Abstract
The prevalence of obstructive sleep apnea (OSA) continues to rise. So too do the health, safety, and economic consequences. On an individual level, the causes and consequences of OSA can vary substantially between patients. In recent years, four key contributors to OSA pathogenesis or “phenotypes” have been characterized. These include a narrow, crowded, or collapsible upper airway “anatomical compromise” and “non-anatomical” contributors such as ineffective pharyngeal dilator muscle function during sleep, a low threshold for arousal to airway narrowing during sleep, and unstable control of breathing (high loop gain). Each of these phenotypes is a target for therapy. This review summarizes the latest knowledge on the different contributors to OSA with a focus on measurement techniques including emerging clinical tools designed to facilitate translation of new cause-driven targeted approaches to treat OSA. The potential for some of the specific pathophysiological causes of OSA to drive some of the key symptoms and consequences of OSA is also highlighted.
Introduction
Obstructive sleep apnea (OSA) is an increasingly common, chronic, sleep-related breathing disorder.Citation1–Citation3 OSA is characterized by periodic narrowing and obstruction of the pharyngeal airway during sleep. Untreated OSA is associated with long-term health consequences including cardiovascular disease,Citation4,Citation5 metabolic disorders,Citation6 cognitive impairment,Citation7 and depression.Citation8 Common symptoms include excessive daytime sleepiness, fatigue, non-refreshing sleep, nocturia, morning headache, irritability, and memory loss.Citation9,Citation10 Untreated OSA is also associated with lost productivity and workplace and motor vehicle accidents resulting in injury and fatality.Citation11–Citation13 The costs of untreated OSA and sleep loss are substantial.Citation14,Citation15 Recommended therapy can relieve symptomsCitation16,Citation17 and reduce some of the associated sequelae.Citation18,Citation19 However, many people with OSA struggle with the first-line therapy, continuous positive airway pressure (CPAP), for which adherence rates remain unacceptably low.Citation20,Citation21 Non-CPAP therapies (e.g., oral appliance therapy and upper airway surgery) are beneficial in many cases but have variable and unpredictable efficacy.Citation22–Citation25 Thus, new approaches to treat OSA are required.
Indeed, most people with OSA are undiagnosed and untreated.Citation26–Citation28 In some cases, this may be attributed to, at least in part, a lack of awareness of the disorder.Citation29 Other potential barriers to seek treatment include stigma related to some of the features of the disease such as snoring, access to polysomnography (PSG) and diagnostic services (particularly in remote communities and in the developing world),Citation27,Citation29 perceived lack of enthusiasm with existing treatment options, and, in some cases, concern that driving licenses will be revoked. In addition, primary care physicians may not be prompted to explore an early diagnosis of OSA. This is especially true if patients do not present with subjective sleepiness and the classic characteristics of a high body mass index. Symptoms such as fatigue or sleepiness may also be attributed to comorbid disease that is common in people with OSA.Citation30 However, we know that absence of subjective sleepiness does not rule out substantial sleep-disordered breathing and up to 50% of people with OSA are not obese.Citation2,Citation31 Indeed, 25% of individuals with moderate OSA have neither subjective nor objective sleepiness.Citation32,Citation33 Nonetheless, given the burden of disease, the shortcomings of existing diagnostic and treatment approaches, and the substantial health, safety, and economic consequences of untreated OSA, there is a pressing need to continue to raise awareness and develop new strategies to manage and treat this common chronic health condition.
There are multiple contributors to OSA.Citation34,Citation35 Each contributor represents a therapeutic target.Citation34,Citation35 While these new research findings offer hope for new therapies, identification of these new targets has not yet translated to new models of care for OSA.Citation36 However, there has been recent progress toward achieving this goal. For example, strategies to extract information from existing clinical PSG studies to help inform treatment decisions according to a cause-driven targeted therapy model for OSA have been developed.Citation37–Citation41 In accordance with this objective, simple wakefulness upper airway and respiratory physiology tests may also be useful.Citation42–Citation45 These concepts are the focus of the current review.
Existing clinical measures of OSA
The gold standard method used to diagnose sleep-disordered breathing is a comprehensive in-laboratory PSG. The main outcome used to define OSA severity is the apnea-hypopnea index (AHI). This index represents the number of breathing stoppages (apneas) and periods of reduced airflow (hypopneas) lasting greater than 10 seconds that result in a brief awakening (arousal) or reduced oxygenation that occur per hour of sleep. While severity cutoffs vary, mild sleep apnea is typically defined as 5–15, moderate 15–30, and severe more than 30 respiratory events/h sleep.
While in-laboratory PSG is comprehensive, it is also labor intensive, time-consuming, and costly (see the study by Edwards et alCitation46 for a review). To facilitate the diagnosis process, home-monitoring technologies have emerged. These range from a replication of the same measurements used in the laboratory (a level 2 unattended study) to limited channel devices that focus on a few core signals (e.g., oxygen and an airflow sensor). These tend to be most useful for the detection of severe disease, provided patients do not have severe comorbidity.Citation47–Citation49
Despite the quantity of neurophysiological signals obtained during an overnight PSG, most of the data collected is ignored and treatment decisions rely heavily on the AHI. While the AHI remains a widely used measure of OSA severity clinically and for research purposes, it has several limitations. For example, a patient with very long respiratory events may experience substantial hypoxemia but have a relatively low AHI. Conversely, another patient may have more frequent events and therefore a much higher AHI, but minimal exposure to hypoxemia.Citation50 Thus, the effects of hypoxia from OSA and its adverse impact on the cardiovascular system in the patient with a low AHI may be more pronounced.Citation51,Citation52 In addition, non-apneic respiratory events that do not meet the scoring criteria for a hypopnea are associated with heart rate changes and increased expiratory pharyngeal resistance.Citation53 Breathing disruptions that do not cause major hypoxemia are also associated with objective daytime sleepiness.Citation54 Furthermore, the total AHI correlates poorly with the key causes and consequences of the disease.Citation35,Citation55 Conversely, recent studies indicate that REM sleep apnea may be more important in mediating insulin resistance and the cardiovascular consequences of OSA.Citation56–Citation59 Thus, these examples highlight the heterogeneity in the various clinical manifestations of OSA and its consequences and some of the limitations with currently used diagnostic methods.
OSA pathophysiology
Similar to the clinical heterogeneity, OSA pathogenesis is also multifactorial. There are “anatomical” and “non- anatomical” causesCitation34,Citation35,Citation60 (). In recent years, the potential role that factors beyond pharyngeal anatomy and craniofacial structure play in OSA pathophysiology has been recognized. Indeed, OSA can develop due to multiple contributors, the combination of which likely varies substantially between patients. These concepts have been the focus of several recent review articles (e.g., studies by Eckert,Citation34 Carberry et al,Citation36 Edwards et al,Citation61 and Eckert and WellmanCitation62) and are therefore only briefly summarized here.
Figure 1 Schematic of the anatomical and non-anatomical causes of OSA.
Abbreviations: EEG, electroencephalography; EMG, genioglossus electromyography; MTA, 100 ms moving time average of the rectified raw EMG signal; OSA, obstructive sleep apnea.
