the attachment of cationic or anionic moieties can alter a cell’s membrane potential and depending on the cell type can affect cell proliferation, viability, and apoptosis.Citation83
RNA delivery
Free, unmodified small interfering RNA (siRNA) is notoriously unstable and sensitive to nucleases when delivered into nonembryonic mammalian cells.Citation84,Citation85 A variety of chemically modified siRNAs, especially methylation at the 2′-OH hydroxyl group,Citation86 peptide conjugation,Citation87 or PEGylation,Citation88 have partly addressed the stability issue. However, attachment of siRNA to GNPs or other nanomaterials will provide important delivery as well as stabilization advantages, ideally without excessively modifying the oligo- or nanoparticle. GNPs may also possess a more efficient loading or assemblage for accomplishing RNA interference, since the radial arrangement of siRNA about the surface of a GNP uniquely creates extra working area per unit particle. Although it is possible to unload siRNA from the GNP by laser stimulation inside the cell, it is not necessary because researchers have successfully triggered RNA interference without that step.
Experiments with GNPs coated with siRNA via thiolmodified oligoethylene glycol bridges have allowed for Luciferase knockdown in HeLa cells without affecting cell viability.Citation89 For efficacy, a layer-by-layer approach to siRNA deposition onto GNPs has been developed for identical unit preparation; also showing successful delivery into cells.Citation90 It is interesting that both gene therapy and siRNA experiments have shown success in cells without direct attempts to liberate the RNA from the complex. Further experiments may clarify how a RISC loading complex is able to interact with siRNA on a GNP and whether it is capable of dissociating them or forming a cluster of multiple catalytic sites. In addition to more research required on GNPs in terms of their advantages for cell entry and protection of the RNA molecules from degradation, other possible limitations may be endosomal release and/or nuclear delivery as well known for other systems.
There is intense interest in GNP delivery of nucleic acids to induce immune response and this has been shown recently for GNRs and their delivery of single-stranded RNA (5′-PPP-ssRNA).Citation91 Another double-stranded RNA (dsRNA) molecule, polyinosinic:polycytidilic acid (poly I:C) has been extensively clinically tested and there is a great deal of interest in its formulation via nanoparticles since it is such a powerful immunostimulant and activator of toll-like receptor.Citation92,Citation93
Conclusion and future
In summary, presents an overview of the wide variety of approaches used by researchers to date in order to functionalize GNPs for the purpose of binding, stabilizing, and delivery of DNA, RNA, and other biological macromolecules. This includes methods such as adding various sulfur and linker chemistries, amino acids and peptides, ligand exchange, electrostatic interactions, etc in order to create a surface which will bind these macromolcules. A variety of factors have now been identified which upon such functionalization will influence the bioactivity of the GNPs. This includes their size, shape, charge and electron density, concentration, solvent, spatial coordinates, pH, and of course their method of preparation. Characterization chemistries are another important area of active research including but not limited to light scatter and a variety of spectroscopic and fluorimetric techniques, plasmon resonance, infra red and X-ray spectroscopy, QCM, centrifugation, gel electrophoresis, HPLC, and NMR.
Table 1 Summary of the approaches, variables, characterization and obstacles for GNPs
There are still, however, a number of challenges or obstacles to be overcome for GNPs to reach their full potential as delivery reagents for therapeutic DNA, RNA, and other macromolecules. Despite the apparent breakthrough of getting siRNA into the cell and preserving its integrity, structure, and activity, the toxic effects of different GNP-conjugates elicit mixed results depending on particle size and other factors.Citation81–Citation83 This is problematic, but development of safe GNP–siRNA delivery complexes is hopeful and research is ongoing. It has been shown that macrophages will internalize GNPs without triggering the inflammatory response,Citation91 though the final excretory fate in multicellular organisms is still under study. Clinical trials for other siRNA delivery systems such liposomes, polymers, nongold cationic polyelectrolyte complexes, and virus-based vectors have been delayed because of their variability in cytotoxicity tests,Citation92 so research into GNP-conjugation offers a promising alternative. For the moment it seems that gene therapy in humans is realizable, if not with GNPs then with another agent, drawing from similar chemistries.
