Abstract
Hyperarousal is a key component in all modern etiological models of insomnia disorder. Overall patterns in the literature suggest that over-active neurobiological and psychological systems contribute to difficulty sleeping. Even so, mixed results regarding the specific mechanisms linking hyperarousal to sleep disturbance limit current etiological conceptualizations. Similar basal arousal profiles between individuals with high vs low risk for insomnia in the absence of stress exposure suggest that dysregulated stress “response” rather than general hyperarousal may be a more pertinent marker of risk. In this report, we discuss evidence for hyperarousal in insomnia and explore the role of sleep reactivity. A trait characteristic, sleep reactivity is the degree to which stress disrupts sleep, manifesting as difficulty falling and staying asleep. Premorbid sleep reactivity has been shown to identify individuals at risk for future insomnia disorder, such as highly reactive sleepers (whose sleep systems are sensitive to stress) who are at elevated disease risk. Research points to genetics, family history of insomnia, gender, and environmental stress as factors that influence sleep reactivity. Importantly, stress-related cognitive–emotional reactivity (e.g., rumination, worry) may exploit the vulnerability of a highly reactive sleep system. We propose that sleep reactivity and cognitive–emotional reactivity may share a bidirectional relationship, conferring an insalubrious environment for sleep in response to stress. Future research on sleep reactivity is needed to identify its neurobiology, characterize its relationship with cognitive–emotional reactivity, and explore the potential clinical utility of sleep reactivity in treatment planning.
Introduction
Insomnia is the most common sleep disorder in the US with prevalence rates estimated between 9% and 20% among adults.Citation1,Citation2 Despite advances in pharmacological and behavioral interventions,Citation3–Citation8 many treated insomniacs relapseCitation9 and those with severe forms of insomnia are resistant to treatment.Citation10 Understanding the course of insomnia is difficult, in part, because its complex psychoneurobiology is not yet fully understood. However, recent advances using hyperarousal and stress dysregulation models of insomnia have offered critical insights into insomnia disease processes.
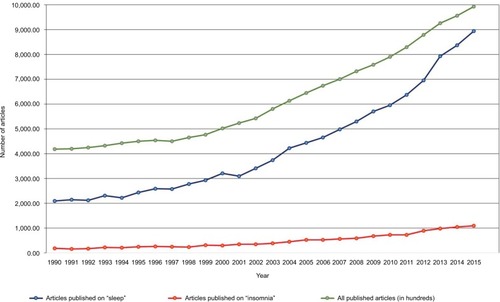
The concept of hyperarousal as an important etiological factor in insomnia has been long held, with serious scientific inquiry into this conceptualization, as well as the issue of cause and effect, undertaken more than 50 years ago. Several important factors have led to an increasing interest in this broadly defined area of sleep science. One factor is the near exponential rise of sleep science over the last 2 decades (). Another contributing factor is the now wide availability and low cost of tools to study aspects of hyperarousal including its physiological, cognitive, and neuronal correlates.
Figure 1 All published articles including keywords “sleep” and “insomnia” from 1990 to 2015.
A critical challenge for our current understanding of hyperarousal in insomnia is to synthesize these often-disparate findings both across and within these broad domains. Similar to terms such as stress, reactivity, and other broad constructs that cross the boundaries of psychology, physiology, and medicine, the term “hyperarousal” is difficult, if not impossible, to define without sufficient context. Indeed, many conceptualizations exist that vary not only between but also within disciplines. Thus, there has been an expansion in the scientific study of all aspects of sleep-related hyperarousal becoming practically as broad as the concept itself. While this expansion both across and within domains has led to numerous important findings, it has also produced an ever-widening gap between researchers who are essentially studying the same construct.
There have been many excellent and encompassing reviews of hyperarousal in insomnia since 2000 (see studies by Riemann,Citation11 Bonnet and Arand,Citation12 Roth et al,Citation13 Harvey et al,Citation14 Kay and Buysse,Citation15 Drummond et al,Citation16 and Vgontzas et al, Citation17 among others); therefore, we will attempt to not retread here. Some have focused on physiological components, some have provided new insights regarding neuroimaging and cognitive findings, and others have explored the role of hyperarousal in insomnia phenotyping. In the current review, we will begin by briefly considering the literature on hyperarousal in insomnia, and then shift focus to explore a recent and quickly emerging area of insomnia research that is proving to be an integral component of the hyperarousal model of insomnia: sleep reactivity.
