Abstract
Narcolepsy is a life-long, underrecognized sleep disorder that affects 0.02%–0.18% of the US and Western European populations. Genetic predisposition is suspected because of narcolepsy’s strong association with HLA DQB1*06-02, and genome-wide association studies have identified polymorphisms in T-cell receptor loci. Narcolepsy pathophysiology is linked to loss of signaling by hypocretin-producing neurons; an autoimmune etiology possibly triggered by some environmental agent may precipitate hypocretin neuronal loss. Current treatment modalities alleviate the main symptoms of excessive daytime somnolence (EDS) and cataplexy and, to a lesser extent, reduce nocturnal sleep disruption, hypnagogic hallucinations, and sleep paralysis. Sodium oxybate (SXB), a sodium salt of γ hydroxybutyric acid, is a first-line agent for cataplexy and EDS and may help sleep disruption, hypnagogic hallucinations, and sleep paralysis. Various antidepressant medications including norepinephrine serotonin reuptake inhibitors, selective serotonin reuptake inhibitors, and tricyclic antidepressants are second-line agents for treating cataplexy. In addition to SXB, modafinil and armodafinil are first-line agents to treat EDS. Second-line agents for EDS are stimulants such as methylphenidate and extended-release amphetamines. Emerging therapies include non-hypocretin-based therapy, hypocretin-based treatments, and immunotherapy to prevent hypocretin neuronal death. Non-hypocretin-based novel treatments for narcolepsy include pitolisant (BF2.649, tiprolisant); JZP-110 (ADX-N05) for EDS in adults; JZP 13-005 for children; JZP-386, a deuterated sodium oxybate oral suspension; FT 218 an extended-release formulation of SXB; and JNJ-17216498, a new formulation of modafinil. Clinical trials are investigating efficacy and safety of SXB, modafinil, and armodafinil in children. γ-amino butyric acid (GABA) modulation with GABAA receptor agonists clarithromycin and flumazenil may help daytime somnolence. Other drugs investigated include GABAB agonists (baclofen), melanin-concentrating hormone antagonist, and thyrotropin-releasing hormone agonists. Hypocretin-based therapies include hypocretin peptide replacement administered either through an intracerebroventricular route or intranasal route. Hypocretin neuronal transplant and transforming stem cells into hypothalamic neurons are also discussed in this article. Immunotherapy to prevent hypocretin neuronal death is reviewed.
Introduction
Narcolepsy is a chronic disabling condition that is estimated to affect 25–50/100,000 people.Citation1 “Narcolepsy robs you of your life’s goals and dreams”.Citation2 “There is no magic pill that completely controls narcolepsy. Even with the proper dose of my medications and lifestyle modifications, I still have to work hard to function anywhere close to normal”.Citation2 These laments articulate the devastating impact narcolepsy wields on the lives of patients. Surprisingly, there is low awareness and knowledge of narcolepsy among the general public, primary care physicians, and sleep specialists who responded to the AWAKEN survey.Citation3 Recognizing symptoms early, confirming the diagnosis, and treating narcolepsy effectively remains a challenge.
Clinical features
The narcolepsy pentad consists of excessive daytime sleepiness (EDS), cataplexy, hypnogogic hallucinations, sleep paralysis, and disrupted nocturnal sleep.Citation4–Citation6 Cognitive dysfunction (“brain fog”) includes difficulty focusing, thinking, and concentrating along with automatic behavior and blurry vision.Citation2 Depression and anxiety affect both adults and children. Hyperactive/aggressive behavior, problems interacting with peers, and exhibiting psychotic features may be noted in children.
Recurrent sleep attacks lasting less than 10 minutes is the most common presentation, while cataplexy is the most pathognomonic. Extended sleep time, weight gain, and increased body mass index (BMI) may be noted in children.Citation7 Cataplexy in adults involves paroxysmal episodes of loss of antigravity muscle tone, while the childhood phenotype consists of a complex movement disorder with persistent hypotonia and prominent buccofacial involvement (jaw opening, eyelid drooping, head rolling, or tongue thrusting movements), also called “cataplectic facies”.Citation5,Citation7 Cataplexy is triggered by laughing, sharp remarks, telling or listening to jokes, being tickled or tickling others, surprise, chuckling, meeting famous persons, feeling angry, stressed, or embarrassed/ashamed. Cataplexy attacks can occur spontaneously or during orgasm. Failure to recognize symptoms may delay diagnoses from 8.7 years up to 22.1 years.Citation8
Diagnosis, classification, and epidemiology
The International Classification of Sleep Disorders (ICSD-3) classifies narcolepsy into Type 1 (narcolepsy with cataplexy) and Type 2 (narcolepsy without cataplexy).Citation9 Type 1 is prevalent in 0.02%–0.18% in the US and Western European populations and in 0.16%–0.18% in the Japanese populations.Citation9 The prevalence of Type 2 disease is uncertain, but a point prevalence of 20.5/10,000 has been suggested.Citation9 The risk of narcolepsy Type 1 occurring in first-degree relatives of affected individuals is 1%–2%.Citation9
Narcolepsy Type I criteria require cataplexy plus either 1) two sleep-onset REM periods (SOREMPS) on multiple sleep latency test (MSLT) or a SOREMP on nocturnal polysomnogram (PSG) plus one SOREMP on MSLT or 2) cerebrospinal (CSF) hypocretin-1 (hcrt-1) concentration ≤110 pg/mL or <1/3 of mean values obtained in normal subjects with same standardized assay in addition to EDS.Citation9,Citation10 Type 2 narcolepsy patients do not have cataplexy but have EDS confirmation by MSLT or PSG + MSLT and CSF hcrt-1 concentration is not measured, or CSF hcrt-1 >110 pg/mL, or >1/3 of mean values obtained in normal subjects.Citation9 Differentiating narcolepsy from other disorders of excessive somnolence may be problematic.Citation11
Pathophysiology and genetics
Loss of up to 95% of hypocretin-producing neurons in the lateral hypothalamus resulting in low CSF hcrt-1 levels (Type I narcolepsy) appears to be the underlying major pathophysiology.Citation10,Citation12
Narcolepsy is strongly associated with HLA DQB1*06:02, and genome-wide association studies have identified polymorphisms in the T-cell-receptor-α (TCRA) locus on chromosome 14, TNFSF4 (also called OX40L), Cathepsin H (CTSH) the purinergic receptor P2RY11, the DNA methyltransferase DNMT1, and carnitine palmitoyltransferase (CPT1B).Citation12–Citation14 TCRA encodes the α chain of the α β-heterodimer of the T-cell receptor on CD4+ T-helper cells and CD8+ T-cytotoxic cells.Citation15 A third gene mutation is usually suggested as being needed for the autoimmune destruction of the hypocretin/orexin neurons. A combination of genetic and environmental factors could trigger immunological pathways through molecular mimicry or bystander activation, thereby leading to cell death of hypocretin-producing neurons.
Management
Narcolepsy is a life-long illness for which there is currently no cure. Treatment focuses on the following: 1) reduce EDS for fullest return of normal function; 2) minimize nocturnal sleep disruption; 3) treat cataplexy, hypnagogic hallucination (HH), and sleep paralysis (SP); and 4) consider risk–benefit ratio of the drug, cost of medications and ongoing care, and convenience of administration.Citation16 Quality measures formulated for the care of patients with narcolepsy include three outcome measures – 1) reducing EDS, 2) improving diagnostic accuracy, and 3) reducing adverse events.Citation17 Seven process measures were designated based on proportion of patients who met criteria that reflect these outcomes.Citation17 “Functionality” is included in process 5, but it is unclear how “functionality” is defined/quantified and to what extent the patients and their caregivers contribute to this parameter.
