Abstract
Objective
Many cases of obstructive sleep apnea (OSA) involve collapse of the tongue base and soft palate during sleep, causing occlusion of the upper airway and leading to oxygen desaturation. Existing therapies can be effective, but they are plagued by patient adherence issues and the invasiveness of surgical approaches. A new, minimally invasive implant for OSA has been developed, which is elastic and contracts a few weeks after deployment, stabilizing the surrounding soft tissue. The device has had good outcomes in preclinical testing; this report describes the preliminary feasibility and safety of its implementation in humans.
Patients and methods
A prospective, multicenter, single-arm feasibility study was conducted. Subjects were adults with moderate-to-severe OSA who had previously failed or refused conventional continuous positive airway pressure treatment. Intraoperative feasibility data, postoperative pain, and safety information were collected for a 30-day postoperative period.
Results
Forty subjects participated (37 men, three women; average age of 46.1 years); each received two tongue-base implants and two soft-palate implants. Surgical procedure time averaged 43 minutes. Postsurgical pain resolved readily in most cases; at 30 days post implantation, <20% of subjects reported pain, which averaged less than two out of ten. Adverse events were generally the mild and expected sequelae of a surgical procedure with general anesthesia and intraoral manipulation. The device was well tolerated. Implant extrusions were reported with soft-palate implants (n=12), while tongue-base implants required few revisions (n=2). Quantitative and qualitative sleep effectiveness outcomes (including full-night polysomnographic and quality-of-life measures) will be presented in a subsequent report.
Conclusion
Implantation of the device was feasible. Although a relatively high rate of extrusions occurred in the now-discontinued palate implants, tongue-base implants were largely stable and well tolerated. The minimally invasive and maintenance-free implant may provide a new alternative to higher morbidity surgical procedures.
Introduction
Sleep disorders have been described in the medical literature since the 19th century.Citation1 Obstructive sleep apnea (OSA) is a treatable but markedly underdiagnosed condition of frequent breathing pauses during sleep. OSA is defined by recurrent episodes of apnea (complete cessation of airflow) or hypopnea associated with persistent respiratory effort during sleep.Citation2 OSA is associated with a number of significant health consequences, such as cardiovascular disease, atherosclerosis, hypertension, heart failure, arrhythmias, stroke, diabetes, headaches, depression and impaired motor/visual skills, accidents, and all-cause mortality.Citation1,Citation3–Citation12 The US/European prevalence of OSA is 2%–11%, and its health care costs are estimated at $34 billion.Citation5,Citation13,Citation14
One of the more common etiologies of OSA is the collapse of the soft palate and/or tongue base during sleep.Citation15 Continuous positive airway pressure (CPAP) provides a “pneumatic splint” that holds the airway open and remains the standard of care, reducing cardiovascular risk.Citation6,Citation7,Citation16 The effectiveness of CPAP, although high with perfect usage, is less under typical conditions because up to 40% of patients are unable to tolerate it or are nonadherent.Citation17–Citation19 The effectiveness of oral appliances for mandibular advancement is similar to that of CPAP in the short term, but adherence rates likewise decrease over time.Citation20,Citation21 Surgical options may be investigated upon failure or intolerance to these interventions, as most do not require daily equipment use and therefore do not depend on patient adherence.Citation22 Surgeons aim to improve upper airway patency by widening the airway, removing anatomical obstruction, stiffening the pharyngeal wall, and/or increasing muscle tone without compromising normal functions such as breathing, speaking, and swallowing.