Hyperarousal and stress dysregulation in insomnia
The many studies of physiological measures associated with insomnia disorder point to general signs of hyperarousal in insomnia, but with important caveats and inconsistencies. For example, although stressful life events have long been shown to be important triggers of insomnia,Citation18–Citation20 traditional physiological markers of stress and arousal such as hypothalamic–pituitary–adrenal (HPA) axis (e.g., cortisol, adre-nocorticotropic hormone) and sympathetic nervous system (SNS) activation have produced varied findings over the past 3 decades of insomnia research.Citation21–Citation28 Neuroimaging studies of insomnia also show disparate findings with respect to any concept of “general hyperarousal” with several studies suggesting higher activation in insomnia,Citation29–Citation31 although some evidence suggests hypoarousal.Citation32 A recent review of this literature emphasized dysregulation in separate components of wake drive, sleep drive, and conscious awareness that likely contribute to these seeming inconsistencies.Citation15 The idea that wake drive may be dysregulated independent of sleep drive (and vice versa) and that independent alterations within these systems may differentially impact nighttime insomnia symptoms (eg, sleep complaints) highlights a complexity that has continued to challenge the scientific understanding of hyperarousal in insomnia.
As in most areas of science, findings in insomnia have produced a more complex array of questions. What is the underlying neurophysiology that accounts for insomnia symptoms? If it is increased neurophysiological brain activation (ie, central nervous system hyperarousal), what areas of brain are critical for the perception of poor sleep? And do those brain areasCitation29 or specific pathwaysCitation33 also account for polysomnography (PSG)-defined insomnia-related sleep disturbances (ie, objective insomnia)?
One approach to clarifying some of the complexities in understanding the role of hyperarousal in insomnia has been to study the neurophysiological and cognitive processes in individuals at risk for insomnia prior to the development of chronic sleep symptoms (ie, before insomnia develops). This approach has been used to disentangle potential causes from consequences of insomnia and has yielded several important findings along with additional questions over the past decade. For example, recent findings have shown undamaged SNS functioning both basally and in response to standardized cognitive and physiological stress challenges along with concomitant dysregulation in HPA axis reactivity in insomniacs and individuals at elevated risk for insomnia.Citation26,Citation27 Interestingly, despite a dysregulated HPA axis response in individuals at risk for insomnia, no differences in the cognitive response to the laboratory stress were present, challenging the notion that the critical vulnerability to insomnia is a purely a cognitive phenomenon.Citation27 Indeed, previous studies show that normal sleeping individuals at risk for insomnia also have a differential sleep alteration following predominantly physical sleep challenges. Do these findings reflect hyperarousal as a potential etiological risk factor for the development of symptoms in insomnia disorder? The absence of differences in basal measures of hyperarousal (cortisol, alpha amylase, heart rate, blood pressure) prior to stressful challenges suggests that dysregulated stress “response” rather than general hyperarousal may be a critical marker of risk.
Stress response is a broad concept that encompasses cognitive, emotional, and neurobiological reactions. Stress exposure is equally myriad, varying by source (eg, mental stress, physiologic pain, circadian challenge, substance consumption), potency (mild to severe), and duration or schedule (acute, chronic, intermittent, and so on). Not only do stressors vary greatly, but also individuals vary greatly in their response to stressors. That is, different individuals react differently even to uniform stressors, thus emphasizing the complex machinations of stress regulation.Citation34–Citation36 And it is precisely these differences in stress dysregulation that contribute to interindividual differences in risk for insomnia.Citation35–Citation37
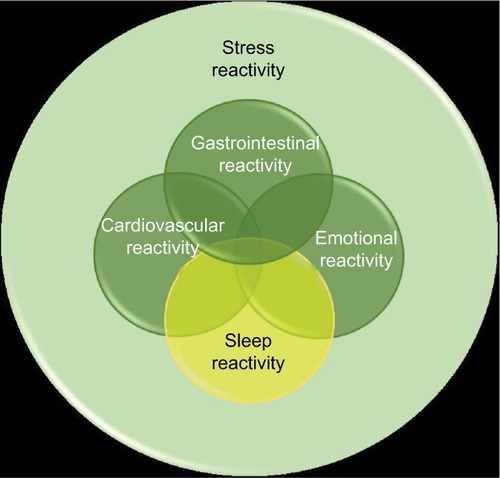
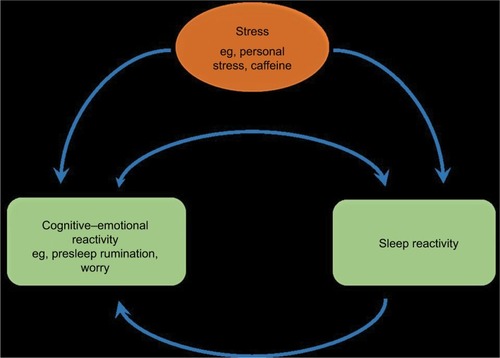