Symptom-based current therapy
Treatment of narcolepsy patients is both a science and an art of balancing drug efficacy, convenience of administration, development of drug tolerance, managing drug effects, considering comorbidities, monitoring for evidence of drug abuse, and, finally, choosing drugs partly based on insurance carrier’s “allowed” drugs and patient’s copay. It is important that clinicians are familiar with the various narcolepsy medications, mechanisms of action, dosing regimen, rationale for their use, and any drug–drug interactions.Citation5,Citation18–Citation25 shows various medications used to treat narcolepsy symptoms along with their relevant information. In addition to drug therapy, behavior therapy should be discussed with the patient and should include such topics as regular bedtimes, allowing enough time in bed at night, and taking scheduled naps during the day.
Table 1 Medications for narcolepsy
First-line therapy for daytime sleepiness
In adults with narcolepsy, modafinil or its r-enantiomer, armodafinil, are first-line therapies.Citation16 Modafinil selectively activates wake-generating sites in the hypothalamus. Fos immunoreactivity is increased in the tuberomammillary nucleus and in the hypocretin neurons in the perifornical area of the hypothalamus.Citation26 Modafinil binds competitively to dopamine transporter in the cell membrane and is dependent on catecholaminergic (dopamine and adrenergic) signaling for wake promotion.Citation27 Modafinil is a very weak but also very selective dopamine transporter inhibitor.Citation27 Relative to other stimulants that act through catecholaminergic mechanisms, modafinil has low abuse potential, produces wakefulness with an attenuated compensatory sleep thereafter, and does not improve cataplexy. Modafinil (200–400 mg/d) improved daytime somnolence, increased mean sleep latencies on maintenance of wakefulness test (MWT), and improved scores on Clinical Global Improvement of Change (CGI-C).Citation28–Citation30 Split dosing (200 mg at 7 am and 200 mg at noon) may be more effective than a single dose in the morning; 600 mg/d split dosing may be needed by some.Citation28 Somnolence was very much improved in 80% and 92%, of users at doses of 400 and 600 mg, respectively.Citation28 Armodafinil has a longer duration of action compared to modafinil; at doses of 100–250 mg/d, mean ESS (Epworth Sleepiness Scale) decreased from 16.9 to 12.6 after 12 months of therapy and percentage of patients with Epworth Sleepiness Scale (ESS) <10 increased from 3.4% at baseline to 31.3%.Citation31 “Worst fatigue” scores also declined from 7.8 (7.7 is severe) at baseline to 6.4 after a month’s treatment.Citation31 A cohort analysis of medical and pharmacy claims reported that mean monthly drug-specific pharmacy costs were lower for the armodafinil cohort vs modafinil cohort ($11,363 vs $13,775, P=0.005) and that lower total health care costs were also noted with armodafinil.Citation32
First-line therapy for EDS and cataplexy
Sodium oxybate is a metabolite of γ-amino butyric acid (GABA) that acts as putative neurotransmitter and neuromodulator.Citation16,Citation33 It may also act via specific non-GABAergic receptors; it also reduces dopamine release. Sodium oxybate (SXB) is first-line therapy for daytime sleepiness and cataplexy.Citation16,Citation33 It may improve HH and SP; it may also help consolidate nocturnal sleep. Compared to placebo, SXB effectively reduced daytime sleepiness and improved cataplexy.Citation34–Citation36 Sleep attack frequency and duration were significantly reduced at 6 and 9 gram doses. But effect on sleepiness takes at least 8 weeks before it becomes evident. The 9-gram dose significantly reduced nocturnal awakenings.Citation34 The addition of oxybate to modafinil resulted in the most improvement in subjective and objective measures of sleepiness when compared to modafinil alone in a level 1 study.Citation36
Although SXB is usually well tolerated, mild-to-moderate side effects can occur. Confusion, anxiety, and dizziness at the onset of therapy may be due to the dose being insufficient to induce sleep rapidly (up to 2 hours delay). A faster dose titration may be helpful. If nausea is prominent, adding flavored water may mask its salty taste; the dose may also be reduced. If nausea is severe, a 5-HT3 antagonist, such as ondansetron, may be prescribed.
Second-line therapy for EDS
Stimulants have traditionally been used to alleviate daytime somnolence; they are indirect sympathomimetic compounds that share a benzene ring with an ethylamine side chain. These drugs include methylphenidate, amphetamines (dextroamphetamine or amphetamine–dextromethamphetamine combination, or amphetamine sulfate). These traditional stimulants increase the release of noradrenaline, dopamine, and serotonin; they inhibit reuptake of amines by dopamine transporter, and synaptic concentration of amines is higher. Wakefulness may be promoted by increased amine signaling through direct effects on the cortex or via subcortical pathways. Central nervous system (CNS) effects increase threefold with the d-isomer of amphetamine compared to the l-isomer. Rebound hypersomnia can occur at the end of transmitter availability for release. Amphetamine, methamphetamine, and methylphenidate have high abuse potential, and so tolerance may occur.Citation19,Citation20,Citation24,Citation25 For these reasons, they are used only under special circumstances. For disabling daytime sleepiness that does not respond to first-line therapy, an intermediate-release formulation of methylphenidate may be useful. For patients who are partial responders to modafinil or armodafinil but who have difficulty performing in the late afternoon, in addition to behavioral measures (two naps), a supplemental dose of a short-acting stimulant, (we prefer methylphenidate) may be added.
For patients who are nonresponders to modafinil/armodafinil or SXB and who have been using amphetamines for their EDS, switching to extended-release formulations provide longer duration of action and help alleviate concerns about recreational abuse. MES-amphetamine extended release (Adderall XR) combines the neutral salts of dextroamphetamine and amphetamine and uses drug-containing beads for a double-pulsed delivery of amphetamines resulting in longer duration of action. This makes it suitable for administration at home with parental supervision and relieves the adolescent patient of the need to take additional medication outside of the home. Another once-daily amphetamine medication is the d-amphetamine prodrug lisdexamfetamine dimesylate (LDX; Vyvanse, Shire Pharmaceuticals, Dublin, Republic of Ireland). Common side effects are reduced appetite and insomnia. Children and adolescents taking LDX may have lower gains in weight, height, and BMI.
Second-line therapy for cataplexy, SP, HH
Antidepressant reuptake inhibitor medications or tricyclic antidepressants are second-line alternatives to treat cataplexy, SP, and HH. Drug categories are: 1) Serotonin–norepinephrine reuptake inhibitors – venlafaxine, which has a short duration of action, but extended-release formulation is available; desmethyl venlafaxine or atomoxetine are alternatives, 2) selective serotonin reuptake inhibitors (fluoxetine), or 3) tricyclic antidepressants (protriptyline, imipramine, or clomipramine) (). Of the above medications, venlafaxine is our preferred add-on to SXB.