A large variety of surgical options exist, including reduction of nasal obstruction, oropharyngeal enlargement (uvulopalatopharyngoplasty or modifications with/without tonsillectomy, expansion sphincter pharyngoplasty, or transpalatal advancement pharyngoplasty), hypopharyngeal dilation, maxillomandibular advancement, rigid tongue implants, tongue suture techniques and tongue/hyoid suspension techniques, and hypoglossal nerve stimulation.Citation22–Citation28 Effectiveness of these surgical approaches ranges from 30% to 66%, depending on technique, although definitive assessment is difficult because the field of published literature in this interventional area is primarily composed of small cohort studies of varying methodology.Citation29–Citation33 Radio frequency tissue ablation is gaining popularity as an OSA treatment, and a recent meta-analysis concluded its effectiveness for base-of-tongue procedures.Citation34 However, randomized, controlled trials have not demonstrated consistent evidence for the effectiveness of surgical and implant approaches, and a systematic review on surgery for sleep apnea concluded that the overall significant benefit of surgery has not been demonstrated.Citation35,Citation36
Regardless of their clinical effectiveness, all of these interventions – like any surgical approach – carry morbidity risks including infection in addition to significant recovery time. In OSA surgeries, there is a perioperative complication rate of ∼7%, including serious ones such as hemorrhage, edema, and upper-airway obstruction, which can carry legal implications.Citation37,Citation38 In addition, approximately half of the patients report long-term problems with dysphagia, voice changes, and a foreign-body sensation in the throat.Citation35 Swallowing dysfunction has a reported incidence as high as 87% in the early postoperative period, resolving to ∼12% (and generally of mild severity) after 6 months.Citation39,Citation40 Moreover, the applicability of OSA surgeries is limited by the technical difficulty of performing the procedures and their considerable cost.
Thus, there remains a clear need for an OSA solution that can address issues of patient adherence and provide a significant and lasting effect with minimal disruption of tissue. To be effective, an OSA treatment must address all potential sources of upper-airway obstruction (principally collapse of the soft palate and/or tongue base during sleep) or be compatible with other OSA interventions to allow utilization in multilevel disease and have reproducible outcomes for a broad user base with minimal training. Furthermore, the design of any implanted device must incorporate materials with long-term biocompatibility and lifetime stability, be easy to implant and remove (if desired), possess sufficient strength to provide soft tissue support but have sufficient elasticity to allow for normal speech and swallowing without any awareness of the device, use well-accepted and readily available biomaterials, and be easily and reproducibly manufactured at a reasonable cost.
A novel device for the treatment of OSA has been developed in accordance with these aims and was tested in laboratory conditions and in animal models. Results indicated that it is biocompatible and well tolerated.Citation41 This report describes the feasibility and acute safety data of the first trial of this novel device in human subjects; clinical and patient-reported effectiveness outcomes will be presented in a forthcoming report.
Patients and methods
Study design
This prospective, multicenter, single-arm feasibility study was conducted to evaluate the safety and feasibility of a novel implantable device for the treatment of OSA.
Four Ethics Committee-approved investigational centers in Germany, Canada, and the Czech Republic were involved. All site personnel were trained before the study, including implantation training in cadavers if necessary. Administrative staff were instructed in proper data collection, storage, and study procedures. The study was conducted in conformance with the Declaration of Helsinki and the laws and regulations of the participating country, whichever afforded the greater protection to the human subject.
Patient selection
Study candidates were recruited from the investigators’ clinical practices and through referrals and advertising. Subjects gave their full written informed consent prior to any study activities. Appropriate subjects were adults with moderate-to-severe OSA (defined as an Apnea-Hypopnea index [AHI] of >15 events/h to ≤40 events/h) who had previously refused or failed treatment with CPAP. To maximize the generalizability of the study, no subjects were excluded on the basis of ethnicity or other demographic factors. Key inclusion/exclusion criteria are listed in . Subjects received reimbursement for travel-related expenses as required.
