Abstract
Study objectives
To evaluate sex differences in predictors of obstructive sleep apnea (OSA) as per outcomes from home sleep apnea testing.
Design
This was a retrospective analysis of a large repository of anonymous test results and pretest risk factors for OSA.
Setting and patients
A total of 272,705 patients were referred for home sleep apnea testing from a variety of clinical practices for suspected sleep disordered breathing across North America from 2009 to 2013.
Interventions
Not applicable.
Measurements and results
Predictors of OSA (apnea hypopnea index4%≥5) were evaluated by multiple logistic regression; sex differences were evaluated by interaction effects. Middle age was the single most robust predictor of OSA for both sexes and was particularly foretelling for females (P<0.001) even after controlling for measures of adiposity and medical conditions. Females over the age of 45 years were much more likely to have OSA compared to their younger counterparts (78.7% vs 42.5%, respectively; odds ratio: 5.0) versus males (88.1% vs 68.8%, respectively; odds ratio: 3.4). Snoring, although more frequently reported by males, was similarly predictive of OSA for both sexes. Witnessed apneas and measures of adiposity were better predictors of OSA for males than females. Insomnia, depression, and use of sleep medication, although more commonly reported in females, did not predict OSA. Hypertension, although equally reported by both sexes, performed better as a predictor in females (P<0.001), even after controlling for age, measures of adiposity, and other medical conditions. Diabetes, heart disease, stroke, and sleepiness did not contribute unique variance in OSA in adjusted models.
Conclusion
This study found that males and females report different symptoms upon clinical evaluation for suspected sleep apnea, with some of the “classic” OSA features to be more common in and robustly predictive for males. The finding that advancing age uniquely and robustly predicted OSA in females reinforces our understanding that age-related changes in sex hormones play a role in the development and/or manifestation of sleep disordered breathing. Need exists for sex-specific prediction models and quantification of menopausal status in OSA screening tools.
Keywords:
Introduction
Obstructive sleep apnea (OSA) syndrome is a serious medical condition associated with increased morbidity, mortality, and high direct and indirect costs.Citation1 Diagnosing OSA has traditionally required an attended polysomnogram. However, home sleep apnea testing (HSAT) is an alternative for carefully selected patients with a high pretest probability of moderate to severe OSA.Citation2 Although “high pretest probability” is not a clear-cut phenotype,Citation3 practitioners are encouraged to rely on well-known risk factors for OSA, such as obesity, male sex, advancing age, snoring, gasping/witnessed apneas, and sleepiness.Citation4 Male sex is a known risk factor for OSA with a male-to-female ratio conservatively estimated at between 3 and 5:1 in the general population.Citation5,Citation6 However, there appears to be a general underrecognition of OSA in females because studies on clinical samples suggest a much sex wider sex gap in presentation for the evaluation of sleep disordered breathing (between 6 and 10:1).Citation5,Citation6 Further, data suggest that females have a more delayed diagnosis as they are typically older, heavier, and report more medical comorbidities at the time of clinical evaluation.Citation6 Sex differences in the prevalence of OSA have been attributed to a number of factors, including differences in body fat distribution, craniofacial anatomy/airway mechanics, and lifestyle risk factors, such as alcohol and tobacco use.Citation7
Although male risk for OSA is apparent, recent data have suggested that the male–female gap in OSA may be smaller than once believed. Instead, it has been hypothesized that females with OSA may not present the same clinical symptoms as their male counterparts and, thus, may be less likely to present to a sleep practitioner and/or may be less likely to be referred for sleep testing. For example, females with OSA may be less likely to report some of the aforementioned classic “high pretest” features of OSA, including witnessed apneas,Citation8 and instead report symptoms of disrupted sleep, depression, and insomnia.Citation8,Citation9
Furthermore, female risk for OSA, and thus clinical symptoms, is likely moderated by menopausal status. Generally speaking, the menopausal period is associated with a 3.5–4 factor increase in OSA risk for females controlling for other factors that can influence OSA risk, such as age and body mass index (BMI).Citation7,Citation10 Although the relationship between menopause and OSA is complex, risk for sleep disordered breathing is at least partially attributed to estrogen and progesterone-mediated changes in body fat distribution and airway anatomy and mechanics.Citation11,Citation12 Since the OSA testing landscape has undergone a paradigm shift toward unattended sleep testing where clinicians are encouraged to rely on high pretest indicators of OSA, it is worthwhile to reevaluate the utility of said indicators for males and females on actual HSAT outcomes. The following domains were investigated: 1) self-reported OSA symptoms, 2) adiposity, 3) common medical comorbidities, and 4) age.