Not included in is reboxetine, a selective norepinephrine reuptake inhibitor that reduced cataplexy in 12 subjects with Type 1 narcolepsy; this medication is, however, not available in the United States.Citation16 Selegiline, a MAO Type B inhibitor, at a dose of 10 mg 2 times/d suppressed REM sleep and increased sleep-onset and REM-onset latencies on both PSG and MSLT, and patients reported significant improvement in daytime sleepiness, reduced number of naps needed, and reduced frequency of cataplexy.Citation37 Although selegiline is listed as an option for narcolepsy therapy,Citation16 it is not usually prescribed because the high dose required makes it a nonselective MAO inhibitor with increased risk of food/drug interactions.
A large proportion of narcolepsy patients complain about cognitive issues (50%)Citation2 and severe fatigue (45%–62.5%);Citation2,Citation38 mood disorder (27%)Citation39 and anxiety (21.1%)Citation39 are also common. Fatigued patients have higher depression scores, but ESS scores do not differ from the nonfatigued narcoleptics. Severe fatigue significantly correlated with more functional impairment; the most impaired domains were in mobility, ambulation, social interaction, recreation, and pastimes.Citation38 Complaints of fatigue, anxiety, or depression should be investigated and addressed.
Other comorbidities
Hypercholesterolemia (odds ratio [OR], 1.51), diseases of the gastrointestinal tract (OR, 3.27), heart disease (OR, 2.07), upper respiratory tract diseases (OR, 2.52), and hypertension (OR, 1.32) are frequently observed;Citation39 care for these conditions have to be coordinated with primary and specialty care. Obesity affects more than 50% of children with narcolepsy; 9% of obese children and 3% of nonobese children with narcolepsy met sleep apnea criteria.Citation40 Weight management programs with diet and exercise should be undertaken, and sleep apnea should be treated before starting SXB.
Nocturnal sleep can be disrupted by periodic leg movements of sleep (PLMS); associated restless legs syndrome (RLS); disturbing dreams sometimes associated with abnormal behavior, and thus called “REM sleep behavior Disorder (RBD)” despite the very different etiologic background and pathophysiology of the classical RBD syndrome; nocturnal eating disorder; and nocturnal awakenings. RLS symptoms can be moderately severe, occur every night, and may be present diurnally. RLS, PLMS, and violent dreaming may be exacerbated by SXB or antidepressant usage and may require a change in therapy, behavioral measures, and iron supplementation (if serum ferritin levels are low). These associated comorbidities may require specific syndromic treatments.
Narcolepsy management during pregnancy
Management of narcolepsy during pregnancy requires a full discussion with the patient of risks and benefits of continued use of their usual narcolepsy drugs during pregnancy, delivery, and while nursing compared to risks of not taking their usual drugs. Most of the standard narcolepsy therapies are FDA pregnancy category C drugs (). Thorpy et alCitation41 reviewed the literature and conducted a survey that revealed variability in how pregnant narcoleptics are managed. Most narcolepsy patients have vaginal deliveries without complications; although some required cesarean section, surgical or anesthetic risk did not increase.Citation41
New developments and future therapies
Emerging therapy for narcolepsy focuses on a multipronged approach: nonhypocretin therapies, hypocretin-based therapy, and immunotherapy to prevent hypocretin neuronal loss.
Nonhypocretin therapies
Nonhypocretin therapies include 1) histamine receptor H3 antagonists/inverse agonists, 2) novel monoaminergic reuptake inhibitors that may target specific neurotransmitters, 3) drugs developed to treat other conditions like idiopathic hypersomnia or attention-deficit-hyperactivity-disorder (ADHD) or stimulant abuse that may target multiple reuptake sites, 4) novel slow-wave sleep (SWS) enhancers may help consolidate disrupted night sleep, 5) thyrotropin-releasing hormone (TRH) analogs, and 6) new formulations and/or delivery systems or pediatric indications for existing medications.
Histamine receptor antagonists
Histaminergic neurons in the posterior hypothalamus stimulated by hypocretin neurons, control wakefulness, feeding, learning, and memory via four aminergic G-protein-coupled receptors: H1, H2, H3, and H4.Citation42,Citation43 H3 acts as an autoreceptor and as a presynaptic heteroreceptor.Citation43 H3 suppresses histamine neuronal firing and inhibits synthesis and release of histamine. H3 also inhibits the release of neurotransmitters acetylcholine, noradrenaline, and dopamine.Citation43
Pitolisant (Wakix, Bioprojet Pharma, Paris, France) previously called (BF2.649) is an inverse agonist of the H3 receptor.Citation44–Citation46 Oral pitolisant has orphan drug status in the EU and in the US. Pitolisant received approval in EU in March 2016 for the treatment of adults with narcolepsy Type I or Type II.Citation45,Citation46 Pitolisant is initiated at 9 mg/d and titrated weekly to a maximum of 36 mg/d; dosage can also be reduced by 4.5 mg/d if needed. The maximum dose is 18 mg/d if renal or moderate hepatic impairment are present.Citation45 It is contra-indicated if there is severe hepatic impairment. As shown in Phase III trials, HARMONY [-1, -Ibis, -III), pitolisant reduced daytime sleepiness (with ESS and MWT confirmation), improved level of attention on sustained attention to response tasks – No-Go index, and decreased frequency of cataplexy attacks.Citation44–Citation46 Pitolisant reduced ESS to –5.8±6.2 compared to –6.9±6.2 with modafinil and –3.4±4.2 with placebo, when compared to baseline values.Citation44 CGI-C analysis showed 73%, 86%, and 56% improvement for pitolisant, modafinil, and placebo, respectively.Citation44 In HARMONY CTP (NCT 1800045), pitolisant resulted in 64% reduction of cataplexy episodes, significant improvement in ESS scores, and increased MWT sleep latency by 80%. No drug abuse potential was observed. Much higher doses (108–216 mg) produced slight prolongation of QTc interval. Pitolisant may reduce efficacy of oral contraceptives, and so alternative methods of contraception should be utilized. A double-blind trial to evaluate safety and efficacy in narcoleptic children 6 years to less than 18 years of age (P11-06, NCT02611687) followed by a prolonged open-label period trial (P11-11) is being conducted.Citation47 A 12-month long-term safety and efficacy study, HARMONY III, (NCT01399606) is ongoing. There are also trials with pitolisant as an add-on drug: 1) to sodium oxybate in HARMONY IV (NCT 01789398) and 2) to modafinil in (NCT01067235); both trials have been completed but results are not available.Citation47
Other histamine receptor antagonists
Several H3 antagonists have advanced to clinical trials with completion of Phase I studies (BF2.649, PF-03654746, GSK189254, GSK239512, MK-0249, MK-3134, JNJ-17216498, and ABT-286) to evaluate the pharmacokinetics and tolerability of single- and multiple-dose administration to treat various neurologic conditions.Citation48 An open-label, dose-escalation study with a double-blind, randomized, placebo-controlled withdrawal to examine the effects of H3 antagonist GSK189254 in narcolepsy patients was terminated, and the study results are not posted. The efficacy of H3 antagonists in addressing insomnia issues in narcolepsy has not been determined.
JNJ-17216498 (NCT00424931) completed Phase II study enrolled 16 patients to evaluate efficacy of a single dose of JNJ-17216498 (10 mg, 50 mg) compared to placebo and modafinil 200 mg 2 times/d, using polysomnography, MWT, and questionnaires.Citation47 Results are not yet available.