Evidence suggests that insomniacs exhibit exaggerated and maladaptive neurobiological and cognitive–emotional reactivity to stress.Citation11,Citation19,Citation38–Citation41 However, it is unclear whether this stress dysregulation has a sleep-specific component (). And while growing evidence suggests that stress dysregulation typically preexists and even contributes to insomnia development, it had been unclear until recently whether this stress dysregulation produces a uniquely sleep-specific vulnerability or rather a broad cognitive–emotional response that can impact a myriad of stress-linked physiological systems, such as sleep regulation, HPA axis, and autonomic nervous system (ANS). In recent years, research has identified that a sleep-specific component of stress reactivity exists, and the degree to which stress reactivity manifests in the sleep system has been referred to as situational insomniaCitation42 and, now more commonly, “sleep reactivity”.Citation38
Sleep reactivity
Sleep reactivity is the degree to which individuals exhibit acute sleep disturbance in response to stress exposure. Important to note here is that other terms used to describe sleep reactivity include “sleep system sensitivity”, “stress-reactivity of the sleep system”, and “vulnerability to stress-related insomnia” (in the current review, we will largely use the term “sleep reactivity” for the sake of consistency). Sleep reactivity is a ubiquitous phenomenon as all individuals experience some degree of difficulty sleeping when faced with a sufficiently potent sleep challenge. Even minor challenges, including slight changes in sleep timing and ill-timed low-dose stimulants, produce sleep disturbance in some individuals.Citation42 This reactivity exhibits trait-like characteristics such that individuals’ sleep responses to stress are consistent over time and across a variety of stressful stimuli.Citation38,Citation42,Citation43
The first efforts to distinguish between individuals with high vs low sleep reactivity relied on PSG characteristics after stress exposure (caffeine use, circadian shift, PSG adaptation night).Citation38,Citation42 Individuals who had greater nocturnal wakefulness and lower sleep efficiency and yet elevated alertness on the Multiple Sleep Latency Test per PSG following laboratory stress were identified as highly reactive sleepers, whereas those whose sleep was largely unaffected by laboratory stress were characterized as having low sleep reactivity. Laboratory challenges, coupled with PSG, yield high validity for measuring sleep reactivity. Further, this approach benefits from being affectively neutral and is therefore unlikely to be contaminated by cognitive and emotional processes.
A validated assay for a priori measurement of sleep reactivity in large epidemiological samples is the Ford Insomnia Response to Stress Test (FIRST), a self-report instrument that was originally validated in a large adult sample in the USA (see Drake et al 2004Citation38 for the full instrument). The FIRST has also been validated for use in early pregnancyCitation44 and translated into Spanish,Citation44 Japanese,Citation45 European Portuguese,Citation46 French,Citation47 German,Citation48 and Italian languages.Citation49 Many studies comparing high vs low sleep reactivity in individuals have relied on using median splits to distinguish the two groups with good results. However, as medians are highly sample dependent, this has resulted in a lack of consistency regarding cutoffs across studies. To date, the sole effort to establish empirical cutoffs was based on an epidemiological sample of 2,892 good lifetime sleepers without a history of depression or insomnia,Citation50 revealing cutoffs of FIRST ≥16 reflecting moderate risk for future insomnia, and FIRST ≥18 reflecting high risk for future insomnia.
A burgeoning literature supports sleep reactivity as a moderating influence of stress on the sleep system,Citation38,Citation42,Citation51,Citation52 which has drawn much attention in insomnia etiology. Despite being a normal variant, sleep reactivity offers critical insight into insomnia progression as excessive sleep reactivity yields substantial pathogenicity for insomnia development. Individuals with high sleep reactivity are vulnerable to having difficulty falling and staying asleep during and even following the removal of stress, whereas those with low sleep reactivity experience mild sleep disruptions but often return to normal without serious or persistent complications.
Sleep reactivity predicts future insomnia
Two recent prospective epidemiological studies have demonstrated high premorbid sleep reactivity as a trait-like diathesis of insomnia. In 2014, Jarrin’s team assessed 1,449 lifetime good sleepers and showed that good sleepers with high sleep reactivity were at elevated risk for insomnia symptoms and chronic insomnia disorder across the following 2 years than those with low sleep reactivity. Further, evidence suggested that sleep reactivity predicted future insomnia independently of general trait hyperarousal, thus supporting sleep-specific stress reactivity as a unique factor that may be related to hyperarousal but is also a unique and independent insomnia risk factor.