Methods
Sleep data were acquired from a large repository of anonymous HSAT outcomes from 2009 to 2013. The study was evaluated and approved by the Schulman Institutional Review Board. Home apnea testing was completed using the Apnea Risk Evaluation System (ARES) model 610, which simultaneously records airflow by via nasal cannula, oxygen saturation (SpO2) and heart rate by forehead reflectance pulse oximetry, snoring via a calibrated acoustic microphone, and head position/movement via forehead accelerometry. The device’s algorithm estimates sleep time using behavioral indicators of quiescence (nonmovement and regularity in nasal flow and/or snoring).Citation13 The device and associated autoscoring algorithm have demonstrated adequate sensitivity (0.98) and specificity (0.84) when compared to simultaneously recorded polysomnogram using an apnea hypopnea index (AHI) cutoff of ≥5 events per hour.Citation14 As per standard protocol, all raw data were autoscored, edited by a registered sleep technologist, and interpreted by a physician.
The AHI and a respiratory disturbance index were calculated as the average number of apneas (no apparent airflow) and hypopneas (≥50% reduction in flow plus a ≥4% desaturation) per hour of valid recording time and the average number of apneas, hypopneas (4%), plus flow-limited events (≥50% reduction in flow that terminated with increased movement, snoring, and/or pulse rate) and ≥1% desaturation per hour of valid recording time, respectively. For the ARES, valid recording time is defined as the length of the sleep period minus wake after sleep onset (actigraphy based) and periods of poor signal integrity. Risk factors for OSA were extracted from the ARES OSA screening questionnaire,Citation15 which assesses a variety of self-reported symptoms of OSA as well as anthropomorphic risk factors (BMI and neck circumference) and common comorbid medical risk factors. Self-reported OSA symptoms were assessed with the question “on average in the past month, how often have you snored or been told that you snore […] do you wake up choking or gasping […] have you ever been told that you stop breathing in your sleep or wake up choking or gasping?” and dichotomized “at least frequently (≥3–4 times/week; yes)”/“sometimes or less (≤1–2 times/week; no).” Self-reported comorbidities (yes/no) were assessed with the question “have you been diagnosed or treated for any of the following conditions?” Data on sleepiness were acquired from an embedded (in the ARES OSA screening questionnaire) Epworth Sleepiness Scale (ESS).Citation16 In predictive models, sleepiness was considered present if ESS ≥10. Studies were excluded from analyses if there was missing questionnaire data or if valid recording time was <2 hours.
Analyses were performed using SPSS 23.0 (IBM Corporation, Armonk, NY, USA). Descriptive analyses were completed to analyze the shape, central tendency, and dispersion of all variables. Sex differences in continuous variables were analyzed using univariate analysis of variance with eta squared (η2) to estimate effect size. Descriptive differences between nominal or dichotomous variables were analyzed using chi-square (χ2) with phi coefficient (Φ) to estimate the effect size. Phi coefficients <0.10 were considered negligible. Unadjusted and adjusted multivariable logistic regression with odds ratios and Nagelkerke R2 to estimate effect size were used to evaluate predictors of OSA. Odds ratios represent the odds that an outcome will occur given a particular exposure compared to the odds of the outcome occurring in the absence of that exposure.Citation17 For logistic analyses, we defined the presence of OSA as an AHI ≥5 and sex differences were evaluated using interaction terms. P-values <0.05 were considered statistically significant and all comparisons were two-tailed.
Results
The final sample size consisted of 272,705 patients. A total of 19,909 patients (6.6%) were excluded from analyses because of missing anthropomorphic or demographic data. A total of 10,295 (3.4%) studies were excluded from analyses due to insufficient recording time (defined as <2 hours) and an additional 1,590 studies (<1%) were removed due to missing questionnaire data.