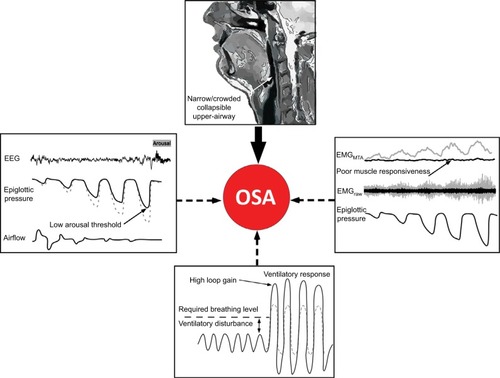
Non-anatomical contributors include impaired pharyngeal dilator muscle function, premature awakening to mild airway narrowing (low respiratory arousal threshold), and unstable control of breathing (high loop gainCitation3,Citation34,Citation35) (). As highlighted in later sections, when combined with a pharyngeal airway that is susceptible to closure during sleep, impairment in one or more of these non-anatomical contributors can perpetuate OSA severity. Given that airway obstruction in OSA only occurs during sleep, the combination of an anatomical predisposition combined with state-dependent changes in non-anatomical contributors is crucial in driving this common disorder.Citation34,Citation35,Citation63
OSA is largely a sleep-dependent anatomical problem
As highlighted, OSA is a multifactorial disorder. However, some level of upper airway anatomical impairment is essential.Citation34,Citation35 Thus, it is logical that most existing therapies for OSA aim to correct the anatomical problem. Imaging studiesCitation64–Citation66 have identified key pharyngeal anatomical abnormalities in people with OSA. For example, a narrow pharyngeal airway, increased airway length, and certain pharyngeal lumen shapes are all associated with the propensity for pharyngeal collapse during sleep.Citation67–Citation69 The upper airway can collapse at one or multiple sites.Citation70 The pharyngeal structures that can contribute to airway crowding and collapse include the dilator muscles such as genioglossus, soft palate, lateral pharyngeal walls, and the epiglottis. Obesity is an important risk factor.Citation71 Neck circumference is routinely measured in the clinic and has been used to help predict OSA risk.Citation72,Citation73 Craniofacial morphology,Citation74,Citation75 position of the hyoid bone,Citation76 airway surface tension,Citation77 tongue scalloping,Citation78 and tongue fatCitation79 are some of the factors associated with OSA risk and its severity. While these approaches have provided insight into OSA pathogenesis, limitations for clinical use include 1) high cost of imaging procedures and 2) awake static imaging provides limited insight into the properties of a dynamic structure that closes involuntarily during sleep.
Upper airway collapsibility
Pharyngeal critical closing pressure (Pcrit) is a well-established technique used to quantify upper airway collapsibility during sleep.Citation80–Citation82 Pcrit has been used to describe differences in upper airway collapsibility across the sleep-disordered breathing spectrum (from snoring to OSACitation80). It is considered as the gold standard approach to quantify “functional anatomy” during sleep.Citation34 The Pcrit technique allows the pharyngeal airway to be examined under conditions of reduced,Citation82 although not absent,Citation83 neuromuscular input compared to wakefulness. Once a therapeutic CPAP level that prevents airway obstruction or narrowing is established, brief reductions (5 breaths) in the holding pressure are applied during stable sleep.Citation70 This procedure is repeated at different levels of mask pressure until airflow limitation and closure occurs. The pressure-flow relationship between peak inspiratory flow for flow-limited breaths and the corresponding mask pressure is compared. These values are then extrapolated to determine the “Pcrit”, the mask pressure at zero airflow. Within an individual, Pcrit measurements are stable over time (days to months).Citation84 However, factors such as weight gain over a longer period would be expected to increase airway collapsibility (Pcrit).Citation85,Citation86
On average, OSA patients tend to have Pcrit values near atmospheric pressure. This indicates that their airway closes at or near 0 cmH2O during sleep.Citation35,Citation76,Citation86 However, there is substantial variability in Pcrit in OSA and therefore anatomical vulnerability to pharyngeal collapse. Indeed, Pcrit can range from approximately −5 to greater than +5 cmH2O in OSA. A Pcrit at or near +5 cmH2O indicates a highly collapsible airway, whereas a sub-atmospheric Pcrit indicates a relatively stable upper airway as suction pressure is required to close the upper airway during sleep. OSA is very rare in people with Pcrit values less than −5 cmH2O.Citation35,Citation86 However, within the sub-atmospheric range (0 to −5 cmH2O), there is considerable overlap in Pcrit between people with and without OSA. Indeed, approximately 20% of OSA patients have similar pharyngeal collapsibility during sleep compared to people without OSA.Citation35 In this group, the interaction between mild anatomical susceptibility and impairment in one or more of the non-anatomical causes of OSA is crucial in driving OSA pathogenesis.Citation34,Citation35 These patients are more likely to benefit from targeted non-CPAP therapies compared to those with very high Pcrits.Citation34,Citation36 Thus, given the key role that upper airway anatomy/collapsibility plays in driving OSA pathogenesis, a simple measure of airway collapsibility would be invaluable to inform targeted treatment decisions. The problem, however, is that the Pcrit technique is not clinically viable as the protocol is technically challenging, somewhat invasive (requires CPAP and ideally a pharyngeal pressure catheter), time-consuming, and requires skilled personnel to collect and analyze the data.
New simplified methods to estimate upper airway collapsibility
There has been recent progress toward development of simple and reliable methods to estimate the extent of anatomical/ airway collapsibility contribution to OSA. The first technique involves an existing tool traditionally used to assess expiratory flow limitation in patients with chronic obstructive pulmonary disease.Citation42 Participants are fitted with a nasal breathing mask and brief (2 second) periods of negative pressure (−5 cmH2O) are delivered during early expiration.Citation42 This elicits a transient increase in expiratory airflow the extent to which is mediated, at least in part, by upper airway collapsibility/anatomy. The average response is quantified as the ratio between the exhaled volumes (during the first 0.2 seconds) for at least 4 breaths prior to the expiratory pressure application versus the expiratory pressure breaths for 10 replicate trials. An increase in this ratio suggests a collapsible airway. A modest relationship with Pcrit and other important anatomical components that contribute to OSA was detected.Citation42 Thus, this technique alone is unlikely to be helpful in informing treatment decisions, but if combined with other simple measures it may play a role.
Preliminary data from our group indicate that a 10–15 minute protocol in which brief pulses of suction are applied during early inspiration through a nasal mask during wakefulness correlates well with Pcrit.Citation43 The prescribed CPAP level from a routine CPAP titration study is also associated with passive Pcrit.Citation37 Thus, the therapeutic CPAP level may be useful in distinguishing patients with mildly versus highly collapsible upper airways.Citation37 Genta et alCitation39 have also recently demonstrated that analysis of the shape of the inspiratory flow curve during airflow limitation during sleep and the degree of negative effort dependence (extent to which the airway narrows during inspiration) can inform the site of upper airway collapse. This was determined using endoscopy to locate the site of collapse while simultaneously monitoring nasal airflow and pharyngeal pressures. Averaging multiple flow-limited breaths revealed characteristic flow patterns that were associated with different sites of airway narrowing/collapse.Citation39 In addition, simply quantifying peak flow during routine polysomnography has recently been shown to be associated with active Pcrit (a measure that encompasses upper airway collapsibility and neuromuscular compensationCitation40). Thus, there are several new promising approaches to estimate the extent of anatomical impairment in people with OSA. Given their relative simplicity, one or more of these approaches may be preferable compared to more invasive procedures such as drug-induced endoscopy, which is becoming increasingly used to help inform patient selection for upper airway surgery.Citation87
The upper airway muscles
The human pharynx is unique in that it lacks rigid bony support. Its predominant soft tissue structure enables it to change cross-sectional area with varying intraluminal pressures. However, depending on the dynamic balance of intraluminal pressure and neural drive to the upper airway dilator muscles, the human pharynx is vulnerable to collapse during sleep.Citation88 There are over 20 muscles in the upper airway. These are involved in respiratory and non-respiratory tasks (speech, mastication, swallowing, and breathing). A subset of these muscles plays a predominant role in airway stability during breathing.Citation89 In healthy individuals and people with OSA during wakefulness, activation of the upper airway dilator muscles is effective in opposing the collapsing pressures generated during inspiration. However, during sleep, state-dependent reductions in muscle activity when combined with anatomical susceptibility (narrow/crowded/collapsible airway) can induce airway collapse.Citation88 Thus, understanding the neural control of the airway muscles and their mechanical consequences is important for development of new treatments and preventative measures to improve upper airway function in OSA.