JNJ 28583867 is a potent H3 antagonist and is a highly selective inhibitor of serotonin transporter. In rats, it significantly increased extracellular levels of serotonin with smaller increases of norepinephrine and dopamine; it decreased NREM sleep, increased wake-after-sleep onset, and potently suppressed REM sleep.Citation49 It has not been tested in humans.
PF-03654746 (NCT01006122) has been tested in a randomized, double-blind, placebo-controlled, crossover assignment, safety and efficacy study with starting dose of 0.25 mg titrated to a maximum of 2 mg to evaluate effects on daytime somnolence using MWT 20-minute test. The results are available at clinicaltrials.gov but have not been published. Changes in MWT 20-minute test mean sleep latency did not appear to significantly differ from controls, and changes in cataplexy episodes from baseline were no better than the placebo group. These results suggest that PF-03654746, at the doses utilized, may not be useful in the treatment of either EDS or cataplexy in narcolepsy.Citation47
Novel monoaminergic reuptake inhibitors
Dopamine reuptake inhibitors are likely to be mild stimulants, while inhibitors of adrenergic reuptake may improve cataplexy. Drugs that target multiple reuptake sites that have been developed for other conditions like idiopathic hypersomnia or ADHD or treatments for stimulant abuse may subsequently be considered for narcolepsy treatment.
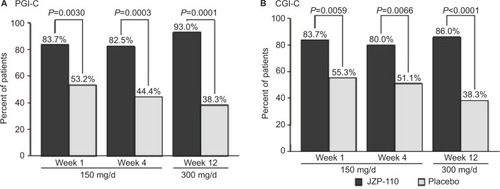
JZP-110 (ADX-N05) is a phenylalanine-derived ([R]-2-amino-3-phenylpropylcarbamate hydrochloride) wake-promoting agent that is a dopamine and norepinephrine reuptake inhibitor. It does not release monoamines nor does it inhibit serotonin reuptake; it also does not inhibit MAO-A enzymatic activity.Citation50 In randomized, double-blind, placebo-controlled clinical trials, JZP-110 underwent Phase II (NCT01485770)Citation50 and Phase II B (NCT01681121)Citation51 testing in adults with narcolepsy with and without cataplexy.Citation50,Citation51 After 4 weeks of JZP-110 at doses of 150 mg (weeks 1, 3) and 300 mg (weeks 2, 4), ESS decreased –6.7 in the treatment group (n=33) compared to –2.4 in the placebo group (n=33). MWT (40-minute test) mean-sleep-latency (MWT SL) after 2 weeks at the 300 mg dose was 12.7±10.6 minutes longer compared to placebo results of 0.9±6.0 minutes.Citation50 A parallel group trial using 150 mg × 4 weeks and 300 mg × 8 weeks showed significant improvements in MWT SL, ESS, and CGIC in treated patients (n=44) compared to placebo (n=49). – are from the Ruoff et al’sCitation51 study demonstrating the effects of JZP-110 on MWT, ESS, and CGIC. The results from these two studies are encouraging and demonstrate JZP-110 may be a new effective treatment for daytime somnolence. JZP_110 is currently recruiting for a Phase III Safety and Efficacy trial (NCT02348593) of 75, 150, and 300 mg of JZP-110 compared to placebo.Citation47
Figure 1 Mean change from baseline in sleep latency on MWT for each of the individual periods at 4 weeks on 150 mg/day (A) and 12 weeks on 300 mg/day of JZP-110 (B) and mean difference in change from baseline between JZP -110 and placebo at 4 weeks and 8 weeks (C).
Abbreviations: MWT, maintenance of wakefulness test; d, day; SE, standard error.
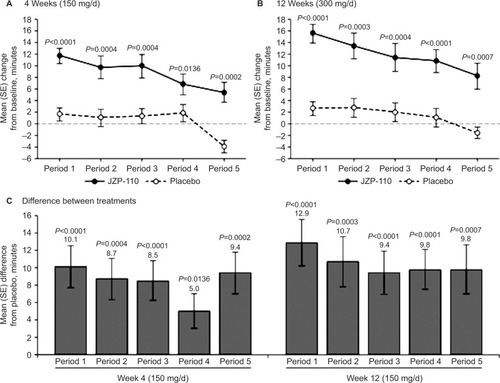
Figure 2 Change in ESS scores with JZP-110 compared to placebo
Abbreviations: ESS, Epworth Sleepiness Scale; SE, standard error; d, day.
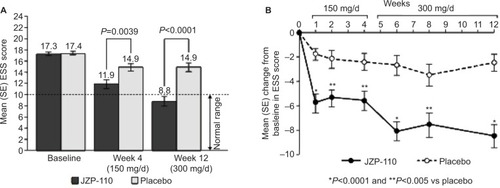
Figure 3 Percentages of patients who achieved improvement on global impression of change measures at weeks 1, 4, and 12 from the patient and clinician’s perspective.
Notes: (A) PGI-C (B) CGI-C. Reproduced from Ruoff C, Swick TJ, Doekel R, et al. Effect of oral JZP-110 (ADX-N05) on wakefulness and sleepiness in adults with narcolepsy: a phase 2b study. Sleep. 2016;39(7):1379–1387, by permission of Oxford University Press.Citation51
GABA modulation
GABA is an inhibitory neurotransmitter that binds to specific receptors in the plasma membrane of both pre- and postsynaptic neurons causing an influx of chloride ions intracellularly or an efflux of potassium extracellularly. There are two general classes of GABA receptors: GABAA receptors are part of a ligand-gated ion channel complex while GABAB receptors are G-protein-coupled receptors that open or close ion channels via G proteins.
GABAB receptor agonists
At pharmacological doses, γ hydroxybutyric acid (GHB) binds to GABAB receptors. GHB is a GABAB receptor agonist; its sodium salt, sodium oxybate, is used to treat the various symptoms of narcolepsy. Baclofen is another GABAB receptor agonist. A parallel group study in teen-agers with narcolepsy Type 1 compared SXB 3–6 g/night (n=13) treatment to baclofen at 5–20 mg/night (n=13); each medicine was added to the patients’ usual dose of modafinil (300 mg/d). Both SXB and baclofen increased delta sleep and total sleep time. However, baclofen had no significant effect on daytime sleepiness nor on cataplexy, while SXB improved both daytime sleepiness and cataplexy, suggesting that another mechanism other than GABAB may be at work.Citation52 Renewed interest in other GABAB agonists as potential therapy for narcolepsy was fueled by different findings in narcoleptic mice using R-baclofen. R-baclofen has a much higher affinity (three-fold) for GABAB receptor than the racemic version. In murine narcolepsy, R-baclofen increased NREM sleep time and deepened sleep intensity and consolidation during the light period; wake-bout duration increased and cataplexy decreased during the subsequent dark period to a greater extent than with GHB.Citation53 These results are only positive in narcolepsy-rodent models, and further studies in human narcolepsy are needed. Several preparations of R-baclofen (arbaclofen, STX209) have already been studied in other conditions such as multiple sclerosis, autism, and gastroesophageal reflux disease; results from these studies could help in designing studies to test R-baclofen’s efficacy and safety in narcolepsy.
GABAA receptor modulators
In some patients with hypersomnia, CSF examination showed potentiation of GABAA receptors. On this basis, clinical trials using GABAA receptor antagonists (clarithromycin, flumazenil) have been undertaken to determine whether somnolence is improved.