In conclusion, a variety of conjugation chemistries for modifying surfaces have resulted in the fabrication of many GNP derivatives with multifunctional purposes. Applications of these range from homing devices and molecular “antennae” to platforms for the delivery of drugs of all kinds, including DNA. In the case of drug and gene delivery for GNRs, this was recently reviewed by Pissuwan et al.Citation93 With advances in surface chemistry, there come novel applications for characterizing the GNP bionanoconjugates such as plasmon absorption, light scatter and other optical methods, QCM, electrophoresis and HPLC, and many more. Certainly for protein GNP bioconjugates, these chemistries are now well established, though they do not ensure conjugate enhancement. A conformational change in the biomolecule in response to GNP binding or functional group linkage could enhance, stabilize, or reduce its activity as can be seen by studies on different complexes.Citation24 Research in our lab and many others across the world is now engaged in determining whether these conjugation and characterization chemistries and approaches for stabilization and delivery can be applied to DNA vaccines and therapeutic RNA molecules, such as siRNA, splice-site switching oligomers, the double-stranded RNA immunostimulant polyinosinic polycytidilic acid (poly I-C), RNA vaccines, and others. It is clear that these nucleic acid nanoparticles have very potent and specific bioactivities and represent a powerful new generation with potential for molecular therapy stabilized and delivered via the emerging exciting field of biomedical nanotechnology.
Acknowledgments
Dr Wanekaya and Dr DeLong are supported by an AREA/R15 grant from the National Cancer Institute entitled, “Anticancer RNA Nanoconjugates” (1 R15 CA139390-01).
Disclosure
The authors report no conflicts of interest in this work.
References
- ElghanianRStorhoffJJMirkinCASelective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticlesScience1997277107810819262471
- ShenharRRotelloVMNanoparticles: scaffolds and building blocksAcc Chem Res20033654956112859216
- CheonJLeeJHSynergistically integrated nanoparticles as multimodal probes for nanobiotechnologyAcc Chem Res2008121630164018698851
- GoelRShahNVisariaRPaciottiGFBischofJCBiodistribution of TNF-α-coated gold nanoparticles in an in vivo model systemNanomedicine (Lond)2009440141019505243
- NikoobakhtBEl-SayedMASurface-enhanced Raman scattering studies on aggregated gold nanorodsJ Phys Chem A200310733723378
- JansHLiuXHuoQDynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studiesAnal Chem2009819425943219803497
- DuboisandLHNuzzoRGSynthesis, structure, and properties of model organic surfacesAnnu Rev Phys Chem199243437463
- UsonRThe chemistry of goldSynthesis and Reactivity in Inorganic, Metal-organic, and Nano-metal Chemistry19788503504
- KanarasAGKamounahFSBrustMThioalkylatedtetraethylene glycol: a new ligand for water soluble monolayer protected gold clustersChem Commun20022022942295
- PellegrinoTKuderaSParakWOn the development of colloidal nanoparticles towards multifunctional structures and their possible use for biological applicationsSmall20051486317193348
- ParkSYLeeJSMirkinCAStructures of DNA-linked nanoparticle aggregatesJ Phys Chem B2006110126731268116800601
- XiangyangSSuheWBakerJRDendrimer-entrapped gold nanoparticles as a platform for cancer-cell targeting and imagingSmall200731245125217523182
- KariukiNNLuoJZhongCJAssembly of bimetallic gold-silver nanoparticles via selective interparticle dicarboxylate-silver linkagesChem Mater200518123132
- MayerARMarkJEColloidal Gold Nanoparticles Protected by Cationic PolyelectrolytesJ Macromol Sci A19973421512164
- ChoHBakerBRTokJHAptamer-based SERRS sensor for thrombin detectionNano Lett200884386439019367849
- HuangCCHuangYFChangHTAptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptorsAnal Chem2005775735574116131089
- MedleyCDSmithJETanWGold nanoparticle-based colorimetric assay for the direct detection of cancerous cellsAnal Chem2008801067107218198894