The upper airway muscles have complex patterns of neural activation that differ between muscles. For example, the genioglossus, the largest pharyngeal dilator muscle located at the base of the tongue, receives up to six different patterns of drive.Citation90 It receives central input from the brainstem (respiratory pattern generator neurons) and reflex input from pharyngeal mechanoreceptors and chemoreceptors. The summation of drive to genioglossus typically results in a phasic pattern of activation (i.e., more activity during inspiration and less during expiration, –). Genioglossus activity is reduced at sleep onsetCitation91 and varies between sleep stages.Citation83 However the tensor palatini muscle (a palatal muscle) displays predominantly tonic (constant throughout the breathing cycle) patterns of activation.Citation92 It is less sensitive to small changes in pharyngeal pressure compared to genioglossus but can be activated in a similar manner with larger transient pressure swingsCitation93 and is sensitive to sleep state but has minimal change across sleep stages in the absence of upper airway resistance.Citation83 The combinations of a loss in central drive and reflex input to the upper airway muscles during sleep are thought to be important contributors to OSA pathogenesis.Citation94,Citation95 Similarly, the ability to increase reflex drive to airway narrowing during sleep is important in OSA pathogenesis.Citation35,Citation96,Citation97 Indeed, approximately 30% of OSA patients have poor genioglossus muscle responsiveness to airway narrowing during sleepCitation35 (). Many patients have a high recruitment threshold to respiratory stimuli during sleep that is not reached without awakening from sleep (arousal).Citation96 Conversely, others are able to restore airflow during sleep via pharyngeal muscle recruitment without arousal (). In addition, enhanced muscle responsiveness can protect certain obese individuals from developing OSA despite their anatomical compromise.Citation98 Thus, the combination of non-anatomical and anatomical compromise is crucial in preventing or promoting OSA.
Figure 2 Example of minimal genioglossus muscle responsiveness.
Abbreviations: EEG, electroencephalography; EMG, genioglossus electromyography; MTA, 100 ms moving time average of the rectified raw EMG signal.
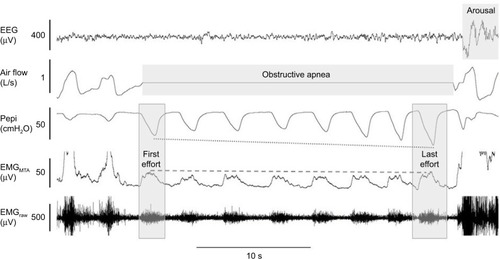
Figure 3 Example of robust genioglossus muscle responsiveness and restoration of airflow without cortical arousal.
Abbreviations: EEG, electroencephalogram; EMG, genioglossus electromyography; MTA, 100 ms moving time average of the rectified raw EMG signal.
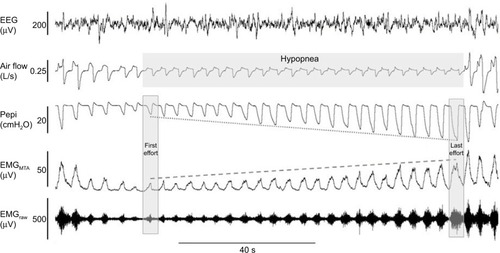
Figure 4 Example of robust genioglossus muscle responsiveness without restoration of airflow.
Abbreviations: EEG, electroencephalogram; EMG, genioglossus electromyography; MTA, 100 ms moving time average of the rectified raw EMG signal.
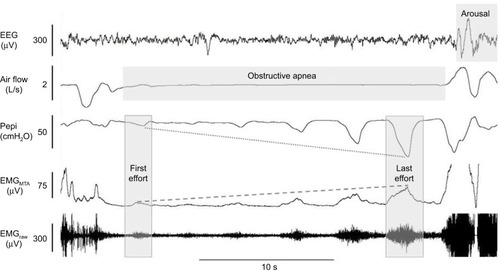
In addition, recent findings suggest a mismatch between central neural drive to the genioglossus muscle and the mechanical response of the muscles in OSA patients.Citation99 Indeed, in healthy individuals, dynamic magnetic resonance imaging shows anterior movement of this fan-shaped muscle during inspiration and increased cross-sectional area (CSA) of the pharynx.Citation100 However, tongue movement patterns during quiet breathing vary in people with OSA.Citation101 Some patients have counterproductive motion characterized by anterior motion at the base of the tongue followed by airway narrowing at the level of the soft palate, while others have little to no movement during inspiration.Citation101 These patterns of movement are dependent, at least in part, on OSA severity whereby minimal movement is most common in severe OSA.Citation101 Compensatory mechanisms in healthy individuals who have a narrow airway CSA compared to controls display larger anterior movement of the tongue during inspiration.Citation102 Accordingly, breathing stability is associated with greater genioglossus activity.Citation103 However, increased genioglossus activity is sometimes insufficient to re-open the airway (). Thus, the contribution of the other upper airway muscles may also play a contributing role.Citation104 How the various components of upper airway muscle function change over time is unknown. However, increased weight gain over time and fat accumulation in the tongue are predicted to worsen upper airway motion.Citation79
Treatments to target the upper airway muscles
One approach to activate the upper airway muscles during sleep is to deliver current to the muscles via direct stimulation or via stimulation of the hypoglossal nerve. Clinically, this is achieved via surgical implantation of a stimulation device connected to a cuff placed around the nerve.Citation105 Mechanistic studies have also used fine-wire electrodes or non-invasive methods such as transcutaneous electrodes (see recent review to compare different methodsCitation106). Improvements in inspiratory airflow, AHI, apnea duration, oxygen saturation, arousal index, sleep architecture, and excessive daytime sleepiness have all been reported to varying degrees using these methods.Citation107–Citation111 Clinical trial follow-up studies show long-term sustained reductions in AHI (~50–66%).Citation111–Citation114 However, it is difficult to predict responders. The STAR trial used endoscopy for pre-screening to eliminate patients with concentric airway collapse, which may have increased the response rate to therapy.Citation105 However, one-third of patients were still classified as non-responders.Citation105 Thus, other factors such as airway shape and Pcrit are also likely to be important considerations to optimize treatment response rates.Citation105,Citation110
To date, attempts to develop pharmacotherapy to increase upper airway muscle activity to treat OSA have not been successful. Targets have included serotonergic, noradrenergic and GABAergic systems, as well as potassium channels (for reviews see).Citation115,Citation116 However, recent studies with the tricyclic antidepressant desipramine which has strong noradrenergic, mild antimuscarinic, and mild serotonergic effects, have shown preservation of sleep-related reductions in genioglossus activity.Citation117 Desipramine also yields improvements in airway collapsibility and OSA severity in patients with poor muscle responsiveness.Citation118 Thus, this combined approach may be superior to single system targets.Citation115,Citation119–Citation121 Interestingly, the hypnotic zolpidem (which acts on the GABAergic system) shows potential to increase pharyngeal muscle responsiveness during airway narrowing without impairing the other key causes of OSA.Citation122 Designer Receptors Exclusively Activated by Designer Drugs that allow for selective targeting of a group of neurons via introduction of an engineered macromolecule (designer receptor) with viral vectors that can be activated with a specific drug (designer drug) have also recently been tested in animal models with the objective to increase pharyngeal muscle activity during sleep.Citation123,Citation124 These exciting findings demonstrate lasting increases in genioglossus activity and offer promise that these new concepts will ultimately translate to humans.
Other strategies to improve pharyngeal muscle function include training modalities. Indeed, regular didgeridoo playing and oropharyngeal exercises can reduce snoring and OSA severity (~50% reduction in AHI) and daytime sleepiness.Citation125–Citation128 However, the mechanisms are largely unknown. No studies have investigated upper airway muscle tone or muscle properties pre-training versus post-training. Longitudinal follow-up studies are also lacking. Understanding the mechanisms may help to inform which OSA phenotypes are most likely to respond to this form of therapy.