Clarithromycin is an antibiotic as well as a negative allosteric modulator of GABAA receptors. Clarithromycin 500 mg twice a day for 5 weeks significantly reduced daytime sleepiness with a four-point reduction in ESS, significantly improved Functional Outcomes of Sleep Questionnaire scores, and significantly improved SF-36 energy section scores in 20 hypersomnolent nonnarcoleptic–cataplectic patients, 4 of whom had Type 2 narcolepsy, when compared to the placebo group. There was no difference in median reaction time on the psychomotor vigilance task between the two groups.Citation54 The improvements seen with clarithromycin therapy appear modest, and chronic use of an antibiotic with possible emergence of drug-resistant bacteria does not appear warranted.
Flumazenil is another GABAA receptor antagonist. A trial (NCT01183312) utilized retrospective chart review of 153 patients treated with flumazenil transdermal cream and/or sublingual flumazenil titrated up to 12 mg 4×/d.Citation55 The study showed symptomatic reduction in sleepiness in 62.8% of patients, with mean reduction of ESS by 4.7±4.7. Patients in this study either had previously used or were using wake-promoting drugs. Female sex and the presence of sleep inertia differentiated responders from nonresponders.Citation55 Flumazenil may be useful for recalcitrant somnolence in female narcoleptics who do not respond to standard stimulant therapy.
BTD-001 (NCT02512588) is currently recruiting for a Phase II multicenter dose-finding study (ARISE 201) of a pentylene tetrazole medication to treat somnolent patients with narcolepsy or idiopathic hypersomnia. Pentylene tetrazole is a GABAA receptor antagonist.Citation47
Slow-wave sleep enhancers
Narcolepsy is associated with sleep fragmentation, frequent awakenings, and stage shifts; disrupted nocturnal sleep may add to daytime fatigue. Theoretically, drugs that promote slow-wave sleep could be helpful, but they have not undergone clinical trials for this indication, except for SXB. Tiagabine is an inhibitor of GAT-1, a transporter protein that promotes GABA reuptake into the presynaptic terminals; GAT-1 inhibition results in increased synaptic levels of GABA. In sleep-deprived normal subjects, tiagabine increased slow-wave sleep by 41% and improved ratings of the restorative nature of sleep.Citation56 Other potential SWS enhancers include gaboxadol, a selective extrasynaptic GABAA agonist, gabapentin and pregabalin, both of which act on α2-δ voltage-gated calcium channels, and trazodone, which acts on multiple receptors including 5 HT2 antagonist.Citation56 More research is needed to determine the usefulness of these drugs in consolidating nocturnal sleep in narcolepsy patients.
“Standard Drugs for Narcolepsy, new indications, and new formulations” provide other avenues to pursue treatment modalities.
A recent hypothesis based on animal studies is that modafinil acts as a cellular-coupling enhancer in glial cells through modulation of gap junctions constituted by connexins.Citation57 Connexins are gap junction proteins expressed by glial cells. In mice, flecainide is an astroglial connexin inhibitor that enhances the awakening and procognitive effects of modafinil.Citation57 Modafinil combined with flecainide reduced the cataplexy-like phenotype in orexin knockout mice.Citation57 A Phase II trial of THN 102 (NCT02821715) is not yet open for recruitment; it is a randomized, placebo-controlled, three-way crossover trial that will compare a combination drug (300 mg modafinil/3 mg flecainide) to 300 mg modafinil in narcoleptic patients.Citation47
A Phase III open-label, flexible-dose trial of the safety and effectiveness of modafinil treatment for excessive sleepiness (dose of 100 mg titrated to maximum of 400 mg/d) in children 6–16 years of age has concluded, but results have not been posted. (NCT00214968).Citation47
Sodium oxybate use in pediatric patients has been investigated. A retrospective study of tolerance and efficacy of SXB in childhood narcolepsy with cataplexy (n=27, mean age: 10.3±3.2) showed that 18/27 had been prescribed SXB as their first anticataplectic drug. SXB treatment allowed withdrawal from other medications (7/10 patients were on venlafaxine, 5/24 patients on modafinil). A large majority of SXB-treated patients reported improvements in cataplexy, daytime sleepiness, and nocturnal sleep. Three patients reported improved attention and learning in school. Main side effects were weight loss, headache, nausea, disturbed nocturnal sleep, irritability, parasomnias (sleep walking, sleep talking, enuresis), and/or daily episodes of sleep drunkenness. About 15% (n=4) required complete SXB withdrawal because of sleep loss and persistent nausea.Citation58 The Xyrem Pediatric Narcolepsy Study (NCT02221869) is a 52-week, Phase III randomized, double-blind open-label multicenter (US and EU) clinical trial that will evaluate safety and efficacy and pharmacokinetics in pediatric patients (ages 7–17) with narcolepsy with cataplexy. This trial is active but not currently enrolling.Citation47 JZP 13-005 (CT2014-001389-93) is an open label Xyrem pharmacokinetic evaluation and safety extension in children and adolescents under 18 years of age and is ongoing. JZP-386 is deuterium-modified analog of sodium oxybate; it is a dopamine receptor agonist. Results from a Phase I clinical trial in 30 subjects showed a higher serum concentration and increased pharmacodynamics effect when compared to SXB-Xyrem. A Phase IV open-label study (NCT00345800) to evaluate the effects of SXB-Xyrem on the endocrine system has completed, but results have neither been posted nor published.Citation47
A new formulation of SXB that will allow single dosing at night is being tested. This study (NCT02720744, FT218) conducted by Flamel Ireland is not yet open for recruitment. It is a double-blind, randomized, placebo-controlled, two-arm, multicenter study to assess the efficacy and safety of a once-nightly formulation of sodium oxybate for extended-release oral suspension using micropump technology (doses of 4.5, 6.0, 7.5, and 9.0 g) with regard to daytime somnolence and cataplexy.Citation47 If effective and safe, this formulation may sim-plify administration and mask the taste of SXB. Flamel also has “trigger-lock technology” (although not in this study) that can alleviate abuse potential concerns.
TRH and its analogs
TRH is a tripeptide that has endocrinologic and neuromodulatory actions. It stimulates cholinergic turnover, increases neurotransmission of dopamine and norepinephrine, depolarizes spinal motor neurons, promotes CNS arousal, modulates pain perception and locomotor activity, regulates respiration, and modulates seizure threshold.Citation59–Citation61 Hypocretin cells are modulated by TRH. Two receptors that are targets for TRH are TRH-R1 and TRH-R2. TRH-R2 is believed to be responsible for neuropharmacological actions, while TRH-R1 is responsible for endocrinologic effects.Citation62 Clinical applications for TRH are limited due to its short plasma half-life of 5 minutes, low intestinal and CSF permeability, and endocrine side effects.Citation61 Modifications of the C or N terminals have yielded several TRH analogs of varying potencies (CG-3703, CG-3509, and TA-0910) that have been used intravenously in canine narcolepsy; these TRH analogs increased wakefulness, suppressed both slow-wave sleep and REM sleep, and significantly reduced cataplexy.Citation60,Citation61 Drug tolerance was noted with CG-3703 usage after a week of therapy, requiring dose adjustment.Citation61 Although studies in canine narcolepsy have been somewhat successful, we are not aware of any clinical trials of these drugs in human narcolepsy. Researchers have synthesized metabolically stable and more potent selective TRH prodrugs and analogs – taltirelin [TA-0910], montirelin [CG-3703], azetirelin [YM-14673], JTP-2942, DN 1417, MK-771, and posatirelin [RGH-2202] – which have potential applications in various diseases, such as depression, epilepsy, spinocerebellar degeneration, amyotrophic lateral sclerosis, Parkinson’s disease, schizophrenia, Alzheimer’s disease, and cancer-related fatigue.Citation62 Rovatirelin is another TRH analog that binds to TRH receptor with greater affinity than taltirelin (a drug approved for spinocerebellar degeneration treatment) and has increased noradrenergic activity and increased locomotor activity.Citation63 JAK4D binds to a new TRH receptor subtype in human hippocampal tissue and reduced cognitive defects in rat model of neurodegeneration; it also protected against neuronal damage.Citation64 TRH analogs potentially represent new therapies for narcolepsy, but further studies need to be undertaken to determine their safety and efficacy.