Table 1 Inclusion and exclusion criteria
Device and treatment
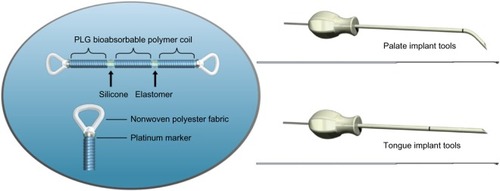
The implant is a linear silicone elastic element with polyester-reinforced loops on each end. At implantation, the elastic element of the implant is held in its extended state by an external sheath of poly(lactide-co-glycolide) bioabsorbable polymer. A radio-opaque marker at each end of the implant allows fluoroscopic visualization if desired. Subjects are placed briefly under general anesthesia for the procedure. Two palate implants are placed through a curved trocar using an intraoral approach, and two tongue implants are placed through a straight trocar using a submental approach (). Soft-palate placement is typically through two 4 mm-long lateral incisions at the hard palate/soft palate junction spaced ∼5 mm to the right and left of the centerline, while tongue placement typically uses a single 8–10 mm incision at the base of the mandible on the centerline. No incision is made inside the mouth for the tongue-base implants. Trocar insertion utilizes tactile feedback at the base of the tongue to gauge the depth of the trocar tip. The first implant is placed along the midline adjacent to the raphe of the tongue parallel to the mandible line, terminating in the submucosa just above the vallecula. The second implant is placed along the midline adjacent to the opposite side of the raphe and terminating in the submucosa 2–3 cm above the first implant. The surgical procedure also includes the optional use of X-ray imaging in the lateral and anterior–posterior directions to assist the placement of the tongue trocars as well as verification of the implant position. Preoperative antiseptic oral rinse, prophylactic antibiotics, and postoperative antibiotics (5–7 days) were administered along with appropriate wound care.
After implantation, normal healing anchors the loop ends into the target tissue. The poly(lactide-co-glycolide) formulation is a bioabsorbable material that dissolves within a few weeks and only after acute healing has occurred, allowing the elastic elements to contract and stabilize the tissue between the anchor sites. During sleep-disordered breathing, this support is intended to maintain patency of the airway by preventing the collapse of the tongue base and supporting the soft palate. Because the implants are flexible, they are expected to be compliant with normal function of the target tissue, eg, swallowing and speech.
Study schedule
Study eligibility was confirmed via baseline physical/otolaryngological examination and standard polysomnographic parameters. Surgical procedural times were recorded. Subjects recorded their daily pain ratings (on a standard 10 cm Visual Analog Scale [VAS]), diet modifications, and pain medication use for the 30 days following implantation. Adverse events (AEs) were managed according to standard care at the study centers and categorized for reporting according to their intensity, relatedness to the device or procedure, outcome, and treatment or action taken. Problems with speaking, swallowing, or breathing were assessed by physician interview, airway examination, and assessment for AEs at each study visit. Reported here are feasibility and safety data from the implant procedure through 30 days post procedure in order to characterize the acute performance and results. Effectiveness outcomes will be presented in a subsequent report.
Data management and statistical analysis
Study data were recorded and stored in compliance with local regulations and were periodically monitored by the study sponsor for quality and completeness. Procedures to prevent compromise of subject confidentiality were employed. Because this was a “first-in-man” trial of a novel device, implantations of ten-subject cohorts were completed and assessed for the feasibility of the implant procedure and any complications before proceeding to implantations of the next ten-subject cohort. Descriptive statistics were calculated. Unless otherwise noted, data are expressed as mean ± standard error of the mean.
Results
Subjects
Forty subjects received implants between May 2011 and February 2013. There were 37 men (92.5%) and three women (7.5%). All subjects were Caucasian. The mean age at procedure was 46.1 years, with a range of 25–65 years. Mean baseline body mass index (BMI) was 28.3 kg/m2, with a protocol deviation granted by the study sponsor for enrollment of one subject whose BMI of 32.7 kg/m2 slightly exceeded the inclusion criterion of 32 kg/m2. OSA-relevant characteristics (ie, palate rating, tongue size, modified Mallampati [MMP] score, tonsillar grade, neck circumference, and soft palate length) were recorded to allow analysis of response and complication rates as a function of these characteristics ().
Table 2 Baseline demographics
Procedural feasibility
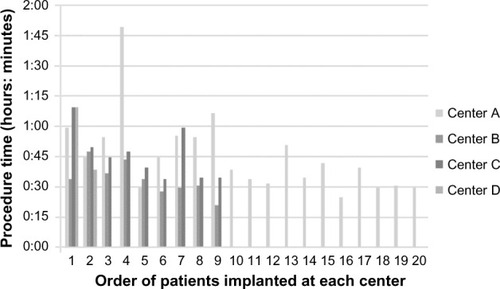
Each subject received two tongue-base implants and two soft-palate implants. The mean surgical time was 43 minutes (range: 21–110 minutes), which included the time needed for anesthesia induction and fluoroscopic imaging. There was a trend at each of the study centers for early procedures to be lengthier than the procedures performed after some practice (). Early procedures involved increased positioning of trocars and evaluation of implant position with fluoroscopic imaging for future evaluation of implant position versus the therapeutic effect of the implants. Later procedures continued to include fluoroscopic evaluation of implant positioning but contained fewer incidence of repositioning of the implant trocar, which was responsible for much of the procedure time decrease.