Prediction tools and simplified methods to estimate pharyngeal muscle function
Identification of patients who have poor pharyngeal muscle function may facilitate development of targeted therapies directed toward this traitCitation118 and improve treatment success rates with existing therapies (e.g., hypoglossal nerve stimulation). However, gold standard methodology to quantify pharyngeal muscle activity is complex, invasive (fine-wire electrodes inserted directly into the muscle), requires specialized personnel and equipment, and is time-consuming.Citation34 There are no simplified tools to estimate pharyngeal muscle function to identify people with this clinical phenotype accurately. However, while multiple variables impact inspiratory flow during sleep, mean peak inspiratory airflow during airflow limitation appears to be a good surrogate for active Pcrit, a measure that incorporates both anatomical and neuromuscular components.Citation40 Thus, if this approach proves useful to predict treatment outcomes, automated signal processing algorithms based on routine PSG data could be implemented.Citation40 Nonetheless, additional practical tools to determine pharyngeal muscle function are urgently required in order to advance personalized treatment approaches that target the upper airway muscles.
Respiratory arousal threshold
The role of the respiratory arousal threshold in OSA pathogenesis has been described in detail.Citation129 Accordingly, these concepts are outlined only briefly here. Historically, given that most respiratory events are associated with a cortical arousal, arousals were considered crucial to reopen the upper airway following a respiratory event in OSA.Citation130,Citation131 However, approximately 20% of respiratory events cease without cortical arousal and an additional 20% occur after the upper airway has already reopened and airflow has been restored.Citation129,Citation132–Citation134 Indeed, 75% of adults with OSA have respiratory events that terminate without an arousal or the arousal occurs following airway reopening at some stage of the night.Citation132 Thus, airway reopening can occur without arousal. Rather, continual unnecessary arousals can worsen OSA and contribute to OSA pathophysiolgy.Citation129,Citation132 Specifically, repetitive arousals can perpetuate blood-gas disturbances and cause sleep fragmentation to promote cyclical breathing and prevent establishment and maintenance of more stable, deeper stages of sleep.Citation135–Citation138
Numerous respiratory stimuli can contribute to arousal from sleep during a respiratory event.Citation129,Citation139–Citation142 Increased respiratory effort due to a narrowed pharyngeal airway increases negative intrathoracic pressure. Although the amount of negative intrathoracic pressure generated can vary greatly between individuals and in different stages of sleep,Citation35,Citation132,Citation143–Citation147 the magnitude of negative pressure required to cause an arousal from sleep is relatively constant within an individual.Citation147–Citation149 This is the case regardless of whether the respiratory disturbance is caused by hypoxia, hypercapnia, or respiratory loading.Citation147
Accordingly, the gold standard method to quantify the threshold for arousal to respiratory stimuli requires an epiglottic or esophageal pressure catheter combined with PSG recording equipment. Specifically, the respiratory arousal threshold is the nadir pressure immediately prior to cortical arousal (, , and ). The respiratory arousal threshold is quantified by averaging multiple pressure values throughout the night to an experimental intervention designed to cause airway narrowing or activate respiratory afferentsCitation35,Citation147 or during naturally occurring respiratory events.Citation144,Citation145
In people with OSA who require large intrathoracic pressure swings to cause an arousal (i.e., patients with high respiratory arousal thresholds ≤25cmH2O), respiratory events are often prolonged, particularly if these patients also have poor upper airway muscle responsiveness.Citation129 Thus, in the absence of neuromuscular compensation, arousal from sleep and reintroduction of wakefulness drive can act as a last line of defense to facilitate rapid reopening of the airway to re-establish airflow and normalize blood-gas levels in these individuals.Citation129,Citation130,Citation150 The consequences of OSA such as sleep deprivation increase, while CPAP therapy decreases the respiratory arousal threshold.Citation151,Citation152 Nonetheless, approximately 30–50% of OSA patients wake to relatively small intrathoracic/epiglottic pressure swings (i.e., patients with low respiratory arousal thresholds ≥−15 cmH2O).Citation35,Citation38,Citation129,Citation145 In these patients, given that the stimuli for arousal are the same as the stimuli required to recruit the pharyngeal dilator muscles (i.e., blood gas changes and negative pressure swings), premature arousal limits the opportunity for neuromuscular compensation mechanisms to overcome airway narrowing and stabilize breathing.Citation132 Frequent arousals can also cause sleep fragmentation and sleep instability, prevent deeper stages of sleep, and perpetuate unstable breathing.Citation132,Citation142 Thus, strategies to reduce arousals in these patients may allow for more stable breathing during sleep.
Treatments to target the respiratory arousal threshold
Given the abovementioned rationale, the potential therapeutic role of hypnotics to treat OSA in patients with a low respiratory arousal threshold phenotype has been an area of recent research focus.Citation150 This strategy requires a careful targeted approach to optimize benefit in those with the desired phenotype and avoid harm in those with high arousal thresholds. In particular, the selected agent to increase the threshold for arousal to respiratory stimuli must do so without impairment in pharyngeal muscle activity. Apart from an early wakefulness benzodiazepine study,Citation22 subsequent studies during sleep have not shown systematic reductions in pharyngeal muscle activity or responsiveness to negative pharyngeal pressure with common doses of zopiclone,Citation96,Citation144 trazodone,Citation153 temazepam,Citation122 or zolpidem.Citation122 While a recent study found more variable effects on genioglossus muscle responsiveness with temazepam, paradoxically, on average, zolpidem increased muscle responsiveness 3-fold in people with and without OSA.Citation122
The other concern with hypnotic use in OSA is prolongation of respiratory events and worsening hypoxemia due to blunted arousal responses in those individuals with a high threshold for respiratory arousal. Indeed, this can occur with high doses or in obese patients with very severe disease.Citation129 By contrast, the hypnotics eszopiclone,Citation145 zopiclone,Citation22 and trazodoneCitation154 can reduce OSA severity as measured by the AHI without worsening hypoxemia. In the eszopiclone study,Citation145 reductions in AHI occurred invariably in those with a low arousal threshold phenotype. The number of arousals per hour of sleep also decreased.Citation145 Given the contrasting effects of hypnotics in OSA, screening tools to distinguish between patients with low and high respiratory arousal thresholds and to determine who will benefit versus be susceptible to harm are important.
Prediction tools and simplified methods to estimate the respiratory arousal threshold
The gold standard approach to quantify the respiratory arousal threshold is impractical for routine clinical use as it is time-consuming, costly, and somewhat invasive (requires an airway pressure catheter). Preliminarily findings indicate that respiratory sensation to inspiratory loading during wakefulness is related to the respiratory arousal threshold during sleep.Citation155 Edwards et al have developed a simple tool to estimate the respiratory arousal threshold with high sensitivity and specificity based on three measures from a standard overnight PSG (AHI, nadir oxygen saturation, and the apnea to hypopnea ratio).Citation38 Thus, while prospective intervention studies are required, this simple approach could easily be implemented in the clinical setting to inform treatment decisions. Given that over 40% of OSA patients may also have insomnia,Citation156–Citation158 simple accurate tools to determine which OSA patients will benefit versus those at risk of harm with hypnotics would be invaluable.