Demographics and comorbidities
The final adult sample was comprised of slightly more males than females (57% male). lists the demographics for the sample, and illustrates the similar distribution shape for age for males and females. Modal number of study nights was 1 (79%) with a mean recording duration of 6.4±1.2 hours and mean valid recording time of 5.5±1.2 hours. Studies with more than one night of data collected were not excluded from analyses; rather, study information (AHI, recording duration, etc) was averaged over the number of study nights collected. Number of study nights (χ2 [1, 272,695]=1.6, P=0.21) and study duration (Δ5 minutes; F [1, 272,703]=58.3, P<0.001; η2=0.00) were similar for males and females. Age, BMI, and ESS were remarkably similar for males and females (although statistically significantly different, measures of effect size were negligible). Females were less likely to report some of the “classic” symptoms of sleep apnea, namely, frequent snoring and witnessed apneas (). Females were more likely to report insomnia, depression, and use of sleep medication. In general, females had test results indicative of less severe OSA compared to males.
Figure 1 Distribution of age for males and females being evaluated for obstructive sleep apnea by home sleep apnea testing.
Table 1 Demographics, self-reported comorbidities, and HSAT test outcomes
Sex differences in OSA prevalence across age
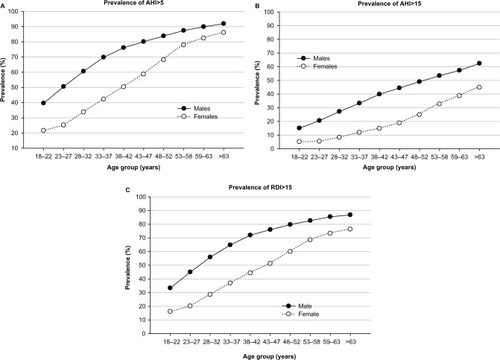
Sex (unadjusted) accounted for 3.6% of the variance in OSA in that males were more likely to have test outcomes indicative of OSA than females, especially moderate-to-severe OSA (AHI ≥15). However, illustrates that the prevalence of OSA in females increases in a more precipitous fashion than males, especially after 45 years of age. In fact, females appear to “catch up” with their male counterparts in late middle age when using AHI ≥5 or respiratory disturbance index ≥15 as a diagnostic cutoff. When defined as an AHI ≥5, the prevalence of OSA in males and females was much more similar after 45 years (88.1% males vs 78.7% females) compared to earlier in life (68.8% males vs 42.5% females). However, the prevalence of moderate-to-severe OSA (AHI ≥15) was always more pronounced in males ().
Figure 2 Sex differences in HSAT outcomes across age.
Domain 1: age
Middle age (≥45) was the single most robust predictor of OSA in unadjusted () and adjusted () analyses for both sexes, accounting for over 10% of the variance in OSA (AHI ≥5). On average, individuals over 45 years of age had four times the odds of having an AHI ≥5, controlling for sex, BMI, neck circumference, and medical conditions. Middle age was particularly foretelling for females (), even after controlling for measures of adiposity (neck circumference and BMI) and medical conditions (). The function was similar for moderate-to-severe OSA (AHI ≥15; females odds ratio: 4.72 vs males odds ratio: 3.03; interaction χ2 [1, 272,705]=726.8, P<0.001).
Table 2 Unadjusted logistic regression analyses of predictors of OSA (AHI ≥5)
Table 3 Adjusted logistic regression analyses of predictors of OSA (AHI ≥5)
Domain 2: adiposity
Obesity (BMI ≥30) and neck circumference performed better as predictors of OSA for males than females (), even after controlling for age and medical conditions (). On average, every inch increase in neck circumference was associated with a >20% increased risk for OSA, controlling for sex, age, BMI, and medical conditions.