Complications and adverse events
Postoperative pain
Of the 40 subjects, 36 completed a pain diary for the 30 days following the implantation procedure. Thirty-two subjects (89%) reported diet modifications, employing a soft diet for 7.8 days (±0.8). Twenty-nine subjects (81%) reported using pain medications for an average of 6.9 days (±0.9). All subjects reported some procedure-related pain (>0). Immediately after the procedure (day 1), the average pain rating was 5.9 out of 10. The severe acute pain of one subject necessitated overnight hospital admission; it resolved without sequelae. In general, pain rapidly resolved, with 30 subjects (83%) reporting pain at day 10 (average of 2.8) and seven subjects (19%) reporting pain at day 30 (average of 1.6; ).
Table 3 Pain, diet modification, and pain medication usage reported by most subjects in the immediate postoperative period
Adverse events
There were 94 AEs among 40 subjects. Seventy-four AEs were determined to be related to the device or procedure. A majority of AEs were the expected sequelae of a minor surgical procedure with general anesthesia and were mild. One event, postsurgical pain that necessitated an inpatient night for observation after implantation, was categorized as a serious adverse event, although its severity was rated as mild.
Two subjects each had one of their tongue-base implants removed; both removals were considered serious adverse events. In one case, the subject removed an implant by himself by manually exploring the submental implant track and pulling on the implant. The site was inspected by the investigator, closed, and resolved without sequelae. In the second case, the subject developed an infection that necessitated explantation and was resolved with a course of antibiotics.
Twelve subjects experienced the extrusion of at least one soft-palate implant. Ten subjects each had one of their soft-palate implants removed, and two subjects required trimming of the implant due to extrusion of the implant end into the airway. All events resolved without sequelae.
Removal of tongue and palate implants typically took just a few minutes under local anesthesia and was without difficulty in all instances. Most removals were performed through the initial incision site. The removal process included access of the implant through the incision, freeing the exposed implant loop from tissue, grasping the exposed implant end with forceps, and removing the implant by pulling on the implant loop to stretch and remove the implant. In cases where the opposite implant end was healed and anchored on to the tissue, the implant was removed by grasping the implant loop with forceps near the incision site, pulling on the implant loop to stretch the elastic implant, dissecting along the length of the implant body with surgical scissors toward the opposite end, and trimming near that implant end, the opposite loop, or the tissue attachment through the loop to remove most or all of the implant. Revision of the tissue surrounding the implant was performed if necessary before closure of the incision site. In all cases, patient symptoms completely and quickly resolved after implant removal.
The device was well tolerated in subjects; no AEs regarding effects on speaking, swallowing, or detrimental effect on breathing were reported, despite specific queries for these events (). Effectiveness outcomes, which will be presented in an upcoming report, included overnight polysomnographic studies at 6-monthly intervals, which will further characterize change in sleep-disordered breathing. Furthermore, the implanter system and the implants functioned as intended during the implantation procedure and did not break or malfunction.
Table 4 Summary of complications
Discussion
The demographics of the subjects enrolled in this study were representative of the OSA patients treated at the referring clinics in terms of age and sex, although it is noted that the proportion of women in this study (7.5%) was smaller than the prevalence in the general population (up to 15%), a fact that is nevertheless complicated by the fact that women are less likely to be diagnosed and aggressively treated.Citation42 This study excluded most obese participants because of the selection criteria taking into account the historically reported low efficacy rates of surgical interventions in the higher BMI population.Citation43 In particular, feedback from surgeons indicated that subjects with lower BMI, small tonsils/absence of lingual tonsils, and no major palate contribution were the ideal candidates in regard to the surgical implantation procedure. The surgical time required for the implantation procedure was short. As would be expected for a “first-in-man procedure”, the procedure times were related to the experience of the implanting physician. Surgeons noted that a considerable portion of the surgical time involved fluoroscopic visualization and consideration of optimal implant placement. Revised implanter tools have been released for the next-generation iteration of the device; this is expected to reduce procedural times by eliminating the need for X-ray guidance. Procedure time is also expected to decrease with continued experience, improved tooling, and fewer evaluations performed for study purposes, although it will vary among the institutions; for example, teaching hospitals may have longer procedure times to instruct and train additional staff.