In the “Evolution of Pathways to Insomnia Cohort (EPIC)” study, our team demonstrated that premorbid sleep reactivity predicted insomnia disorder incidence in a sample of 2,316 individuals without a history of Diagnostic and Statistical Manual of Mental Disorders, 4th edition Citation53 insomnia or major depression.Citation54 In addition, this study offered the first evidence in support of premorbid sleep reactivity moderating the effects of major life stress on insomnia risk; the crux of earlier proposals that high sleep reactivity as an insomnia vulnerability is most pathogenic via its inability to maintain normal sleep patterns during stress. Notably, data from this sample also showed that insomniacs with high premorbid sleep reactivity are 2–3 times more likely to have “sleep onset insomnia” complaints than nonreactive sleepers at time of insomnia onset,Citation50 with highly reactive insomniacs estimating their habitual sleep latency to be nearly twice as long as nonreactive sleepers at disease onset (65 minutes vs 37 minutes). However, symptom profiles did not differ between premorbidly high vs low sleep reactive insomniacs a year “after” onset of the disorder, indicating that phenotypic insomnia differences between high vs low sleep reactivity are most evident early in the disease course.
Although sleep reactivity has trait-like qualities with consistency across stressorsCitation38,Citation42 and time, Citation38,Citation43 researchers have recently proposed that exposure to stress and even to insomnia itself causes changes in the regulation of stress and sleep.Citation11,Citation55,Citation56 As a result, the conditions under which the initial episode of sleep disturbance develops may differ from the conditions that perpetuate its course or give rise to recurrent or chronic insomnia. Evidence from 262 subjects from the EPIC cohort showed that sleep reactivity increased substantially in insomniacs with low baseline sleep reactivity such that 68% of these insomniacs were reclassified as having high sleep reactivity; this phenomenon was dubbed “sleep system sensitization”.Citation57 In addition, exposure to major life stress augmented this sensitization process. And even after insomnia remission, sleep reactivity did not return to preinsomnia baseline levels even after disease remission, indicating that stress exposure and insomnia development have a scarring effect on the sleep system.
The basis of sleep reactivity
Genetics and heritability
Consistent with evidence for a genetic basis of sleep regulation and insomniaCitation55,Citation58–Citation63 and the prognosticity of familial insomnia,Citation57,Citation64–Citation66 early evidence suggests a substantial polygenic influence on sleep reactivity. Twin and sibling studies estimate that 29%–37% of sleep reactivity is heritable,Citation67,Citation68 but that environmental influences play a larger role in sleep reactivity in women than in men.Citation69 Along these lines, results showed that women are substantially more sleep reactive than men, despite similar genetic architecture. Taken together, these findings suggest that greater sensitivity in the sleep system may play a critical role in increased prevalence of insomnia in women.
Stress dysregulation
An imbalance between the SNS and parasympathetic nervous system (PNS) has been implicated in cases of excessive sleep reactivity such that the SNS is hyper-active and the PNS is under-active,Citation12 particularly when under stress,Citation42 which is a similar pattern to what is seen between chronic insomniacs (imbalance) and good sleepers (balance).Citation70 Building on this literature, a daily diary study showed that when individuals worried about stress and exhibited blunted PNS activity, they experienced greater stress-induced sleep disturbance, ie, higher sleep reactivity.Citation71 This finding provides laboratory support for synergistic effects of cognitive–emotional hyperarousal and autonomic imbalance on the sleep system. However, some evidence suggests that ANS profiles may not differ between those with high vs low sleep reactivity,Citation26 thus introducing inconsistencies into the literature that require further exploration.
In addition, HPA axis dysregulation has been posited to underlie highly reactive sleep systems, with data from the insomnia literature indicating that increased nocturnal HPA activity and greater HPA reactivity to stress may constitute trait-like markers for insomnia vulnerability.Citation72 However, recent studies specifically comparing HPA responses to stress between high vs low reactive sleepers offer mixed support for exaggerated and blunted cortisol response to the same laboratory stressors.Citation27,Citation52 So although HPA dysregulation may contribute to stress-induced sleep disturbance,Citation26 more research is needed to clearly understand the role of the HPA axis in sleep system reactivity.