Domain 3: symptoms of OSA
Frequent snoring was a robust predictor of OSA and performed equally for both sexes (), even after controlling for age, measures of adiposity, and medical conditions (). Waking choking “at least frequently” was a fair predictor of OSA in the unadjusted model, especially for males (), but was no longer significant in the adjusted model (males P=0.15, females P=0.05). Witnessed apneas remained robust in the adjusted model but were better for males than females (). Sleepiness, although slightly better in predicting OSA for males than females, was not a reliable predictor of OSA as it accounted for negligible variance (; R2<0.01), and thus was not added to the adjusted model.
Domain 4: medical comorbidities
A history of hypertension performed better as a predictor of OSA for females than males (), even after controlling for age, measures of adiposity, and other medical conditions (). Diabetes and heart disease performed equally for both sexes () but were attenuated in the adjusted model (). Stroke, although appearing to predict OSA in unadjusted models, accounted for negligible variance in OSA outcomes (; R2<0.01), and was thus not added to the adjusted model.
Insomnia, sleep medication, and depression
Neither insomnia nor depression predicted OSA for either sex. Interestingly, the use of sleep medication was associated with slightly less OSA risk, namely, for males, but effect sizes were negligible and thus not added to the adjusted model.
Discussion
To the best of our knowledge, this is the first study to evaluate sex differences in predictors of OSA in patients being evaluated by at-home sleep testing. This study found that males and females report different symptoms upon clinical evaluation for suspected sleep apnea, with some of the classic OSA features, including anthropometric measures of adiposity and witnessed apneas, to be more common in and robustly predictive for males. Females indeed were more likely to report depression, insomnia, and use of sleep medication; however, these factors were not associated with HSAT outcomes. The finding that sex mediated the potency of OSA predictors highlights the need for sex-specific prediction models to maximize the sensitivity and specificity of pretest screening for OSA.
Male risk for OSA
Even in this high OSA risk sample, females were generally less likely to have test results indicative of OSA, especially moderate-to-severe OSA. On average, males in this sample were ~1.6–2 times more likely to have test results indicative of OSA compared to females. Male risk for OSA is likely at least partially attributable to other risk factors not assessed in this study, including craniofacial/airway structure and function. For example, data have suggested that females generally have “more favorable” airway mechanics than males as evidenced by the fact that females consistently have less severe OSA despite smaller pharyngeal airways.Citation18,Citation19 However, an interesting finding was that OSA that was at least mild in nature (AHI ≥5) became much more prevalent in females and was remarkably similar to males later in life, corroborating what has been observed in community samples.Citation4 This, in conjunction with the finding that age was a particularly robust predictor for OSA in females, even when holding measures of adiposity constant, highlights the potential role of age-related changes in hormones in airway patency.Citation12
Self-reported symptoms
Similar to previous reports,Citation8,Citation9 some of the classic high pretest symptoms of OSA, including snoring and witnessed apneas, were less commonly reported as presenting symptoms of OSA in females. Although it is not entirely clear why this is, several possibilities exist. It is possible that females are simply less likely to have these symptoms or that these symptoms require a “severe” OSA phenotype. It is also possible that menopausal status mediates the manifestation of these symptoms in females. For example, reductions in estrogen and progesterone during the menopausal period may allow for OSA (and thus symptoms) to manifest via changes in upper airway dilator activity,Citation12 especially in the presence of other risk factors found to predict female OSA, such as increased adiposity. Also, females may simply be better reporters for their male counterparts’ symptoms as opposed to vice versa. Lastly, it is also a possibility that females underreport snoring and witnessed apneas because of embarrassment or social norms (ie, not “lady-like”).Citation7
The finding that females in this sample were more likely to report “atypical OSA symptoms”, such as a diagnosis of insomnia, depression, and use of sleep medication, was similar to other studies.Citation6,Citation8,Citation9 However, because these factors were not particularly helpful in determining female risk of OSA, this finding suggests a higher base rate of these complaints in females. It does, however, highlight that females with OSA may be at an increased risk of more complicated sleep management, as they may require comanagement by mental health care providers and sleep medicine specialists. It is important to note that we did not assess other “atypical OSA symptoms” found to be more commonly reported in females, including fatigue, tiredness, or lack of energy.Citation19