Loop gain
Loop gain is a term used to describe the stability of a feedback control system. In the context of respiratory physiology, loop gain is the ventilatory response to ventilatory disturbance ratio. It comprises three principal components: 1) plant gain (i.e., tissues, blood, and lungs where CO2 is stored) and 2) delays in circulation (i.e., time it takes for a change in CO2 to mix with the existing blood to arrive and be detected by the chemoreceptors) and 3) controller gain (i.e., chemosensitivity). Any medical condition that modifies one or more of these components (e.g., heart failure) will alter loop gain.Citation34 Components of OSA such as intermittent hypoxia can also alter respiratory control.Citation159 People with high loop gain have exaggerated ventilatory responses to minimal changes in CO2. This is a marker of an unstable control system. This can be reduced with CPAP therapy.Citation160 On the other hand, those with extremely low loop gain often experience hypoventilation during sleep, as is the case in people with obesity hypoventilation syndrome.Citation34
In their landmark study using proportional assist ventilation to induce breathing oscillations during sleep after the airway was stabilized with CPAP, Younes et al showed that severe OSA patients have high loop gain.Citation138 Subsequent studies confirmed that many people with OSA have high loop gain.Citation35,Citation161 When combined with even a modest impairment in upper airway anatomy, high loop gain can drive OSA pathogenesis.Citation35,Citation161 Similar to the other phenotypic traits, loop gain can be quantified using transient reductions in CPAP during sleep to create a disturbance to breathing.Citation161,Citation162 Rapid reintroduction of CPAP is then applied so that the ventilatory response (overshoot) can be quantified (using a breathing mask and pneumotachograph) to calculate loop gain. This procedure is repeated as many times as possible throughout the night. Loop gain is then calculated as the ventilatory response divided by the ventilatory disturbance ratio after scaling for the different levels of ventilatory disturbances presented.Citation161,Citation162 This technique results in a negative number such that more negative numbers reflect higher loop gain.Citation162 Approximately one-third of OSA patients have high loop gain (<−5) which indicates a >5 L/min increase in minute ventilation in response to 1 L/min reduction in minute ventilation.Citation35
Treatments to target loop gain and simplified methods to estimate loop gain
O2 therapy reduces loop gain and OSA severity in people with high loop gain.Citation163 Carbonic anhydrase inhibitors such as acetazolamide also reduce loop gain by approximately 40% and OSA severity.Citation164 Similarly, zonisamide reduces OSA severity.Citation24 In addition, stabilization of CO2 and hypercapnia can prevent hypoventilation and unstable respiratory control during sleep in OSA.Citation165 Strategies that combine O2 therapy to reduce loop gain with a hypnotic to increase the arousal threshold can yield major reductions in OSA severity.Citation146 In addition, recent studies from the Mateika group indicate that several of the key contributors to OSA such as airway collapsibility, arousal threshold, and respiratory control are influenced by circadian phase.Citation166–Citation168 Thus, novel strategies that target the circadian system may have therapeutic potential in OSA.
The current approaches to quantify loop gain during sleep require experienced personnel to perform CPAP manipulations and analyze the data. However, Terrill et al have developed an analysis approach that uses the nasal pressure signal from a standard PSG to estimate loop gain.Citation41 This approach has been used to explain and predict changes to a range of interventions in OSA.Citation30,Citation47,Citation146 Wakefulness tests of respiratory control may also be helpful in estimating responses to pharmacotherapies.Citation44,Citation45
Potential links between phenotypic traits, treatment, and health consequences
In addition to the role that the phenotypic traits play in contributing to OSA pathogenesis, the traits may also provide insight into disease consequences and physiological reasons for treatment failure. For example, a low arousal threshold trait may be a physiological contributor to poor CPAP adherence and compliance.Citation31 A low arousal threshold trait and its consequences such as sleep fragmentation and frequent hypopneas with minimal changes in oxygenation may be a marker for cognitive impairment and daytime sleepiness.Citation54,Citation169 Similarly, patients who tend to have more “intense” arousals, which appear to be an inherent trait,Citation47,Citation170 may experience more daytime sleepiness than those who do not. People who have OSA that is driven by high loop gain may be more vulnerable to the cardiovascular consequences of OSA. Conversely, strategies that target certain components of respiratory control such as mild intermittent hypoxia to elicit respiratory plasticity may help improve CPAP compliance and potentially also directly target the autonomic, cardiovascular, neurocognitive, and metabolic systems.Citation171,Citation172 While possible links between the phenotypic traits and specific disease consequences have not yet been investigated, emergence of simplified tools to estimate the traits will enable these theoretical concepts to be tested systematically in cohort studies.
Conclusion
Characterization of the different causes or phenotypes of OSA in recent years has provided new pathways for targeted therapy. New simplified approaches to estimate each of the key causes of OSA have recently been developed. While more work is required, particularly directed toward the impaired pharyngeal muscle trait, these new tools offer promise for the translation of detailed phenotyping concepts to the clinic. Identification of the traits may also provide insight in to which patients are more likely to develop specific disease consequences.
Disclosure
DJE is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship (1116942), has a Commonwealth Government of Australia Cooperative Research Centre grant (industry partner: Oventus Medical), and serves as a consultant for Bayer. The other authors report no conflicts of interest in this work.
References
- PunjabiNMThe epidemiology of adult obstructive sleep apneaProc Am Thorac Soc20085213614318250205
- PeppardPEYoungTBarnetJHPaltaMHagenEWHlaKMIncreased prevalence of sleep-disordered breathing in adultsAm J Epidemiol201317791006101423589584
- HeinzerRVatSMarques-VidalPPrevalence of sleep-disordered breathing in the general population: the HypnoLaus studyLancet Respir Med20153431031825682233
- KapurVKResnickHEGottliebDJSleep Heart Health Study GroupSleep disordered breathing and hypertension: does self-reported sleepiness modify the association?Sleep20083181127113218714785
- WaliaHKLiHRueschmanMAssociation of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication useJ Clin Sleep Med201410883584325126027
- DragerLFTogeiroSMPolotskyVYLorenzi-FilhoGObstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndromeJ Am Coll Cardiol201362756957623770180
- OlaitheMBucksRSHillmanDREastwoodPRCognitive deficits in obstructive sleep apnea: insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivationSleep Med Rev Epub2017330
- WheatonAGPerryGSChapmanDPCroftJBSleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005–2008Sleep201235446146722467983
- AnticNACatchesidePBuchanCThe effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSASleep201134111111921203366
- RomeroEKrakowBHaynesPUlibarriVNocturia and snoring: predictive symptoms for obstructive sleep apneaSleep Breath201014433734319865841
- MulgrewATRyanCFFleethamJAThe impact of obstructive sleep apnea and daytime sleepiness on work limitationSleep Med200791425317825611
- HowardMEDesaiAVGrunsteinRRSleepiness, sleep-disordered breathing, and accident risk factors in commercial vehicle driversAm J Respir Crit Care Med200417091014102115317672
- StoohsRAGuilleminaultCItoiADementWCTraffic accidents in commercial long-haul truck drivers: the influence of sleep-disordered breathing and obesitySleep19941776196237846460