The device was well tolerated by subjects in this study. The postoperative pain was moderate and well within the lower range expected for ablative procedures.Citation44 Pain diminished considerably during the 30-day postoperative period assessed, with the average duration of soft-food diet modifications and pain medications being ∼7 days. The majority of AEs were expected and mild. The rate of extrusion of tongue-base implants was low and, although the rate of infection was 5%, the overall risk of infection may be lower with this approach as compared to other OSA surgeries because no intraoral incision is required to place the tongue implants. The risk profile with this intervention is similar to or better than with other implants for the tongue (complication rates of 15%–30% for tongue-base suspension and 0%–33% for tongue-base suspension with uvulopalatopharyngoplasty, and ∼25% for mandible tethering) and has a lower morbidity profile than other more invasive surgical methods, although it is recognized that the rate of complications and long-term negative consequences of surgical interventions for OSA is poorly characterized.Citation45–Citation47 Thus, the risks with this novel implant are considered to be acceptable as compared to other surgical approaches treating sleep apnea. Lastly, and perhaps most importantly, no subjects reported instances of foreign-body sensation, swallowing insufficiency, tongue mobility effects, or speech impediments, unlike with other procedures.Citation35,Citation48 In fact, investigators anecdotally report that some subjects have relayed having had no awareness of the implant whatsoever, after the healing process. Overnight polysomnographic studies were conducted in these subjects at baseline and at 6-monthly intervals to measure changes in sleep-disordered breathing; data will be presented in the upcoming effectiveness paper.
Unfortunately, there was a high incidence of device extrusions in the soft palate. Such events have been observed with other palate implants with an occurrence of ∼9%, likely due to the thinness of the palate mucosal tissue.Citation26,Citation49 Although extruding implants were readily addressed with minor procedures to remove or trim the implants, palate implants have been eliminated from the next-generation product configuration, which focuses only on tongue implants. Thus, physicians may perform their preferred procedure for addressing palate-level closure in those patients who require it.
It was noted during the study that two implants in the tongue base appeared insufficient to provide optimal tongue coverage for some subjects. Future studies would involve four implants placed along the midline of the tongue axially adjacent to one another to span a broader area of the tongue base with potentially greater effectiveness at preventing closure. Effectiveness outcomes will be presented in a subsequent report.
Conclusion
In this report, we presented the first human data for a first-generation sleep apnea system and placement procedure designed to treat OSA, a multifactorial, multilevel condition. The minimally invasive nature of the novel implants and their delivery were chosen instead of other more invasive or destructive and irreversible ablative options. The device and implantation tools were demonstrated to be feasible; the reported post-implantation issues (eg, palate extrusions) were due to issues with implant location/depth, placement of device, or infection, rather than device failures per se. The implant is believed to stabilize the tongue base and soft palate to increase the airway caliber and thus decrease the degree of airway collapse during sleep. The feasibility/usability data in this report demonstrates that this device has a safety profile that is favorable compared to other available treatments for tongue-base OSA, such as surgical remodeling of the airway. The tongue implant was well tolerated with no foreign-body sensation or difficulties with speech or swallowing, thus validating the design concept for the device. The soft-palate implant extruded at a significant frequency, and therefore will require technical adaptations and has been discontinued for the near term. Although the study’s conclusions are limited by its single-arm design and sample size, this clinical series provides early indications of the system’s safety and appropriateness for the treatment of tongue-base OSA. Future reports will present clinical effectiveness via comparison of pre- and post-treatment outcome measures.
Acknowledgments
This study was sponsored by ReVENT Medical, Inc. The intellectual contribution of Dr Allison Foster is acknowledged for professional medical writing assistance.