Cortical hyperarousal
Fast electroencephalographic (EEG)-defined cortical activity during the presleep period is associated with delayed sleep onset and represents a marker of insomnia.Citation73,Citation74 However, the field has yet to establish cortical activity profiles associated with high sleep reactivity. Laboratory research is needed to compare cortical arousal between good sleepers with high vs low sleep reactivity. Investigating diurnal patterns, presleep profiles, and stress-induced changes would offer critical insights into potentially dysregulated cortical networks moderating the effects of stress on the sleep system.
Related cognitive and emotional factors
Explorations of the relationship between sleep reactivity and cognitive–emotional reactivity depict two independent yet related responses to stress (see for theoretical depiction). A large number of cognitive and emotional factors correlate with sleep reactivity, including presleep cognitive and somatic arousal, rumination, worry, perceived stress, general arousability, neuroticism, and emotion-oriented coping.Citation20,Citation71,Citation75 And indeed, cognitive–emotional arousal moderates the risk for insomnia and sleep disturbance associated with sleep reactivity and stress dysregulation,Citation20,Citation71 thus highlighting their toxic interactions. This relationship is likely bidirectional such that when stress activates cognitive–emotional activity (eg, presleep rumination), the sensitivity of a highly reactive sleep system will be exploited, thus resulting in nighttime wakefulness and poor-quality sleep (). In the opposite direction, when stress activates a highly reactive sleep system (note: the stressor can be affectively neutralCitation38,Citation42 or otherwise), the resultant nocturnal wakefulness creates an unstructured, typically socially isolated time in bed during which the mind is free to wander, thus representing a breeding ground for ruminative thoughts. It is possible that the interbreeding between sleep reactivity and cognitive–emotional reactivity contributes significantly to the high comorbidity between insomnia and depression.Citation76,Citation77
Future directions
Significant strides in insomnia research over many decades have culminated in the hyperarousal hypothesis, which has been a robust framework for the conceptualization of insomnia etiology, and targets for intervention and prevention. Sleep reactivity is a relatively young area of research but has already proved to be an integral component of the hyperarousal model. Furthermore, consideration of hyperactivity vs hyperreactivity lends crucial insights into disparate findings in the extant hyperarousal and stress dysregulation in insomnia literature. Thus, in the absence of stress, many individuals at risk for insomnia may not exhibit hyperarousal or abnormal stress regulation, which must be countenanced in insomnia etiology research.
Given the pathogenicity of elevated sleep reactivity, future research is needed to better elucidate the fundamental basis of and even peripheral factors influencing highly sensitive sleep systems. Improved characterization of its neurobiology and relation to cognitive and emotional processes will unveil novel pathways in the disease progression of insomnia, paving way for the development of preventive interventions. Recent evidence has already suggested that good sleepers with high sleep reactivity are especially prone to insomnia characterized by predominantly difficulty falling asleep at the time of disease onset, indicating that premorbid sleep reactivity segregates with insomnia phenotypes. Although symptom profiles between insomniacs with high vs low premorbid sleep reactivity do not differ a year after disease onset (indeed, changes in insomnia phenotypes over time is not uncommonCitation78), that highly reactive sleepers primarily struggle to initiate sleep early in the course of disease offers important insights into the unique etiological factors contributing to sleep onset insomnia. Expanding on this literature, insomnia disorder with objective short sleep duration has been identified as the most treatment-resistant and devastating phenotype of insomnia in terms of morbidity and early mortality. Short sleep insomnia is characterized by severe biological hyperarousal and stress dysregulation at its foundation. Research is needed to determine whether sleep reactivity can identify those at future risk for the short sleep insomnia phenotype, as reliable premorbid predictors of this form of insomnia have not yet been established.
Last, the clinical utility of sleep reactivity as measured by the FIRST has yet to be explored. Measuring sleep reactivity in patients presenting with insomnia complaints may have value in directing to different levels of intervention. Stepped-care approaches have been recommended for cognitive-behavioral therapy for insomnia (CBTI),Citation79 and early evidence supports the scalability of CBTI.Citation80 Insomniacs with low sleep reactivity may have less severe forms of insomnia trajectories and require less intensive treatment modalities such as self-guided digital/internet-based delivery of CBTI.Citation81–Citation86 Insomniacs with severely high sleep reactivity scores, however, may require more intensive and personalized cognitive-behavioral and pharmacological treatment provided by expertly trained sleep psychologists and physicians. Future research is needed to evaluate whether sleep reactivity is associated with response to pharmacologic or cognitive-behavioral interventions for insomnia. Notably, the clinical utility of sleep reactivity may extend beyond pretreatment evaluation and may be useful in identifying remitters who may be at elevated risk for future relapse. Even after insomnia remission and the restoration of good sleep, sleep reactivity typically remains in the elevated range, suggestive of high risk for future insomnia even when asymptomatic.Citation57 Therefore, even after treatment success, remitted insomniacs who continue to exhibit elevated levels of sleep reactivity may reduce risk for relapse by adopting ongoing maintenance treatment.