- HillmanDRMurphyASPezzulloLThe economic cost of sleep disordersSleep200629329930516553015
- Sleep Health FoundationAsleep on the job: Costs of inadequate sleep in Australia20171112
- MontserratJMFerrerMHernandezLEffectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placeboAm J Resp Crit Care Med2001164460861311520724
- KnudsenTBLaulundASIngerslevJHomøePPinholtEMImproved apnea-hypopnea index and lowest oxygen saturation after maxillomandibular advancement with or without counterclockwise rotation in patients with obstructive sleep apnea: a meta-analysisJ Oral Maxillofac Surg201573471972625443377
- GeorgeCFReduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAPThorax200156750851211413347
- DongYDaiYWeiGChaLLiXEffect of continuous positive airway pressure on blood pressure in hypertensive patients with coronary artery bypass grafting and obstructive sleep apneaInt J Clin Exp Med201471143084315 eCollection201425550946
- OlsenSSmithSOeiTDouglasJHealth belief model predicts adherence to CPAP before experience with CPAPEur Respir J200832371071718417510
- LibmanEBailesSFichtenCSCPAP treatment adherence in women with obstructive sleep apneaSleep Disord20172017276065028352476
- FergusonKAOnoTLoweAAKeenanSPFleethamJAA randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apneaChest19961095126912758625679
- NollerMWGuilleminaultCGouveiaCJMandibular advancement for adult obstructive sleep apnea: a systematic review and meta-analysisJ Craniomaxillofac Surg Epub2017101327939041
- ChoiJHLeeJYChaJKimKHongSNLeeSHPredictive models of objective oropharyngeal OSA surgery outcomes: success rate and AHI reduction ratioPLoS One2017129e018520128938004
- PhillipsCLGrunsteinRRDarendelilerMAHealth outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trialAm J Respir Crit Care Med2013187887988723413266
- AppletonSLVakulinAMcEvoyRDUndiagnosed obstructive sleep apnea is independently associated with reductions in quality of life in middle-aged, but not elderly men of a population cohortSleep Breath20151941309131625896898
- SimpsonLHillmanDRCooperMNHigh prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controlsSleep Breath201317396797323161476
- KapurVStrohlKPRedlineSIberCO’ConnorGNietoJUnderdiagnosis of sleep apnea syndrome in U.S. communitiesSleep Breath200262495412075479
- JaiswalSJOwensRLMalhotraARaising awareness about sleep disordersLung India201734326226828474653
- BixlerEOVgontzasANLinHMCalhounSLVela-BuenoAKalesAExcessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depressionJ Clin Endocrinol Metab20059084510451515941867
- GrayELMcKenzieDKEckertDJObstructive sleep apnea without obesity is common and difficult to treat: evidence for a distinct pathophysiological phenotypeJ Clin Sleep Med2017131818827655455
- YeLPienGWRatcliffeSJThe different clinical faces of obstructive sleep apnoea: a cluster analysisEur Respir J20144461600160725186268
- ArnardottirESBjornsdottirEOlafsdottirKABenediktsdottirBGislasonTObstructive sleep apnoea in the general population: highly prevalent but minimal symptomsEur Respir J201647119420226541533
- EckertDJPhenotypic approaches to obstructive sleep apnoea – new pathways for targeted therapySleep Med Rev201837455928110857
- EckertDJWhiteDPJordanASMalhotraAWellmanADefining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targetsAm J Respir Crit Care Med20131888996100423721582
- CarberryJCAmatouryJEckertDJPersonalized management approach for OSAChest Epub2017616
- LandrySAJoostenSAEckertDJTherapeutic CPAP level predicts upper airway collapsibility in patients with obstructive sleep apneaSleep2017406
- EdwardsBAEckertDJMcSharryDGClinical predictors of the respiratory arousal threshold in patients with obstructive sleep apneaAm J Respir Crit Care Med2014190111293130025321848
- GentaPRSandsSAButlerJPAirflow shape is associated with the pharyngeal structure causing OSAChest2017152353754628651794
- AzarbarzinASandsSATaranto-MontemurroLEstimation of pharyngeal collapsibility during sleep by peak inspiratory airflowSleep2017401
- TerrillPIEdwardsBANematiSQuantifying the ventilatory control contribution to sleep apnoea using polysomnographyEur Respir J201545240841825323235
- HirataRPSchorrFKayamoriFUpper airway collapsibility assessed by negative expiratory pressure while awake is associated with upper airway anatomyJ Clin Sleep Med201612101339134627448414
- OsmanANguyenCCarberryJCAwake upper airway collapsibility is related to airway collapsibility during sleep (Pcrit) in obstructive sleep apneaJ Sleep Res2016252A57
- WangDMarshallNSDuffinJPhenotyping interindividual variability in obstructive sleep apnoea response to temazepam using ventilatory chemoreflexes during wakefulnessJ Sleep Res201120452653221668806
- WangDSomogyiAAYeeBJThe effects of a single mild dose of morphine on chemoreflexes and breathing in obstructive sleep apneaRespir Physiol Neurobiol2013185352653223207373
- EdwardsBAWellmanAOwensRLPSGs: more than just the AHIJ Clin Sleep Med20139652752823772183
- CairnsAPoulosGBoganRWho is getting tested for obstructive sleep apnea using a portable recording system? Test results from 193, 221 patientsJ Clin Sleep Med201410111193119825325579
- DonovanLMPatelSRMaking the most of simplified sleep apnea testingAnn Intern Med2017166536636728114686
- CollopNAAndersonWMBoehleckeBPortable Monitoring Task Force of the American Academy of Sleep MedicineClinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep MedicineJ Clin Sleep Med20073773774718198809
- Muraja-MurroANurkkalaJTiihonenPTotal duration of apnea and hypopnea events and average desaturation show significant variation in patients with a similar apnea-hypopnea indexJ Med Eng Technol201236839339822953737
- AlexRManchikatlaSMachirajuKEffect of apnea duration on apnea induced variations in cerebral blood flow velocity and arterial blood pressureConf Proc IEEE Eng Med Biol Soc2014201427027325569949
- WuHZhanXZhaoMWeiYMean apnea-hypopnea duration (but not apnea-hypopnea index) is associated with worse hypertension in patients with obstructive sleep apneaMedicine (Baltimore)20169548e549327902610
- SankariAPranathiageswaranSMareshSHosniAMBadrMSCharacteristics and consequences of non-apneic respiratory events during sleepSleep2017401
- KochHSchneiderLDFinnLABreathing disturbances without hypoxia are associated with objective sleepiness in sleep apneaSleep20174011
- WeaverEMWoodsonBTStewardDLPolysomnography indexes are discordant with quality of life, symptoms, and reaction times in sleep apnea patientsOtolaryngol Head Neck Surg2005132225526215692538
- AlzoubaidiMMokhlesiBObstructive sleep apnea during rapid eye movement sleep: clinical relevance and therapeutic implicationsCurr Opin Pulm Med201622654555427583667
- AppletonSLVakulinAMartinSAHypertension is associated with undiagnosed OSA during rapid eye movement sleepChest2016150349550527001264
- AuroraRNCrainiceanuCGottliebDJKimJSPunjabiNMObstructive sleep apnea during rapid eye movement sleep and cardiovascular diseaseAm J Respir Crit Care Med Epub2017117
- ChamiHAGottliebDJRedlineSPunjabiNMAssociation between glucose metabolism and sleep-disordered breathing during REM sleepAm J Respir Crit Care Med201519291118112626200994
- NeelapuBCKharbandaOPSardanaHKCraniofacial and upper airway morphology in adult obstructive sleep apnea patients: a systematic review and meta-analysis of cephalometric studiesSleep Med Rev201731799027039222
- EdwardsBAEckertDJJordanASObstructive sleep apnoea pathogenesis from mild to severe: is it all the same?Respirology2017221334227699919
- EckertDJWellmanAPhysiological phenotypesBarbéFPépinJLEuropean Respiratory Monograph: Obstructive Sleep ApnoeaPlymouth, UKEuropean Respiratory Society2015923
- DempseyJAXieAPatzDSWangDPhysiology in medicine: obstructive sleep apnea pathogenesis and treatment–considerations beyond airway anatomyJ Appl Physiol (1985)2014116131224201709
- CarlisleTCarthyERGlasserMUpper airway factors that protect against obstructive sleep apnoea in healthy older malesEur Respir J201444368569324833768
- SafiruddinFKoutsourelakisIde VriesNUpper airway collapse during drug induced sleep endoscopy: head rotation in supine position compared with lateral head and trunk positionEur Arch Otorhinolaryngol2015272248548825142078