Risk factors stronger for male OSA
Not only were males more likely to report some of the classic features of sleep apnea, but these factors were also better predictors of OSA in males than females. Obesity, as per cutoffs derived from BMI, is likely a better predictor of OSA for males as they are more likely to have visceral as opposed to peripheral (as in females) adiposity.Citation20 Visceral adiposity directly influences OSA risk via excess fat deposition in the parapharyngeal space and/or increased work of breathing.Citation21 If males are more likely to have this upper airway phenotype, then it holds true that choking, which is a direct manifestation of reduced pharyngeal space, would be a better indicator for males. Neck circumference as an anthropometric measure faired more similarly for males and females and is intuitive as it is a more direct measure of pharyngeal restriction than overall body habitus.Citation21
Risk factors stronger for female OSA
In this study, advancing age was the single strongest predictor of OSA for both sexes. Age is a well-known risk factor associated with changes in upper airway tone, pharyngeal fat distribution, and the development of comorbid diseases.Citation4 The finding that advancing age uniquely and robustly predicted OSA in females reinforces our understanding that age-related changes in sex hormones, including estradiol, follicle stimulating hormone, progesterone, and testosterone, play a role in the development and/or manifestation of sleep disordered breathing.Citation11 Most importantly, this highlights the need for quantifying menopausal status in OSA screening tools – something “not” typically assessed. The finding that hypertension was a stronger predictor of female OSA could indicate a longer exposure to abnormal breathing, and thus more advanced disease, or that clinicians were simply more likely to refer female patients if hypertension was present. These data warrant further investigation.
Sex-neutral OSA predictors
Despite males being more likely to report frequent snoring, it was the most robust and sex-neutral self-reported symptom of OSA. These findings support other dataCitation22 and are encouraging as snoring is a salient factor that many clinicians and standardized measures query.Citation23,Citation24 With regard to medical conditions, diabetes was equally predictive for both sexes. The link between OSA and diabetes has been well established both under controlled laboratory and naturalistic conditions and is thought to be attributable to maladaptive response of repetitive sleep fragmentation/restriction and long-term exposure to intermittent hypoxemia on glucose metabolism.Citation25
Factors not associated with OSA
Sleepiness, as assessed by the ESS, was a weak predictor of OSA, especially for females. This has been reported by multiple studies previouslyCitation26,Citation27 and highlights the importance of avoiding sleepiness as the “sole” indicator of sleep apnea.Citation28 These findings have important policy implications as many payors and care utilization companies rely on sleepiness as per the ESS to warrant sleep testing.Citation29 Our data suggested that a history of stroke resulted in a 77% increase in odds ratios of sleep disordered breathing, but the variance accounted for was small (<0.001%). This suggests that risk for OSA in this sample was due to other factors that may coexist with a history of stroke, such as obesity and heart disease.
Conclusions drawn from this study must be made with the study’s limitations in mind. The most pronounced limitation was in regard to the nature of the sample. The sample was not a random sample and consisted of patients who were deemed at risk for sleep apnea, namely, those who were able to successfully complete an at-home sleep test. Thus, prediction models may have been driven by factors outside of what we directly measured in this study, including the clinician’s sense of the patient’s pretest risk for OSA. Also, all questionnaire data were self-reported in nature and may not align with practitioner assessment. It is important to note that there were likely patients who were deemed to have an abnormal AHI as per the HSAT, but were actually normal (specificity 0.76). Another limitation was that we were not able to evaluate race/ethnicity as a predictor of OSA. Recent data have suggested that certain racial groups, especially Asians, are at a particularly high risk for OSA,Citation30 even in the absence of obesity.Citation31 Despite these limitations, the study had a number of strengths, including a very large sample size with estimates of effect size, adequate representation of both sexes, and a variety of factors for which to evaluate OSA risk.
Acknowledgments
This study was funded by SleepMed, Inc.
Disclosure
Doctor Cairns, Doctor Bogan, and Greg Poulos are employed by SleepMed, Inc. The authors report no other conflicts of interest in this work.