Disclosure
VP, BR, JTM, and TV received compensation as clinical investigators for this study. EG is a founder of ReVENT Medical, Inc. In addition, JTM and TV received honoraria for leading a training course for the ReVENT technique. The authors report no other conflicts of interest in this work.
References
- TeschlerHRanderathWSleep-related breathing disorders – historical development, current status, future prospectsPneumologie201064958358920827643
- GreenDESchulmanDAObstructive sleep apnea and cardiovascular diseaseCurr Treat Options Cardiovasc Med201012434235420842558
- PekerYHednerJKraicziHLothSRespiratory disturbance index: an independent predictor of mortality in coronary artery diseaseAm J Respir Crit Care Med20001621818610903224
- YoungTFinnLPeppardPESleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohortSleep20083181071107818714778
- YoungTPaltaMDempseyJSkatrudJWeberSBadrSThe occurrence of sleep-disordered breathing among middle-aged adultsN Engl J Med199332817123012358464434
- JenkinsonCDaviesRJMullinsRStradlingJRComparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trialLancet199935391702100210510382693
- HackMDaviesRJMullinsRRandomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoeaThorax200055322423110679542
- CalhounDAHardingSMSleep and hypertensionChest2010138243444320682533
- RedlineSYenokyanGGottliebDJObstructive sleep apnea-hypopnea and incident stroke: the sleep heart health studyAm J Respir Crit Care Med2010182226927720339144
- GottliebDJWhitneyCWBonekatWHRelation of sleepiness to respiratory disturbance index: the Sleep Heart Health StudyAm J Respir Crit Care Med199915925025079927364
- YaggiHKConcatoJKernanWNLichtmanJHBrassLMMohseninVObstructive sleep apnea as a risk factor for stroke and deathN Engl J Med2005353192034204116282178
- PunjabiNMCaffoBSGoodwinJLSleep-disordered breathing and mortality: a prospective cohort studyPLoS Med200968e100013219688045
- RamSSeirawanHKumarSKClarkGTPrevalence and impact of sleep disorders and sleep habits in the United StatesSleep Breath2010141637019629554
- KapurVKAlfonso-CristanchoRJust a good deal or truly a steal? Medical cost savings and the impact on the cost-effectiveness of treating sleep apneaSleep200932213513619238798
- RamaANTekwaniSHKushidaCASites of obstruction in obstructive sleep apneaChest200212241139114712377834
- BuchnerNJSannerBMBorgelJRumpLCContinuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular riskAm J Respir Crit Care Med2007176121274128017673692
- GroteLHednerJGrunsteinRKraicziHTherapy with nCPAP: incomplete elimination of sleep related breathing disorderEur Respir J200016592192711153593
- WeaverTEGrunsteinRRAdherence to continuous positive airway pressure therapy: the challenge to effective treatmentProc Am Thorac Soc20085217317818250209
- KribbsNBPackAIKlineLRObjective measurement of patterns of nasal CPAP use by patients with obstructive sleep apneaAm Rev Respir Dis199314748878958466125
- PhillipsCLGrunsteinRRDarendelilerMAHealth outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trialAm J Respir Crit Care Med2013187887988723413266
- Walker-EngstromM-LTegelbergAWilhelmssonBRingqvistI4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a randomized studyChest200212173974611888954
- MaurerJTSurgical treatment of obstructive sleep apnea: standard and emerging techniquesCurr Opin Pulm Med201016655255820842037
- KezirianEJHypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine reviewJAMA Otolaryngol Head Neck Surg20061322206213
- BaradaranfarMHEdalatkhahMDadgarniaMHThe effect of uvulopalatopharyngoplasty with tonsillectomy in patients with obstructive sleep apneaIndian J Otolaryngol Head Neck Surg201567suppl 1293325621250
- FriedmanMSchalchPLinHCKakodkarKAJosephNJMazloomNPalatal implants for the treatment of snoring and obstructive sleep apnea/hypopnea syndromeOtolaryngol Head Neck Surg2008138220921618241718
- ChoiJHKimS-NChoJHEfficacy of the pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysisLaryngoscope2013123126927622865236