- CoxsonHOEastwoodPRWilliamsonJPSinDDPhenotyping airway disease with optical coherence tomographyRespirology2011161344321044229
- ItoETsuikiSMaedaKOkajimaIInoueYOropharyngeal crowding closely relates to aggravation of OSAChest2016150234635226997240
- SchwabRJPasirsteinMPiersonRIdentification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imagingAm J Respir Crit Care Med2003168552253012746251
- SegalYMalhotraAPillarGUpper airway length may be associated with the severity of obstructive sleep apnea syndromeSleep Breath200812431131618516636
- MorrisonDLLaunoisSHIsonoSFeroahTRWhitelawWARemmersJEPharyngeal narrowing and closing pressures in patients with obstructive sleep apneaAm Rev Respir Dis199314836066118368630
- YoungTPeppardPETaheriSExcess weight and sleep-disordered breathingJ Appl Physiol1985200599415921599
- LaiCCFriedmanMLinHCClinical predictors of effective continuous positive airway pressure in patients with obstructive sleep apnea/hypopnea syndromeLaryngoscope201512581983198725582398
- PintoJAGodoyLBMarquisVWSonegoTBLeal CdeFArticoMSAnthropometric data as predictors of Obstructive Sleep Apnea SeverityBraz J Otorhinolaryngol201177451652121860980
- CaronCJJMPluijmersBIMaasBDPJObstructive sleep apnoea in craniofacial microsomia: analysis of 755 patientsInt J Oral Maxillofac Surg201746101330133728641899
- SchorrFKayamoriFHirataRPDifferent craniofacial characteristics predict upper airway collapsibility in Japanese-Brazilian and white menChest2016149373774626291487
- SforzaEBaconWWeissTThibaultAPetiauCKriegerJUpper airway collapsibility and cephalometric variables in patients with obstructive sleep apneaAm J Respir Crit Care Med20001612 Pt 134735210673170
- VermaMSeto-PoonMWheatleyJRAmisTCKirknessJPInfluence of breathing route on upper airway lining liquid surface tension in humansJ Physiol2006574Pt 385986616690717
- WeissTMAtanasovSCalhounKHThe association of tongue scalloping with obstructive sleep apnea and related sleep pathologyOtolaryngol Head Neck Surg2005133696697116360522
- KimAMKeenanBTJacksonNTongue fat and its relationship to obstructive sleep apneaSleep201437101639164825197815
- GleadhillICSchwartzARSchubertNWiseRAPermuttSSmithPLUpper airway collapsibility in snorers and in patients with obstructive hypopnea and apneaAm Rev Respir Dis19911436130013032048817
- EastwoodPRSzollosiIPlattPRHillmanDRCollapsibility of the upper airway during anesthesia with isofluraneAnesthesiology200297478679312357141
- SchwartzARO’DonnellCPBaronJThe hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activityAm J Respir Crit Care Med19981574 Pt 1105110579563718
- CarberryJCJordanASWhiteDPWellmanAEckertDJUpper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependentSleep201639351152126612386
- KirknessJPPetersonLASquierSBPerformance characteristics of upper airway critical collapsing pressure measurements during sleepSleep201134445946721461324
- GentaPRSchorrFEckertDJUpper airway collapsibility is associated with obesity and hyoid positionSleep201437101673167825197805
- KirknessJPSchwartzARSchneiderHContribution of male sex, age, and obesity to mechanical instability of the upper airway during sleepJ Appl Physiol (1985)200810461618162418420722
- BacharGFeinmesserRShpitzerTYanivENagerisBEidelmanLLaryngeal and hypopharyngeal obstruction in sleep disordered breathing patients, evaluated by sleep endoscopyEur Arch Otorhinolaryngol2008265111397140218327599
- HornerRLHughesSWMalhotraAState-dependent and reflex drives to the upper airway: basic physiology with clinical implicationsJ Appl Physiol (1985)2014116332533623970535
- KubinLNeural control of the upper airway: respiratory and state-dependent mechanismsCompr Physiol2016641801185027783860
- SaboiskyJPButlerJEFogelRBTonic and phasic respiratory drives to human genioglossus motoneurons during breathingJ Neurophysiol20069542213222116306175
- WilkinsonVMalhotraANicholasCLDischarge patterns of human genioglossus motor units during sleep onsetSleep200831452553318457240
- NicholasCLJordanASHeckelLDischarge patterns of human tensor palatini motor units during sleep onsetSleep201235569970722547896
- EckertDJSaboiskyJPJordanASWhiteDPMalhotraAA secondary reflex suppression phase is present in genioglossus but not tensor palatini in response to negative upper airway pressureJ Appl Physiol (1985)201010861619162420378702
- EckertDJMalhotraALoYLWhiteDPJordanASThe influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleepChest2009135495796419118266
- EckertDJMcEvoyRDGeorgeKEThomsonKJCatchesidePGGenioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy malesJ Physiol2007581Pt 31193120517395627
- LoewenAHOstrowskiMLaprairieJMaturinoFHanlyPJYounesMResponse of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patientsSleep20113481061107321804668
- JordanASWellmanAHeinzerRCMechanisms used to restore ventilation after partial upper airway collapse during sleep in humansThorax2007621086186717412778
- SandsSAEckertDJJordanASEnhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apneaAm J Respir Crit Care Med2014190893093725191791
- DotanYPillarGTovNDissociation of electromyogram and mechanical response in sleep apnoea during propofol anaesthesiaEur Respir J2013411748422556023
- ChengSButlerJEGandeviaSCBilstonLEMovement of the tongue during normal breathing in awake healthy humansJ Physiol2008586174283429418635645
- BrownECChengSMcKenzieDKButlerJEGandeviaSCBilstonLERespiratory movement of upper airway tissue in obstructive sleep apneaSleep20133671069107623814344
- ChengSBrownECHattAButlerJEGandeviaSCBilstonLEHealthy humans with a narrow upper airway maintain patency during quiet breathing by dilating the airway during inspirationJ Physiol2014592214763477425217376
- JordanASWhiteDPOwensRLThe effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapseJ Appl Physiol (1985)2010109246947520507968
- DotanYPillarGSchwartzAROlivenAAsynchrony of lingual muscle recruitment during sleep in obstructive sleep apneaJ Appl Physiol (1985)2015118121516152425814639
- StrolloPJJrSooseRJMaurerJTSTAR Trial GroupUpper-airway stimulation for obstructive sleep apneaN Eng J Med20143702139149
- BisogniVPengoMFDe VitoAElectrical stimulation for the treatment of obstructive sleep apnoea: a review of the evidenceExpert Rev Respir Med201711971172028730908
- HidaWOkabeSMikiHEffects of submental stimulation for several consecutive nights in patients with obstructive sleep apnoeaThorax19944954464528016764
- HofauerBPhilipPWirthMKnopfAHeiserCEffects of upper-airway stimulation on sleep architecture in patients with obstructive sleep apneaSleep Breath201721490190828567688
- SchwartzARBarnesMHillmanDAcute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apneaAm J Respir Crit Care Med2012185442042622135343
- SchwartzARSmithPLOlivenAElectrical stimulation of the hypoglossal nerve: a potential therapyJ Appl Physiol198520141163337344
- EastwoodPRBarnesMWalshJHTreating obstructive sleep apnea with hypoglossal nerve stimulationSleep201134111479148622043118
- SteffenASommerJUHofauerBMaurerJTHasselbacherKHeiserCOutcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market studyLaryngoscope Epub201753128688189
- GillespieMBSooseRJWoodsonBTSTAR Trial InvestigatorsUpper airway stimulation for obstructive sleep apnea: patient-reported outcomes after 48 months of follow-upOtolaryngol Head Neck Surg2017156476577128194999
- WoodsonBTSooseRJGillespieMBSTAR Trial InvestigatorsThree-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR TrialOtolaryngol Head Neck Surg2016154118118826577774
- WhiteDPPharmacologic approaches to the treatment of obstructive sleep apneaSleep Med Clin201611220321227236057
- HornerRLGraceKPWellmanAA resource of potential drug targets and strategic decision-making for obstructive sleep apnoea pharmacotherapyRespirology201722586187328544082
- Taranto-MontemurroLEdwardsBASandsSADesipramine increases genioglossus activity and reduces upper airway collapsibility during Non-REM sleep in healthy subjectsAm J Respir Crit Care Med2016194787888526967681
- Taranto-MontemurroLSandsSAEdwardsBADesipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensationEur Respir J20164851340135027799387