- ChenWBianAKotovANanoparticle superstructures made by polymerase chain reaction: collective interactions of nanoparticles and a new principle for chiral materialsNano Lett200992153215919320495
- OtsukaHNagasakiYKataokaKPEGylated nanoparticles for biological and pharmaceutical applicationsAdv Drug Deliv Rev20035540341912628324
- LévyRWangZBrustMA generic approach to monofunctionalized protein-like gold nanoparticles based on immobilized metal ion affinity chromatographyChem Bio Chem20067592594
- SperlingRAPellegrinoTParakWJElectrophoretic separation of nanoparticles with a discrete number of functional groupsAdv Func Mater200616943948
- AslanKLuhrsCPérez-LunaVControlled and reversible aggregation of biotinylated gold nanoparticles with streptavidinJ Phys Chem B20041081563115639
- GoleAMurphyCJAzide-derivatized gold nanorods: functional materials for “Click” chemistryLangmuir20082426627218052398
- BrennanJHatzakisNBrustMBionanoconjugation via click chemistry: the creation of functional hybrids of lipases and gold nanoparticlesBioconjug Chem2006171373137517105213
- WangLLuoJSchadtMZhongCJThin film assemblies of molecularly-linked metal nanoparticles and multifunctional propertiesLangmuir20102661863219591490
- WangZLévyRFernigDGBrustMThe peptide route to multifunctional gold nanoparticlesBioconjug Chem20051649750015898714
- ShiLLuYShenJSite-selective lateral multilayer assembly of bienzyme with polyelectrolyte on ITO electrode based on electric field-induced directly layer-by-layer depositionBiomacromolecules200341161116712959579
- ChenCZhangPRosiNA new peptide-based method for the design and synthesis of nanoparticle superstructures: construction of highly ordered gold nanoparticle double helicesJ Am Chem Soc2008130135551355718800838
- GoleAMurphyCJPolyelectrolyte-coated gold nanorods: synthesis, characterization and immobilizationChem Mater20051713251330
- El-SayedMASmall is different: shape-, size-, and composition-dependent properties of some colloidal semiconductor nanocrystalsAcc Chem Res20043732633315147173
- El-SayedMASome interesting properties of metals confined in time and nanometer space of different shapesAcc Chem Res20013425726411308299
- KellyLKCoronadoEZhaoLLSchatzGCThe optical properties of metal nanoparticles: the influence of size, shape, and dielectric environmentJ Phys Chem B2003107668677
- JainPKHuangXEl-SayedIHEl-SayedMANoble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicineAcc Chem Res2008411578158618447366
- ZanchetDMicheelCMAlivisatosPElectrophoretic isolation of discrete Au nanocrystal/DNA conjugatesNano Lett200113235
- ZanchetDMicheelCMAlivisatosPElectrophoretic and structural studies of DNA-directed Au nanoparticle groupingsJ Phys Chem B20021061175811763
- MullenDGDesaiAMBakerJRThe implications of stochastic synthesis for the conjugation of functional groups to nanoparticlesBioconjug Chem2008191748175218729391
- LeeJSStoevaSIMirkinCADNA-induced size-selective separation of mixtures of gold nanoparticlesJ Am Chem Soc20061288899890316819885
- AhmadiTSLogunovSLEl-SayedMASize-dependent electron dynamics of gold nanoparticles nanostructured materialsNanostruct Mater1997679125140
- KumarAMandalSSastryMInvestigation into the interaction between surface-bound alkylamines and gold nanoparticlesLangmuir20031962776282
- LandesCFBraunMEl-SayedMAOn the nanoparticle to molecular size transition: fluorescence quenching studiesJ Phys Chem20011051055410558
- BrewerSHGlommWRFranzenSProbing BSA binding to citrate-coated gold nanoparticles and surfacesLangmuir2005219303930716171365
- StorhoffJJElghanianRMirkinCALetsingerRLSequence-dependent stability of DNA-modified gold nanoparticlesLangmuir20021866666670
- SauTKMurphyCJSelf-assembly patterns formed upon solvent evaporation of aqueous cetyltrimethylammonium bromide-coated gold nanoparticles of various shapesLangmuir2005212923292915779967
- KimTLeeKGongMSJooSWControl of gold nanoparticle aggregates by manipulation of interparticle interactionLangmuir2005219524952816207031