- HamansEBoudewynsAStuckBAAdjustable tongue advancement for obstructive sleep apnea: a pilot studyAnn Otol Rhinol Laryngol20081171181582319102126
- WoodsonBTStewardDLMickelsonSHuntleyTGoldbergAMulticenter study of a novel adjustable tongue-advancement device for obstructive sleep apneaOtolaryngol Head Neck Surg2010143458559020869572
- van MaanenJPWitteBIde VriesNTheoretical approach towards increasing effectiveness of palatal surgery in obstructive sleep apnea: role for concomitant positional therapy?Sleep Breath20141834134924014179
- TanKBTohSTGuilleminaultCHoltyJ-ECA cost-effectiveness analysis of surgery for middle-aged men with severe obstructive sleep apnea intolerant of CPAPJ Clin Sleep Med201511552553525700871
- BoydSBWaltersASSongYWangLComparative effectiveness of maxillomandibular advancement and uvulopalatopharyngoplasty for the treatment of moderate to severe obstructive sleep apneaJ Oral Maxillofac Surg201371474375123219145
- HandlerEHamansEGoldbergANMickelsonSTongue suspension: an evidence-based review and comparison to hypopharyngeal surgery for OSALaryngoscope201412432933623729234
- CaplesSMRowleyJAPrinsellJRSurgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysisSleep201033101396140721061863
- BabaRYMohanAMettaVVMadorMJTemperature controlled radiofrequency ablation at different sites for treatment of obstructive sleep apnea syndrome: a systematic review and meta-analysisSleep Breath20151989191025643764
- FranklinKAAnttilaHAxelssonSEffects and side-effects of surgery for snoring and obstructive sleep apnea – a systematic reviewSleep2009321273619189776
- SundaramSLimJLassersonTJBridgmanSASurgery for obstructive sleep apneaCochrane Database Syst Rev2005194CD00100416235277
- PangKPSiowJKTsengPSafety of multilevel surgery in obstructive sleep apnea: a review of 487 casesArch Otolaryngol Head Neck Surg2012138435335722431863
- FouladpourNJesudossRBoldenNShamanZAuckleyDPerioperative complications in obstructive sleep apnea patients undergoing surgery: a review of the legal literatureAnesth Analg2016122114515126111263
- NelsonRECarterJMAnandAGHypopharyngeal airway surgery for obstructive sleep apnea: morbidity in the early postoperative periodOtolaryngol Head Neck Surg20141511 suppl26026124748589
- LiKKRileyRWPowellNBGervacioLTroellRJMinaultCObstructive sleep apnea surgery: patient perspective and polysomnographic resultsOtolaryngol Head Neck Surg200012357257511077343
- GillisERampersaudCPeaseEBuscemiPA novel implantable device for a minimally invasive surgical treatment of obstructive sleep apnea: design and preclinical safety assessmentNat Sci Sleep
- YoungTSkatrudJPeppardPERisk factors for obstructive sleep apnea in adultsJAMA2004291162013201615113821
- JordanASMcSharryDGMalhotraAAdult obstructive sleep apneaLancet2014383991873674723910433
- BlumenMBChalumeauFGauthierABobinSCosteAChabolleFComparative study of four radiofrequency generators for the treatment of snoringOtolaryngol Head Neck Surg200813829429918312874
- BostanciATurhanMA systematic review of tongue base suspension techniques as an isolated procedure or combined with uvulopalatopharyngoplasty in obstructive sleep apneaEur Arch Otorhinolaryngol Epub20151027
- PavelecVHamansEStuckBAA study of the new generation of the advance system tongue implants: three- and six-month effects of tongue to mandible tethering for obstructive sleep apneaLaryngoscope20111212487249321994041
- AuroraRNCaseyKRKristoDAmerican Academy of Sleep MedicinePractice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adultsSleep201033101408141321061864
- StuckBASauterAHormannKVerseTMaurerJTRadiofrequency surgery of the soft palate in the treatment of snoring. A placebo-controlled trialSleep200528784785016124664
- FriedmanMVidyasagarRBliznikasDJosephNJPatient selection and efficacy of pillar implant technique for treatment of snoring and obstructive sleep apnea/hypopnea syndromeOtolaryngol Head Neck Surg200613418719616455363