Melanin-concentrating hormone (MCH) receptor modulation
MCH is a 19-amino acid neuropeptide that results from the cleavage of a precursor preproMCH. MCH neurons inter-mingle with hypocretin neurons in the lateral hypothalamus and project widely throughout the CNS where MCH receptors (MCHR-1 and MCHR-2) are widely distributed. MCH cells play a central role in sleep promotion – they express c-Fos during sleep, discharge action potentials during both NREM and REM sleep, but are preferentially active during REM sleep.Citation65 MCH neurons innervate arousal-promoting regions such as histaminergic TMN cells and the noradrenergic locus coeruleus cells. MCH cells release GABA, thereby inhibiting wake-promoting circuits.Citation65 Optogenetic stimulation of MCH cells triggers rapid onset of sleep; MCH neurons also promote and stabilize REM sleep by inhibiting wake-promoting circuits.Citation65,Citation66 Whether MCH agonists will reduce insomnia and disrupted nocturnal sleep in narcolepsy has not been determined. Meanwhile, MCHR1 antagonists are being investigated as treatments for depression, anxiety, and obesity.
Hypocretin-based therapies
A messenger RNA encodes prepro-hypocretin (orexin). This precursor is cleaved to form two hypothalamic neuropeptides. Hypocretin-1 (orexin A) is a 33-amino acid peptide with an N-terminal pyroglutamyl residue, two intrachain disulfide bonds, and C-terminal amidation, while hypocretin-2 (orexin B) is a 28-amino acid C-terminally amidated linear peptide.Citation67 Hypocretin actions are mediated by 2 orphan G-protein coupled receptors: HCRT1 and HCRT2. HCRT1 has higher affinity for hypocretin-1 than hypocretin-2, while HCRT2 binds both hypocretin-1 and hypocretin-2 with similar affini-ties. Low or undetectable hypocretin peptides are found in almost 95% of patients with narcolepsy Type 1.Citation10 The hypo-cretin ligand deficiency is probably due to postnatal cell death of hypocretin neurons.Citation68
Various hypocretin ligand replacement therapies have been pursued including cell transplantation, peptide replacement, and gene therapy.
Cell transplantation and stem cells
With enhanced transplant media, cells from the posterior hypothalamus of rat pups that were transplanted into the midline pons of adult rats at the level of the locus coeruleus survived up to day 36 (n=9).Citation69 Narcoleptic rats that received transplant grafts showed reduced somnolence.Citation70 However, survival rate of the grafts was poor (~5% of implanted cells).Citation70 Poor graft survival fueled interest in stem cells to produce hypocretin neuroblasts for transplantation.
Merkle et alCitation71 reported the differentiation of both human embryonic stem cells and human-induced pluripotent stem cells into hypothalamic neurons using complementary self-patterning and directed differentiation approaches. This research lays the foundation for in vitro generation of human hypothalamic neurons, which can then enable further investigation into disease modeling, cell transplantation, and efficient drug screening for narcolepsy.
Hypocretin peptide replacement
Hypocretin-1 replacement in ligand-deficient narcoleptic dogs via intracerebroventricular or intravenous methods (up to 6 μg/kg) had no effect on cataplexy or wakefulness. Only very high intravenous doses (96–384 μg/kg) penetrated the blood–brain barrier for a short-lasting anticataplectic effect.Citation72 Intracerebroventricular hypocretin-1 infusion in ligand-deficient mice improved cataplexy and wakefulness for 3 hours.Citation73 These results and the impracticability of intracerebroventricular administration highlight the need to develop hypocretin analogs that can penetrate centrally more effectively, preferably through noninvasive routes of administration.
Hypocretin-1 was applied intranasally to Type I narcolepsy subjects in two studies (n=8, n=14)Citation74,Citation75 – there was no statistically significant effect on wakefulness, although REM sleep quantity was reduced and REM was more stable with fewer wake-REM sleep transition. Participants committed fewer false reactions in the test of divided attention per-formed the next day.Citation75 The addition of 1% phenylephrine into formulations containing hypocretin-1 applied intranasally significantly reduced absorption of hcrt-1 into the blood by 65%, increased deposition into the olfactory epithelium about threefold, and reduced concentration in the trigeminal nerve by 65%. Brain-to-blood concentrations were increased 16–6.8-fold.Citation76 At this time, intranasal hypocretin replacement is not yet a viable treatment.
Gene replacement therapy
Diffuse expression of ligand (transgene with β-actin promoter) improved cataplexy and consolidated REM sleep in ligand-deficient narcoleptic mice.Citation77 Using herpes simplex virus-1 amplicon-based vector, a gene for preprohypocretin was transferred into the lateral hypothalamus of narcoleptic mice: this reduced cataplexy by 60%, and REM sleep levels increased.Citation77 Recombinant adeno-associated virus (rAAV)-orexin gene transfer into the zona incerta neurons suppressed cataplexy while rAAV gene transfer into the striatum did not, suggesting site-specific effects of gene transfer.Citation78 rAAV-orexin gene transfer into the dorsolateral pons improved wake maintenance (wake bouts lasting longer than 32.2 minutes significantly increased to 23% [+180% vs no rAAV; P<0.001]), but overall wake time did not change; cataplexy was also significantly reduced.Citation79 More studies are needed to establish safety and efficacy, but these may be therapies for the future.
Immunotherapy
Since autoimmunity is believed to underlie hypocretin cell destruction, clinical trials have tested immunotherapy as a potential disease modifying therapy. Plasmapheresis,Citation80 corticosteroids,Citation81,Citation82 and intravenous immunoglobulin infusionsCitation83 have been used in case reports and small studies with mixed results (plasmapheresis did not improve narcolepsy; corticosteroids helped daytime somnolence in two cases, did not help in one case; intravenous immunoglobulin (IVIG) infusions helped cataplexy but not other symptoms in ¼ of the cases, but not the other symptoms). Plasmapheresis and steroids were utilized close to onset of symptoms, while IVIG was used within 6 months of diagnosis. Immunotherapy is believed to be helpful when administered close to disease onset to prevent neuronal death. Surprisingly, cataplexy but not other narcoleptic symptoms resolved when a patient who developed lymphoma was treated with alemtuzumab, even though his narcolepsy had started 52 years ago.Citation84 Alemtuzumab is a humanized monoclonal antibody that binds to CD52 and causes lysis of lymphocytes and subsequently a differential recovery of lymphocyte subsets with prolonged suppression of CD4+ T cells.Citation84 We are not aware of any other immunosuppressant therapies utilized in narcolepsy patients. The small numbers and the uncontrolled nature of these various studies as well as differing treatment regimens used do not provide enough bases for guidelines. More controlled studies are indicated.