Disclosure
Dr CL Drake has received research support from Merck & Co., Eisai Co., Aladdin Dreamer, Jazz, Actelion, and Teva and has served on speakers’ bureau for Merck & Co. Dr DA Kalmbach has received research support from Merck & Co. The authors report no other conflicts of interest in this work.
References
- RothTCoulouvratCHajakGPrevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition Criteria: results from the America Insomnia SurveyBiol Psychiatry201169659260021195389
- MorinCMLeBlancMDaleyMGregoireJMeretteCEpidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviorsSleep Med20067212313016459140
- DrakeCLRoehrsTRothTInsomnia causes, consequences, and therapeutics: an overviewDepress Anxiety200318416317614661186
- OkajimaIKomadaYInoueYA meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomniaSleep Biol Rhythms2011912434
- KoffelEAKoffelJBGehrmanPRA meta-analysis of group cognitive behavioral therapy for insomniaSleep Med Rev20151961624931811
- SmithMTPerlisMLParkAComparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomniaAm J Psychiatry2002159151111772681
- RiemannDPerlisMLThe treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapiesSleep Med Rev200913320521419201632
- RoehrsTRothTInsomnia pharmacotherapyNeurotherapeutics20129472873822976558
- MorinCMBélangerLLeBlancMThe natural history of insomnia: a population-based 3-year longitudinal studyArch Intern Med2009169544745319273774
- BathgateCJEdingerJDKrystalADInsomnia patients with objective short sleep duration have a blunted response to cognitive behavioral therapy for insomniaSleep2017401
- RiemannDSpiegelhalderKFeigeBThe hyperarousal model of insomnia: a review of the concept and its evidenceSleep Med Rev2010141193119481481
- BonnetMHArandDLHyperarousal and insomnia: state of the scienceSleep Med Rev201014191519640748
- RothTRoehrsTPiesRInsomnia: pathophysiology and implications for treatmentSleep Med Rev2007111717917175184
- HarveyC-JGehrmanPEspieCAWho is predisposed to insomnia: a review of familial aggregation, stress-reactivity, personality and coping styleSleep Med Rev201418323724724480386
- KayDBBuysseDJHyperarousal and beyond: new insights to the pathophysiology of insomnia disorder through functional neuroimaging studiesBrain Sci20177323
- DrummondSPSmithMTOrffHJChengaziVPerlisMLFunctional imaging of the sleeping brain: review of findings and implications for the study of insomniaSleep Med Rev20048322724215144964
- VgontzasANFernandez-MendozaJLiaoDBixlerEOInsomnia with objective short sleep duration: the most biologically severe phenotype of the disorderSleep Med Rev201317424125423419741
- HealeyESKalesAMonroeLJBixlerEOChamberlinKSoldatosCROnset of insomnia: role of life-stress eventsPsychosom Med19814354394517313035
- MorinCMRodrigueSIversHRole of stress, arousal, and coping skills in primary insomniaPsychosom Med200365225926712651993
- DrakeCLPillaiVRothTStress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomniaSleep20143781295130425083009
- XiaLChenG-HLiZ-HJiangSShenJAlterations in hypothalamus-pituitary-adrenal/thyroid axes and gonadotropin-releasing hormone in the patients with primary insomnia: a clinical researchPloS One201388e7106523951080
- RiemannDKleinTRodenbeckANocturnal cortisol and melatonin secretion in primary insomniaPsychiatry Res20021131172712467942
- GehrmanPRHallMBarillaHStress reactivity in insomniaBehav Sleep Med2016141233325126695
- MikoteitTPawlowskiMSeiffertNHeart rate variability during sleep distinguishes between insomnia and normal sleepSleep Med201740e223e224
- DoddsKLMillerCBKyleSDMarshallNSGordonCJHeart rate variability in insomnia patients: a critical review of the literatureSleep Med Rev2017338810028187954
- ChenIYJarrinDCIversHMorinCMInvestigating psychological and physiological responses to the Trier Social Stress Test in young adults with insomniaSleep Med201740112229221772
- DrakeCLChengPAlmeidaDMRothTFamilial risk for insomnia is associated with abnormal cortisol response to stressSleep20174010