References
- Harvard Medical SchoolThe Price of Fatigue: The Surprising Economic Costs of Unmanaged Sleep ApneaBoston, MAHarvard Medical School2010
- CollopNAAndersonWMBoehleckeBClinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patientsJ Clin Sleep Med20073773774718198809
- CollopNHome sleep testing: appropriate screening is the keySleep201235111445144623115391
- YoungTSkatrudJPeppardPERisk factors for obstructive sleep apnea in adultsJAMA2004291162013201615113821
- YoungTPaltaMDempseyJSkatrudJWeberSBadrSThe occurrence of sleep-disordered breathing among middle-aged adultsN Engl J Med199332817123012358464434
- Quintana-GallegoECarmona-BernalaCCapoteaFGender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patientsRespir Med2004981098498915481275
- LinCMDavidsonTMAncoli-IsraelSGender differences in obstructive sleep apnea and treatment implicationsSleep Med Rev200812648149618951050
- ShepertyckyMRBannoKKrygerMHDifferences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndromeSleep200528330931416173651
- ValipourALothallerHRauscherHZwickHBurghuberOCLaviePGender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaireSleep200730331231917425227
- YoungTFinnLAustinDPetersonAMenopausal status and sleep-disordered breathing in the Wisconsin sleep cohort studyAm J Respir Crit Care Med200316791181118512615621
- JoffeHMasslerASharkeyKEvaluation and management of sleep disturbance during the menopause transitionSemin Reprod Med201028540442120845239
- PopovicRMWhiteDPUpper airway muscle activity in normal women: influence of hormonal statusJ Appl Physiol1998843105510629480969
- PopovicDVelimirovicVAyappaISleep/wake classification using head actigraphy, snoring and airflow signals [abstract]Sleep200832379
- AyappaNNormanRGSeelallVRapoportDValidation of a self-applied unattended monitor for sleep disordered breathingJ Clin Sleep Med200841263718350959
- LevondowskiDOlmsteadRPopovicDCarperDBerkaCWestbrookPRAssessment of obstructive sleep apnea risk and severity in truck drivers: validation of a screening questionnaireSleep Diagnosis Ther2007222026
- JohnsMA new method for measuring sleepiness: the Epworth sleepiness scaleSleep19911465405451798888
- MorganSReichertRHarrisonTFrom Numbers to Words: Reporting Statistical Results for the Social SciencesBoston, MAAllyn & Bacon2001
- MohseninVGender differences in the expression of sleep-disordered breathingChest20011201442144711713117
- ChervinRDSleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apneaChest2000118237237910936127
- TaylorRWGrantAMWilliamsSMGouldingAGender differences in regional body fat distribution from pre- to post pubertyObesity20101871410141619893501
- RedlineSGenetics of obstructive sleep apneaKrygerMRothTDementWPrinciples and Practices of Sleep Medicine5th editionMissouriElsevier201111831193
- YoungTHuttonRFinnLBadrSPaltaMThe gender bias in sleep apnea diagnosis: are women missed because they have different symptoms?Arch Intern Med199615621244524518944737
- ChungFYegneswaranBLiaoPA tool to screen patients for obstructive sleep apneaAnesthesiology200810881282118431116
- NetzerNCStoohsRANetzerCMClarkKStrohlKPUsing the Berlin Questionnaire to identify patients at risk for the sleep apnea syndromeAnn Intern Med199931748549110507956
- TasaliEMokhlesiBVan CauterEObstructive sleep apnea and type 2 diabetes: interacting epidemicsChest2008133249650618252916
- SilABarrGAssessment of predictive ability of Epworth scoring in screening of patients with sleep apnoeaJ Laryngol Otol2012126437237922152589
- ChervinRDAldrichMSThe Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apneaNeurology19995211251319921859
- BixlerEOVgontzasANLinHMCalhounSLVela-BuenoAKalesAExcessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depressionJ Clin Endocrinol Metab20059084510451515941867
- QuanSFAbuse of the Epworth sleepiness scaleJ Clin Sleep Med201391098724127140
- LeongWBAroraTJenkinsonDThe prevalence and severity of obstructive sleep apnea in severe obesity: the impact of ethnicityJ Clin Sleep Med20139985385823997696
- ChenXWangRZeePRacial/ethnic differences in sleep disturbances: the multi-ethnic study of atherosclerosis (MESA)Sleep201538687788825409106