Conclusion
Narcolepsy remains a complex disease whose cure remains elusive despite our expanding knowledge about its pathophysiology. Disease-specific therapies need further development and testing before they can be clinically relevant. The ability to generate hypothalamic neurons from stem cells should facilitate drug screening for narcolepsy. Symptomatic therapy may make a difference in functionality and quality of life. Historically, clinicians choose medications empirically based upon practice guidelines, experience, and personal and patient preferences. As the cost of genotyping becomes more affordable, personalized medicine will come to the fore-ground. Pharmacogenomics will play a greater role clinically in choosing the best drugs for patients, using documented genetic variation to guide medication selection and dosing.Citation85
Disclosure
The authors report no conflicts of interest in this work.
References
- LongstrethWTJrKoepsellTDTonTGHendricksonAFvan BelleGThe epidemiology of narcolepsySleep200730132617310860
- Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration (FDA)The Voice of the Patient. A series of reports from the US Food and Drug Administration’s (FDA’s) Patient Focused Drug Development InitiativeNarcolepsy Public MeetingSeptember 24, 2013 Report date: June 2014. Available from http://www.fda.gov/down-loads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM402907.pdfAccessed October 15, 2016
- RosenbergRKimAYThe AWAKEN survey: knowledge of narcolepsy among physicians and the general populationPostgrad Med2014126788624393754
- DauvilliersYMontplaisirJMolinariNAge at onset of narcolepsy in two large populations of patients in France and QuebecNeurology2001572029203311739821
- GuilleminaultCAbadVCNarcolepsyChokrovertySSleep Disorders Medicine Basic Science, Technical Considerations, and Clinical AspectsPhiladelphia, PASaunders Elsevier2009377396
- RothTDauvilliersYMignotEDisrupted nighttime sleep in narcolepsyJ Clin Sleep Med20139995596523997709
- PizzaFFranceschiniCPeltolaHClinical and polysomnographic course of childhood narcolepsy with cataplexyBrain20131363787379524142146
- ThorpyMJKriegerACDelayed diagnosis of narcolepsy: characterization and impactSleep Med20141550250724780133
- American Academy of Sleep MedicineInternational Classification of Sleep Disorders3rd edDarien, ILAmerican Academy of Sleep Medicine2014
- NishinoSRipleyBOvereemSLow cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsyAnn Neurol20015038138811558795
- BaumannCRMignotELammersGJChallenges in diagnosing narcolepsy without cataplexy: a consensus statementSleep2014371035104224882898
- MahliosJDe la Herran-AritaAKMignotEThe autoimmune basis of narcolepsyCurr Opin Neurobiol20132376777323725858
- LucaGHaba-RubioJDauvilliersYClinical, polysomnographic, and genome-wide association analyses of narcolepsy with cataplexy: a European Network studyJ Sleep Res201322548249523496005
- YamasakiMMiyagawaTToyodaHEvaluation of polygenic risks for narcolepsy and essential hypersomniaJ Hum Genet2016611087387827305985
- FontanaAGastHReithWRecherMBirchlerTBassettiCLNarcolepsy: autoimmunity, effector T cell activation due to infection, or T cell independent, major histocompatibility complex class II induced neuronal loss?Brain2010133Pt 51300131120403960
- MorgenthalerTIKapurVKBrownTPractice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Standards of Practice Committee of the American Academy of Sleep MedicineSleep2007301705171118246980
- KrahnLEHershnerSLoedingLDQuality measures for the care of patients with narcolepsyJ Clin Sleep Med20151133535525700880
- BilliardMBassettiCDauvilliersYEFNS Task ForceEFNS guidelines on management of narcolepsyEur J Neurol2006131035104816987156
- WiseMSArandDLAugerRRBrooksSNWatsonNFAmerican Academy of Sleep MedicineTreatment of narcolepsy and other hyper-somnias of central originSleep2007301712172718246981
- MignotEJA practical guide to the therapy of narcolepsy and hypersomnia syndromesNeurotherapeutics20129473975223065655
- ScammellTTreatment of narcolepsy in adultsBencaREichlerAFUpToDateWaltham, MAAccessed October 23, 2016
- KotagalSNarcolepsy in childrenScammellTEichlerAFUpToDateWaltham, MAAccessed October 23, 2016
- ThorpyMDauvilliersYClinical and practical considerations in the pharmacologic management of narcolepsySleep Med20151691825458251
- PDR.net [homepage on the Internet] Available from: http://www.pdr.netAccessed April 11, 2016
- Drugs.com [homepage on the Internet] Available from: http://www.drugs.comAccessed October 31, 2016
- ScammellTEEstabrookeIVMcCarthyMTHypothalamic arousal regions are activated during modafinil-induced wakefulnessJ Neurosci200020228620862811069971
- WisorJModafinil as a catecholaminergic agent: empirical evidence and unanswered questionsFront Neurol2013413924109471
- SchwartzJRFeldmanNTBoganRKDose effects of modafinil in sustaining wakefulness in narcolepsy patients with residual evening sleepinessJ Neuropsychiatry Clin Neurosci20051740541216179665
- SchwartzJRFeldmanNTBoganRKNelsonMTHughesRJDosing regimen effects of modafinil for improving daytime wakefulness in patients with narcolepsyClin Neuropharmacol20032625225714520165
- SchwartzJRNelsonMTSchwartzERHughesRJEffects of modafinil on wakefulness and executive function in patients with narcolepsy experiencing late-day sleepinessClin Neuropharmacol200427747915252267
- BlackJEHullSGTillerJYangRHarshJRThe long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension studyJ Clin Sleep Med20106545846620957846
- CarltonRLunacsekOReganTCarrollCAHealthcare costs among patients with excessive sleepiness associated with obstructive sleep apnea, shift work disorder, or narcolepsyAm Health Drug Benefits20147633434025558302
- AlshaikhMKTriccoACTashkandiMMamdaniMStrausSEBaHammamASSodium oxybate for narcolepsy with cataplexy: systematic review and meta-analysisJ Clin Sleep Med20128445145822893778
- US Xyrem Multi-Center Study GroupA randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsySleep200225424911833860
- Xyrem International Study GroupA double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsyJ Clin Sleep Med2005139139717564408
- BlackJHoughtonWCSodium oxybate improves excessive daytime sleepiness in narcolepsySleep20062993994616895262
- MayerGMeierKEHephataKSelegiline hydrochloride treatment in narcolepsy. A double-blind, placebo-controlled studyClin Neuropharmacol19951843063198665543
- Droogleever FortuynHAFronczekRSmitshoekMSevere fatigue in narcolepsy with cataplexyJ Sleep Res201221216316921848801
- OhayonMNarcolepsy is complicated by high medical and psychiatric co-morbidities; a comparison with the general populationSleep Med20131448849223643648