- VgontzasANBixlerEOLinH-MChronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implicationsJ Clin Endocrinol Metab20018683787379411502812
- NofzingerEABuysseDJGermainAPriceJCMiewaldJMKupferDJFunctional neuroimaging evidence for hyperarousal in insomniaAm J Psychiatry2004161112126212815514418
- Fernandez-MendozaJLiYVgontzasANInsomnia is associated with cortical hyperarousal as early as adolescenceSleep20163951029103626951400
- Wołyńczyk-GmajDSzelenbergerWWaking EEG in primary insomniaActa Neurobiol Exp201171387392
- SmithMTPerlisMLChengaziVUNeuroimaging of NREM sleep in primary insomnia: a Tc-99-HMPAO single photon emission computed tomography studySleep200225332533512003163
- SpiegelhalderKRegenWBaglioniCRiemannDWinkelmanJWNeuroimaging studies in insomniaCurr Psychiatry Rep2013151140524057158
- EllisBJJacksonJJBoyceWTThe stress response systems: universality and adaptive individual differencesDev Rev2006262175212
- SelyeHThe nature of stressBasal Facts1985713112990402
- PacakKPalkovitsMStressor specificity of central neuroendocrine responses: implications for stress-related disordersEndocr Rev200122450254811493581
- KobasaSCMaddiSRCouringtonSPersonality and constitution as mediators in the stress-illness relationshipJ Health Soc Behav19813683787320474
- DrakeCRichardsonGRoehrsTScofieldHRothTVulnerability to stress-related sleep disturbance and hyperarousalSleep200427228529115124724
- DrakeCLRothTPredisposition in the evolution of insomnia: evidence, potential mechanisms, and future directionsSleep Med Clin200613333349
- Fernández-MendozaJVela-BuenoAVgontzasANCognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomniaPsychosom Med201072439740320368477
- BaglioniCSpiegelhalderKLombardoCRiemannDSleep and emotions: a focus on insomniaSleep Med Rev201014422723820137989
- BonnetMHArandDLSituational insomnia: consistency, predictors, and outcomesSleep20032681029103614746386
- JarrinDCChenIYIversHDrakeCLMorinCMTemporal stability of the ford insomnia response to stress test (first)J Clin Sleep Med20161210137327568895
- GelayeBZhongQ-YBarriosYVRedlineSDrakeCLWilliamsMAPsychometric evaluation of the Ford Insomnia Response to Stress Test (FIRST) in early pregnancyJ Clin Sleep Med201612457958726857055
- NakajimaSOkajimaISasaiTValidation of the Japanese version of the Ford Insomnia Response to Stress Test and the association of sleep reactivity with trait anxiety and insomniaSleep Med201415219620224380783
- MarquesDRAllen GomesADrakeCLRothTde AzevedoMHPAssessing stress-induced sleep reactivity in college students: the European Portuguese version of the Ford insomnia response to stress test (FIRST)Behav Sleep Med201616411227191585
- ChenIJarrinDRochefortALamyMIversHMorinCValidation of the French version of the Ford insomnia response to stress test and the association between sleep reactivity and hyperarousalSleep Med2015161S238
- DieckAHelbigSDrakeCLBackhausJValidation of the German version of the Ford Insomnia Response to Stress TestJ Sleep Res Epub20171019
- PalaginiLBrunoRMPaoloTAssociation between stress-related sleep reactivity and metacognitive beliefs about sleep in insomnia disorder: preliminary resultsBehav Sleep Med201614663664926548894
- KalmbachDAPillaiVArnedtJTDrakeCLIdentifying at-risk individuals for insomnia using the Ford Insomnia Response to Stress TestSleep201639244945626446111
- PetersenHKecklundGD’onofrioPNilssonJÅkerstedtTStress vulnerability and the effects of moderate daily stress on sleep polysomnography and subjective sleepinessJ Sleep Res2013221505722835074
- ChenIJarrinDIversHRochefortAMorinCAssociation between stress-induced arousal and nocturnal sleep: a preliminary studySleep Med201740e55
- American Psychiatric AssociationDiagnostic and Statistical Manual-Text Revision (DSM-IV-TR, 2000)Washington DCAmerican Psychiatric Association2000
- DrakeCLPillaiVRothTStress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomniaSleep201337812951304
- PalaginiLBiberKRiemannDThe genetics of insomnia – evidence for epigenetic mechanisms?Sleep Med Rev201418322523523932332
- MeerloPSgoifoASucheckiDRestricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivitySleep Med Rev200812319721018222099
- KalmbachDAPillaiVArnedtJTAndersonJRDrakeCLSleep system sensitization: evidence for changing roles of etiological factors in insomniaSleep Med201621636927448474
- GehrmanPRByrneEGillespieNMartinNGGenetics of insomniaSleep Med Clin Rev20116191202
- GehrmanPRMeltzerLJMooreMHeritability of insomnia symptoms in youth and their relationship to depression and anxietySleep20113412164122131600
- LindMJGehrmanPRGenetic pathways to insomniaBrain Sci20166464
- LaneJMLiangJVlasacIGenome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traitsNat Genet201749227427992416
- HammerschlagARStringerSde LeeuwCAGenome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traitsNature Genetics201749111584159228604731
- PackAIKeenanBTByrneEMGehrmanPRGenetics and genomic basis of sleep disorders in humansKrygerMHRothTDementWCPrinciples and Practice of Sleep Medicine6th edSt Louis (MO)Elsevier2016
- SingareddyRVgontzasANFernandez-MendozaJRisk factors for incident chronic insomnia: a general population prospective studySleep Med201213434635322425576
- BastienCHMorinCMFamilial incidence of insomniaJ Sleep Res200091495410733689
- DauvilliersYMorinCCervenaKFamily studies in insomniaJ Psychosom Res200558327127815865952
- DrakeCLScofieldHRothTVulnerability to insomnia: the role of familial aggregationSleep Med20089329730217825612
- Fernandez-MendozaJShafferMLOlavarrieta-BernardinoSCognitive-emotional hyperarousal in the offspring of parents vulnerable to insomnia: a nuclear family studyJ Sleep Res201423548949824889269
- DrakeCLFriedmanNPWrightKPJrRothTSleep reactivity and insomnia: genetic and environmental influencesSleep20113491179118821886355
- BonnetMArandDHeart rate variability in insomniacs and matched normal sleepersPsychosom Med19986056106159773766
- GouinJWenzelKDeschenesSDang-VuTHeart rate variability predicts sleep efficiencySleep Med201314e142
- RiemannDSpiegelhalderKFeigeBThe hyperarousal model of insomnia: a review of the concept and its evidenceSleep Med Rev2010141193119481481
- PerlisMLMericaHSmithMTGilesDEBeta EEG activity and insomniaSleep Med Rev200155365376
- Fernandez-MendozaJLiYVgontzasAInsomnia is associated with cortical hyperarousal as early as adolescenceSleep20163951029103626951400
- Fernandez-MendozaJVela-BuenoAVgontzasANCognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomniaPsychosom Med201072439740320368477
- RiemannDVoderholzerUPrimary insomnia: a risk factor to develop depression?J Affect Disord200376125525912943956
- TsunoNBessetARitchieKSleep and depressionJ Clin Psychiatry2005661254126916259539
- PillaiVRothTDrakeCLThe nature of stable insomnia phenotypesSleep201538112713825325468
- EspieCA“Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatmentSleep200932121549155820041590
- ManberRSimpsonNSBootzinRRA step towards stepped care: delivery of CBT-I with reduced clinician timeSleep Med Rev2015193525454675
- ZachariaeRLybyMSRitterbandLMO’TooleMSEfficacy of internet-delivered cognitive-behavioral therapy for insomnia – a systematic review and meta-analysis of randomized controlled trialsSleep Med Rev20163011026615572
- EspieCAHamesPMcKinstryBUse of the internet and mobile media for delivery of cognitive behavioral insomnia therapySleep Med Clin201383407419
- FeuersteinSHodgesSEKeenaghanBBessetteAForseliusEMor-ganPTComputerized cognitive behavioral therapy for insomnia in a community health settingJ Clin Sleep Med201713226727427784409
- PillaiVAndersonJRChengPThe anxiolytic effects of cognitive behavior therapy for insomnia: preliminary results from a web-delivered protocolJ Sleep Med Disord201522a7
- LanceeJvan StratenAMorinaNKaldoVKamphuisJHGuided online or face-to-face cognitive behavioral treatment for insomnia: a randomized wait-list controlled trialSleep201639118319126414893
- BlomKTillgrenHTWiklundTInternet-vs. group-delivered cognitive behavior therapy for insomnia: a randomized controlled non-inferiority trialBehav Res Ther201570475525981329