- KrpetiZNativoPPortaFBrustMAMultidentate peptide for stabilization and facile bioconjugation of gold nanoparticlesBioconjug Chem20092061962419220052
- LiuYFranzenSFactors determining the efficacy of nuclear delivery of antisense oligonucleotides by gold nanoparticlesBioconjug Chem2008191009101618393455
- MassichMDGiljohannDAMirkinCARegulating immune response using polyvalent nucleic acid-gold nanoparticle conjugatesMol Pharm200961934194019810673
- AkamatsuKKimuraMSugimotoNA DNA duplex with extremely enhanced thermal stability based on controlled immobilization on gold nanoparticlesNano Lett2006649149516522049
- SeferosDSPrigodichAEMirkinCAPolyvalent DNA nanoparticle conjugates stabilize nucleic acidsNano Lett2009930831119099465
- SrivastavaSFrankampBLRotelloVMControlled plasmon resonance of gold nanoparticles self-assembled with PAMAM dendrimersChem Mater200517487490
- FurushoHKitanoKHamaguchiSNagasakiYPreparation of stable water-dispersible PEGylated gold nanoparticles assisted by nonequilibrium atmospheric-pressure plasma jetsChem Mater20092135263535
- LiZZhangYFullhartPMirkinCAReversible and chemically programmable micelle assembly with DNA block-copolymer amphiphilesNano Lett2004410551058
- SastryMRaoMGaneshKElectrostatic assembly of nanoparticles and biomacromoleculesAcc Chem Res20023584785512379137
- LévyRThanhNKFernigDGRational and combinatorial design of peptide capping ligands for gold nanoparticlesJ Am Chem Soc2004126100761008415303884
- HanMSLytton-JeanARMirkinCAA gold nanoparticle based approach for screening triplex DNA BindersJ Am Chem Soc20061284954495516608320
- RocaMPandyaNHNathSHaesAJLinear assembly of gold nanoparticle clusters via centrifugationLangmuir2010262035204120099925
- PengZWaltherTKleinermannsKInfluence of intense pulsed laser irradiation on optical and morphological properties of gold nanoparticle aggregates produced by surface acid-base reactionsLangmuir2005214249425316032828
- JainPrashant KQianWEl-SayedMAUltrafast cooling of photoexcited electrons in gold nanoparticle-thiolated DNA conjugates involves the dissociation of the gold-thiol bondJ Am Chem Soc20061282426243316478198
- PetersenSBarcikowskiSConjugation efficiency of laser-based bioconjugation of gold nanoparticles with nucleic acidsJ Phys Chem20091131983019835
- LeeSELiuGLKimFLeeLPRemote optical switch for localized and selective control of gene interferenceNano Lett2009956257019128006
- DvirTBanghartMRKohaneDSPhoto-targeted nanoparticlesNano Lett20101025025419904979
- MaliPBhattacharjeeNSearsonPCElectrochemically programmed release of biomolecules and nanoparticlesNano Lett200661250125316771589
- HillHDMillstoneJEBanholzerMJMirkinCAThe role radius of curvature plays in thiolated oligonucleotide loading on gold nanoparticlesACS Nano2009341842419236080
- WongIYMeloshNADirected hybridization and melting of DNA linkers using counterion-screened electric fieldsNano Lett200993521352619606816
- CharrierACandoniNThibaudauFDNA detection method based on the two-dimensional aggregation and selective desorption of nanoparticle probesJ Phys Chem B200626128961290016805588
- DemersLinette MMirkinChad AThibaudauFA fluorescence-based method for determining the surface coverage and hybridization efficiency of thiol-capped oligonucleotides bound to gold thin films and nanoparticlesAnal Chem2000725535554111101228
- RayPCDarbhaGKBalarezoAGold nanoparticle based surface energy transfer probe for accurate identification of biological agents DNAACS Symp Ser20091016115129
- ChithraniBDGhazaniAAChanWCDetermining the size and shape dependence of gold nanoparticle uptake into mammalian cellsNano Lett2006666266816608261
- GiljohannDASeferosDSMirkinCAOligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticlesNano Lett200773818382117997588
- GhoshPSKimCKRotelloVMEfficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticlesACS Nano200822213221819206385
- SvarovskySAGonzalez-MoaMJSykesKSelf-assembled micronanoplexes for improved biolistic delivery of nucleic acidsMol Pharm200961927193319754152