- InocenteCOLavaultSLecendreuxMImpact of obesity on children with narcolepsyCNS Neurosci Ther20131952152823574649
- ThorpyMZhaoCGDauvilliersYManagement of narcolepsy during pregnancySleep Med201314436737623433999
- BaronioDGanchoroskiTCastroKZanattaGGottfriedRiesgoRHistaminergic system in brain disorders: lessons from the translational approach and future perspectivesAnn Gen Psychiatry20141313425426159
- SwickTJTreatment paradigms for cataplexy in narcolepsy: past, present, and futureNat Sci Sleep2015715916926715865
- DauvilliersYBassettiCLammersGJHARMONY I study group. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double blind, randomized trialLancet Neurol201312111068107524107292
- Summary of Product Characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002616/WC500204746.pdfAccessed November 30, 2016
- SyedYYPitolisant: first global approvalDrugs201676131327438291
- ClinicalTrials.gov Available from: https://clinicaltrials.gov/ct2/results?term=narcolepsy&cond=%22Narcolepsy%22Accessed October 25, 2016
- BrioniJDEsbenshadeTAGarrisonTRBitnerSRCowardMDDiscovery of histamine H3 antagonists for the treatment of cognitive disorders and Alzheimer’s diseaseJ Pharmacol Exp Ther2011336384620864505
- BarbierAJAluisioLLordBPharmacological characterization of JNJ-28583867, a histamine H(3) receptor antagonist and serotonin reuptake inhibitorEur J Pharmacol20075761–3435417765221
- BoganRKFeldmanNEmsellemHAEffect of oral JZP-110 (ADX-N05) treatment on wakefulness and sleepiness in adults with narcolepsySleep Med20151691102110826298786
- RuoffCSwickTJDoekelREffect of oral JZP-110 (ADX-N05) on wakefulness and sleepiness in adults with narcolepsy: a phase 2b studySleep20163971379138727166238
- HuangYSGuilleminaultCNarcolepsy: action of two γ-aminobutyric acid type B agonists, baclofen and sodium oxybatePediatr Neurol200941191619520267
- BlackSWMorairtySRChenTMGABAB agonism promotes sleep and reduces cataplexy in murine narcolepsyJ Neurosci201434196485649424806675
- TrottiLMSainiPBliwiseDLFreemanAAJenkinsARyeDBClarithromycin in γ-aminobutyric acid-Related hypersomnolence: a randomized, crossover trialAnn Neurol201578345446526094838
- TrottiLMSainiPKoolaCLaBarberaVBliwiseDLRyeDBFlumazenil for the treatment of refractory hypersomnolence: clinical experience with 153 patientsJ Clin Sleep Med201612101389139427568889
- WalshJKEnhancement of slow wave sleep: implications for insomniaJ Clin Sleep Med200952 SupplS27S3219998872
- DuchêneAPerierMZhaoYImpact of astroglial connexins on modafinil pharmacological propertiesSleep20163961283129227091533
- LecendreuxMPoliFOudietteDTolerance and efficacy of sodium oxybate in childhood narcolepsy with cataplexy: a retrospective studySleep201235570971122547897
- NicollRAExcitatory action of TRH on spinal motor neuronsNature1977265242243401949
- NishinoSArrigoniJSheltonJEffects of thyrotropin-releasing hormone and its analogs on daytime sleepiness and cataplexy in canine narcolepsyJ Neurosci199717640164089236248
- RiehlJHondaKKwanMChronic oral administration of CG-3703, a thyrotropin-releasing hormone analog, increase wake and decreases cataplexy in canine narcolepsyNeuropsychopharmacology200023344510869884
- KhomaneKSMeenaCLJainRBansalAKNovel thyrotropin-releasing hormone analogs: a patent reviewExpert Opin Ther Pat201121111673169122017410
- IjiroTNakamuraKOgataMEffect of rovatirelin, a novel thyrotropin-releasing hormone analog, on the central noradrenergic systemEur J Pharmacol201576141342226142830
- KellyJABoyleNTColeNFirst-in-class thyrotropin-releasing hormone (TRH)-based compound binds to a pharmacologically distinct TRH receptor subtype in human brain and is effective in neurodegenerative modelsNeuropharmacology20158919320325281210
- FraigneJJPeeverJHMelanin-concentrating hormone neurons promote and stabilize sleepSleep201336121767176824293745
- PelluruDKonadhodeRShiromaniPJMCH neurons are the primary sleep-promoting groupSleep201336121779178124293750
- SakuraiTMiedaMConnectomics of orexin-producing neurons: inter-face of systems of emotion, energy homeostasis and arousalTrends Pharmacol Sci201132845146221565412
- NishinoSOkuroMKotoriiNHypocretin/orexin and narcolepsy: new basic and clinical insightsActa Physiol2000198209222
- Arias-CarriónOMurillo-RodriguezEXuMBlanco-CenturionCDrucker-ColinRShiromaniPJTransplantation of hypocretin neurons into the pontine reticular formationSleep20042781465147015683135
- Arias-CarreonOMurillo-RodriguezEEffects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in ratsPLoS One201494e9534224736646
- MerkleFTMaroofAWatayaTGeneration of neuropeptidergic hypothalamic neurons from human pluripotent stem cellsDevelopment2015142463364325670790
- FujikiNYoshidaYRipleyBMignotENishinoSEffects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dogSleep200326895395914746374
- MiedaMWillieJTHaraJSintonCMSakuraiTYanagisawaMOrexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in miceProc Natl Acad Sci U S A2004101134649465415070772
- BaierPCHallschmidMSeeck-HirschnerMEffects of intranasal hypocretin-1 (orexin A) on sleep in narcolepsy with cataplexySleep Med2011121094194622036605
- WeinholdSLSeeck-HirschnerMNowakAHallschmidMGöderRBaierPCThe effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexyBehav Brain Res201426281324406723
- DhuriaSVHansonLRFreyWH2ndNovel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous systemJ Pharmacol Exp Ther2009328131232018945930
- LiuMThankachanSKaurSOrexin (hypocretin) gene transfer diminishes narcoleptic sleep behavior in miceEur J Neurosci2008281382139318973565
- LiuMBlanco-CenturionCKonadhodeROrexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic miceJ Neurosci2011316028604021508228
- Blanco-CenturionCLiuMKonadhodeRPelluruDShiromaniPJEffects of orexin gene transfer in the dorsolateral pons in orexin knock-out miceSleep2013361314023288969
- ChenWBlackJCallPMignotELate-onset narcolepsy presenting as rapidly progressing muscle weakness: response to plasmapheresisAnn Neurol200558348949016130098
- HechtMLinLKushidaCAReport of a case of Immunosuppression with Prednisone in an 8-year-old boy with an acute onset of hypocretin-deficiency narcolepsySleep200326780981014655912
- CoelhoFMPradella-HallinanMARodriguesGBittencourtLRTufikSReport of two narcoleptic patients with remission of hypersomnolence following use of prednisoneArq Neuropsiquiatr2007652A33633717607439
- PlazziGPoliFFranceschiniCIntravenous high-dose immunoglobulin treatment in recent onset childhood narcolepsy with cataplexyJ Neurol2008255101549155418769859
- DonjacourCELammersGJA remarkable effect of alemtuzumab in a patient suffering from narcolepsy with cataplexyJ Sleep Res201221447948022142323
- MrazekDAPsychiatric pharmacogenomics testing in clinical practiceDialogues Clin Neurosci201012697620373668