- MarshallNSYeeBJDesaiAVTwo randomized placebo-controlled trials to evaluate the efficacy and tolerability of mirtazapine for the treatment of obstructive sleep apneaSleep200831682483118548827
- KraicziHHednerJDahlöfPEjnellHCarlsonJEffect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apneaSleep199922161679989366
- Taranto-MontemurroLSandsSAAzarbarzinAEffect of 4-aminopyridine on genioglossus muscle activity during sleep in healthy adultsAnn Am Thorac Soc20171471177118328387543
- CarberryJCFisherLPGrunsteinRRRole of common hypnotics on the phenotypic causes of OSA: paradoxical effects of zolpidemEur Respir J2017506pii1701344
- HortonGAFraigneJJTorontaliZAActivation of the hypoglossal to tongue musculature motor pathway by remote controlSci Rep2017745860
- Fleury CuradoTFishbeinKPhoHChemogenetic stimulation of the hypoglossal neurons improves upper airway patencySci Rep2017744392
- IetoVKayamoriFMontesMIEffects of oropharyngeal exercises on snoring: a randomized trialChest2015148368369125950418
- GuimarãesKCDragerLFGentaPRMarcondesBFLorenzi-FilhoGEffects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndromeAm J Respir Crit Care Med20091791096296619234106
- PuhanMASuarezALo CascioCZahnAHeitzMBraendliODidgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trialBMJ2006332753626627016377643
- CamachoMCertalVAbdullatifJMyofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysisSleep201538566967525348130
- EckertDJYounesMKArousal from sleep: implications for obstructive sleep apnea pathogenesis and treatmentJ Appl Physiol (1985)2014116330231323990246
- PhillipsonEASullivanCEArousal: the forgotten response to respiratory stimuliAm Rev Respir Dis19781185807809736352
- RemmersJEdeGrootWJSauerlandEKAnchAMPathogenesis of upper airway occlusion during sleepJ Appl Physiol Respir Environ Exerc Physiol1978446931938670014
- YounesMRole of arousals in the pathogenesis of obstructive sleep apneaAm J Respir Crit Care Med2004169562363314684560
- ReesKSpenceDPEarisJECalverleyPMArousal responses from apneic events during non-rapid-eye-movement sleepAm J Respir Crit Care Med19951523101610217663777
- YounesMOstrowskiMAtkarRLaprairieJSiemensAHanlyPMechanisms of breathing instability in patients with obstructive sleep apneaJ Appl Physiol (1985)200710361929194117823298
- RatnavadivelRChauNStadlerDYeoAMcEvoyRDCatchesidePGMarked reduction in obstructive sleep apnea severity in slow wave sleepJ Clin Sleep Med20095651952420465017
- WhiteDPYounesMKObstructive sleep apneaCompr Physiol2012242541259423720258
- YounesMRole of respiratory control mechanisms in the pathogenesis of obstructive sleep disordersJ Appl Physiol (1985)200810551389140518787092
- YounesMOstrowskiMThompsonWLeslieCShewchukWChemical control stability in patients with obstructive sleep apneaAm J Respir Crit Care Med200116351181119011316657
- PuddyAGiesbrechtGSaniiRYounesMMechanism of detection of resistive loads in conscious humansJ Appl Physiol (1985)1992726226722701629082
- YounesMJungDPuddyAGiesbrechtGSaniiRRole of the chest wall in detection of added elastic loadsJ Appl Physiol (1985)1990685224122452361929
- BanzettRBLansingRWBrownR‘Air hunger’ from increased CO2 persists after complete neuromuscular block in humansRespir Physiol19908111172120757
- ChamberlinLBrain circuitry mediating arousal from obstructive sleep apneaCurr Opin Neurobiol201323577477923810448
- BerryRBGleesonKRespiratory arousal from sleep: mechanisms and significanceSleep19972086546759351134
- CarterSGBergerMSCarberryJCZopiclone increases the arousal threshold without impairing genioglossus activity in obstructive sleep apneaSleep201639475776626715227
- EckertDJOwensRLKehlmannGBEszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal thresholdClin Sci (Lond)20111201250551421269278
- EdwardsBASandsSAOwensRLThe combination of supplemental oxygen and a hypnotic markedly improves obstructive sleep apnea in patients with a mild to moderate upper airway collapsibilitySleep201639111973198327634790
- GleesonKZwillichCWWhiteDPThe influence of increasing ventilatory effort on arousal from sleepAm Rev Respir Dis199014222953002382892
- SforzaEKriegerJPetiauCArousal threshold to respiratory stimuli in OSA patients: evidence for a sleep-dependent temporal rhythmSleep199922169759989367
- BerryRBAsyaliMAMcNellisMIKhooMCWithin-night variation in respiratory effort preceding apnea termination and EEG delta power in sleep apneaJ Appl Physiol (1985)1998854143414419760338
- JordanASO’DonoghueFJCoriJMTrinderJPhysiology of arousal in obstructive sleep apnea and potential impacts for sedative treatmentAm J Respir Crit Care Med2017196781482128399379
- BerryRBKouchiKGDerDEDickelMJLightRWSleep apnea impairs the arousal response to airway occlusionChest19961096149014968769499
- Haba-RubioJSforzaEWeissTSchröderCKriegerJEffect of CPAP treatment on inspiratory arousal threshold during NREM sleep in OSASSleep Breath200591121915785916
- EckertDJMalhotraAWellmanAWhiteDPTrazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal thresholdSleep201437481181924899767
- SmalesETEdwardsBADeyoungPNTrazodone effects on obstructive sleep apnea and non-REM arousal thresholdAnn Am Thorac Soc201512575876425719754
- CarberryJCFisherLPCarterSGRespiratory sensation during wakefulness is related to the respiratory arousal threshold during sleep in obstructive sleep apnoeaSleep Biol Rhythms20151358
- BenetóAGomez-SiuranaERubio-SanchezPComorbidity between sleep apnea and insomniaSleep Med Rev200913428729319246219
- KrakowBRomeroEUlibarriVAKiktaSProspective assessment of nocturnal awakenings in a case series of treatment-seeking chronic insomnia patients: a pilot study of subjective and objective causesSleep201235121685169223204611
- KrakowBMelendrezDFerreiraEPrevalence of insomnia symptoms in patients with sleep-disordered breathingChest200112061923192911742923
- MateikaJHPanzaGAlexREl-ChamiMThe impact of intermittent or sustained carbon dioxide on intermittent hypoxia initiated respiratory plasticity. What is the effect of these combined stimuli on apnea severity?Respir Physiol Neurobiol Epub20171031
- LoewenAOstrowskiMLaprairieJDeterminants of ventilatory instability in obstructive sleep apnea: inherent or acquired?Sleep200932101355136519848364
- WellmanAEdwardsBASandsSAA simplified method for determining phenotypic traits in patients with obstructive sleep apneaJ Appl Physiol198520131147911922
- WellmanAEckertDJJordanASA method for measuring and modeling the physiological traits causing obstructive sleep apneaJ Appl Physiol19852011110616271637
- WellmanAMalhotraAJordanASStevensonKEGautamSWhiteDPEffect of oxygen in obstructive sleep apnea: role of loop gainRespir Physiol Neurobiol2008162214415118577470
- EdwardsBASandsSAEckertDJAcetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoeaJ Physiol201259051199121122219335
- XieATeodorescuMPegelowDFEffects of stabilizing or increasing respiratory motor outputs on obstructive sleep apneaJ Appl Physiol1985201311512233
- El-ChamiMShaheenDIversBTime of day affects chemoreflex sensitivity and the carbon dioxide reserve during NREM sleep in participants with sleep apneaJ Appl Physiol (1985)2014117101149115625213638
- El-ChamiMShaheenDIversBTime of day affects the frequency and duration of breathing events and the critical closing pressure during NREM sleep in participants with sleep apneaJ Appl Physiol (1985)2015119661762626183479
- WainsSAEl-ChamiMLinHSMateikaJHImpact of arousal threshold and respiratory effort on the duration of breathing events across sleep stage and time of nightRespir Physiol Neurobiol2017237354128040523
- DjonlagicISaboiskyJCarusonaAStickgoldRMalhotraAIncreased sleep fragmentation leads to impaired off-line consolidation of motor memories in humansPLoS One201273e3410622470524
- AmatouryJAzarbarzinAYounesMJordanASWellmanAEckertDJArousal intensity is a distinct pathophysiological trait in obstructive sleep apneaSleep201639122091210027784404
- MateikaJHEl-ChamiMShaheenDIversBIntermittent hypoxia: a low-risk research tool with therapeutic value in humansJ Appl Physiol (1985)2015118552053225549763
- MateikaJHKomnenovDIntermittent hypoxia initiated plasticity in humans: a multipronged therapeutic approach to treat sleep apnea and overlapping comorbiditiesExp Neurol2017287Pt 211312927170208