- DeLongRKAkhtarUSalleeMCharacterization and performance of nucleic acid nanoparticles combined with protamine and goldBiomaterials2009306451645919726081
- SandhuKKMcIntoshCMRotelloVMGold nanoparticle-mediated transfection of mammalian cellsBioconjug Chem2002133611792172
- DelongRKKnowleRWernerAR4 peptide-pDNA nanoparticle coated HepB vaccine microparticles: sedimentation, partitioning, and spray freeze dry bioprocessesJ Nanosci Nanotechnol200662783278917048483
- AhmedMDengZNarainRCationic glyconanoparticles: their complexation with DNA, cellular uptake, and transfection efficienciesBioconjug Chem2009202169217619919109
- AhmedMDengZNarainRStudy of transfection efficiencies of cationic glyconanoparticles of different sizes in human cell lineACS Appl Mater Interf2009119801987
- VolodkinDVMadaboosiNBlacklockJSurface-supported multilayers decorated with bio-active material aimed at light-triggered drug deliveryLangmuir200925140371404319670892
- LiangMLinICTothICellular uptake of densely packed polymer coatings on gold nanoparticlesACS Nano2010440341319947583
- DenigerDCKolokoltsovAADaveyRATargeting and penetration of virus receptor bearing cells by nanoparticles coated with envelope proteins of Moloney murine leukemia virusNano Lett200662414242117090066
- ShukoorMINatalioFKsenofontovVDouble-stranded RNA polyinosinic-polycytidylic acid immobilized onto gamma-Fe2O3 nanoparticles by using a multifunctional polymeric linkerSmall200781374137817583549
- PanYNeussSJahnen-DechentWSize-dependent cytotoxicity of gold nanoparticlesSmall200731941194917963284
- ConnorEEMwamukaJWyattMDGold nanoparticles are taken up by human cells but do not cause acute cytotoxicitySmall2005132532717193451
- LeroueilPRHongSBanaszak HollMMNanoparticle interaction with biological membranes: Does nanotechnology present a Janus face?Acc Chem Res20074033534217474708
- BrondaniVZhangHFilipowiczWSpecific interference with gene expression induced by long, double stranded RNA in mouse embryonalteratocarcinoma cell linesProc Natl Acad Sci U S A200198144281443311724966
- PaddisonPJCaudyAAHannonGJStable suppression of gene expression by RNAi in mammalian cellsProc Natl Acad Sci U S A2002991443144811818553
- CzaudernaFFechtnerMKaufmannJStructural variations and stabilising modifications of synthetic siRNAs in mammalian cellsNucleic Acids Res2003312705271612771196
- MokHParkTGSelf-crosslinked and reducible fusogenic peptides for intracellular delivery of siRNABiopolymers20088988188818521895
- KimHRKimIKParkTGCationic solid lipid nanoparticles reconstituted from low density lipoprotein components for delivery of siRNAMol Pharm2008562263118461969
- GiljohannDASeferosDSMirkinCAGene regulation with polyvalent siRNA-nanoparticle conjugatesJ Am Chem Soc20091312072207319170493
- ElbakryAZakyABreunigMLayer-by-layer assembled gold nanoparticles for siRNA deliveryNano Lett200992059206419331425
- ShuklaRBansalVSastryMBiocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overviewLangmuir200521106441065416262332
- JeongJHMokHOhYKParkTGsiRNA conjugate delivery systemsBioconjug Chem20092051419053311
- PissuwanDNiidomeTCortieMBThe forthcoming applications of gold nanoparticles in drug and gene delivery systemsJ Control Release Epub2009
- NohSMKimWKKimSJEnhanced cellular delivery and transfection efficiency of plasmid DNA using positively charged biocompatible colloidal gold nanoparticlesBiochim Biophys Acta2007574775217324519
- HuCPengQChenFZhongZZhuoRLow molecular weight polyethylenimine conjugated gold nanoparticles as efficient gene vectorsBioconjug Chem2010583684320438071
- ArvizoRRMirandaORThompsonMAEffect of nanoparticle surface charge at the plasma membrane and beyondNano Lett Epub2010
- ChakravarthyKVBonoiuACDavisWGGold nanorod delivery of an ssRNA immune activator inhibits pandemic H1 N1 influenza viral replicationProc Natl Acad Sci U S A201022101721017720498074
- StoneGWBarzeeSSnarskyVNanoparticle-delivered multimeric soluble CD40 L DNA combined with Toll-like receptor agonists as a treatment for melanomaPLoS One2009410e733419812695