Abstract
Purpose
Orofacial myofunctional therapy (OMT) is a modality of treatment for children and adults with obstructive sleep apnea (OSA) to promote changes in the musculature of the upper airways. This review summarizes and discusses the effects of OMT on OSA, the therapeutic programs employed, and their possible mechanisms of action.
Methods
We conducted an online literature search using the databases MEDLINE/PubMed, EMBASE, and Web of Science. Search terms were “obstructive sleep apnea” in combination with “myofunctional therapy” OR “oropharyngeal exercises” OR “speech therapy”. We considered original articles in English and Portuguese containing a diagnosis of OSA based on polysomnography (PSG). The primary outcomes of interest for this review were objective measurement derived from PSG and subjective sleep symptoms. The secondary outcome was the evaluation of orofacial myofunctional status.
Results
Eleven studies were included in this review. The studies reviewed reveal that several benefits of OMT were demonstrated in adults, which include significant decrease of apnea–hypopnea index (AHI), reduced arousal index, improvement in subjective symptoms of daytime sleepiness, sleep quality, and life quality. In children with residual apnea, OMT promoted a decrease of AHI, increase in oxygen saturation, and improvement of orofacial myofunctional status. Few of the studies reviewed reported the effects of OMT on the musculature.
Conclusion
The present review showed that OMT is effective for the treatment of adults in reducing the severity of OSA and snoring, and improving the quality of life. OMT is also successful for the treatment of children with residual apnea. In addition, OMT favors the adherence to continuous positive airway pressure. However, randomized and high-quality studies are still rare, and the effects of treatment should also be analyzed on a long-term basis, including measures showing if changes occurred in the musculature.
Introduction
Health depends on various factors, with breathing, eating, hydration, and sleep being the primordial among them. However, each of them is susceptible to changes caused by a large number of variables with different degrees of physical and mental consequences. Sleep-disordered breathing (SDB) is a relatively common problem, with obstructive sleep apnea (OSA) being its most severe manifestation.Citation1
OSA is a relevant health problem that involves repeated upper airway collapse during sleep, causing a reduction (hypopnea) or cessation (apnea) of airflow, oxygen desaturation, and fragmented sleep, accompanied by respiratory effort.Citation2,Citation3 Snoring is a characteristic signal, but not when it occurs in an isolated manner. The disease must be diagnosed based on laboratory full-night polysomnography (PSG), considering that criteria are different for children younger than 18 years and adults.Citation4
The pathophysiology of OSA during childhood is poorly known, although adenotonsillar hypertrophy and the installation of oral breathing are the major factors contributing to its occurrence.Citation5–Citation9 In general, studies report that the problem affects 1%–5% of the child population,Citation2 with a peak of incidence among preschoolers, that is, in the age range when tonsil hypertrophy is more common.Citation10 The consequences of OSA in children are low school performance, attention deficit and hyperactivity,Citation11,Citation12 low weight–height development,Citation13 and cardiovascular dysfunction.Citation2
During adulthood, anatomical and nonanatomical factors interact and contribute to the manifestation of OSA, such as narrow pharynx, increased upper airway length, specific pharyngeal lumen shapes, and collapsible upper airway. The factors that may play a role in OSA pathogenesis are changes in the activity of oropharyngeal muscles occurring during sleep, a poor genioglossus muscle responsiveness to negative pharyngeal pressure, a low respiratory arousal threshold, and an oversensitive ventilatory control system.Citation14–Citation18
The disease is more common among men than women and its prevalence increases with age and in obese persons.Citation17,Citation19 However, menopause is a risk factor for women regardless of age or body mass index (BMI),Citation20 a factor that tends to reduce the difference from men, especially when no hormone replacement therapy is used.Citation21 The symptoms of OSA include loud snoring, sleep disruption, excessive daytime sleepiness, nocturia,Citation3,Citation22,Citation23 fatigue, morning headache, irritability, decreased concentration, and memory loss.Citation24
Considered to be a progressive and incapacitating chronic disease,Citation4 OSA impairs the quality of life and results in comorbidities such as arterial hypertension, cardiovascular diseases, and diabetes.Citation25–Citation27 However, the clinical manifestations are heterogeneous, and if the patients do not present characteristics such as a high BMI and subjective sleepiness, the symptoms may also be attributed to comorbid diseases and OSA will not be investigated and diagnosed.Citation18
The first-line treatment during childhood is adenotonsillectomy, with reported cure or improvement of the disorder in most cases.Citation9 Orthodontic treatment for correction of mandibular or maxillomandibular anomalies has been shown to improve OSA.Citation28 Continuous positive airway pressure (CPAP) is the treatment of choice for adults with OSA,Citation20 especially in severe cases, to relieve symptoms and to reduce the sequelae.Citation18,Citation29 However, mandibular advancement device (MAD), an intraoral dental splint used to protrude the mandible in a forward position during sleep and thus enlarge the upper airway,Citation30,Citation31 is mainly indicated as the first-stage treatment of adults with mild-to-moderate OSA and in severe cases in which attempts with CPAP treatment fail.Citation30–Citation32 MAD has been considered as a treatment option for children,Citation33 although it is still under study, and it requires accurate indication and follow-up due to the craniofacial growth and development.
Still, surgical interventions are recommended for the correction of anatomical and morphological problems or as a second option for adult patients who fail to adhere or respond to noninvasive treatments. Surgical procedures include uvulopalatopharyngoplasty,Citation34 surgically assisted rapid maxillary expansion,Citation35 and maxillomandibular advancement.Citation31,Citation36,Citation37
Despite the success rate of these interventions in reducing apnea–hypopnea index (AHI) and symptoms related to OSA,Citation20 the need for new or complementary therapeutic modalities for OSA has been pointed out. The main reasons for this are the percentage of patients who do not respond satisfactorily to available treatments, the reduced adherence to CPAP, especially when the severity of the disease is moderate,Citation2,Citation18,Citation29 and the possible complications of surgical procedures, even when limited.Citation2,Citation28,Citation34
The orofacial myofunctional therapy (OMT), or oropharyngeal exercises, with one of the focal points in the promotion of changes in dysfunctional upper airway muscles, was proposed with success for reducing OSA severity and associated symptoms in adults.Citation38 Since then, the potential of OMT has also been investigated to promote reduction of snoring,Citation39 improvement of quality of life,Citation40 and adherence to the use of CPAP,Citation29 as well to treat residual OSA in children.Citation41 A retrospective study summarized the clinical data of children with OSA and concluded that the OMT after surgery improved the outcome of treatment.Citation42 Previously, systematic reviews,Citation43 meta-analysis,Citation44,Citation45 and narrative reviewsCitation46,Citation47 analyzed the myofunctional therapy for patients with OSA.
The difference in the current review from the previous ones is the emphasis on the strategies of therapeutics used by the various authors and their possible implications regarding the pathophysiology of OSA, in addition to suggestions for future investigations.
The objective of the present review is to summarize and discuss the available literature about OMT for patients with OSA, the benefits described, the strategies employed, and the way they can act on the functionality of the upper airway.
Methods
We conducted a bibliographic search up to December 3, 2017, of the following electronic databases: MEDLINE/PubMed, EMBASE, and Web of Science, limited to humans. A manual search of the references of selected studies was conducted as well. Articles in English and Portuguese were accepted. Search terms were “obstructive sleep apnea” in combination with “myofunctional therapy” OR “oropharyngeal exercises” OR “speech therapy”.
Inclusion criteria
Participants
This review was restricted to studies with participants meeting the following criteria: (1) diagnosis of OSA according to the measurement derived from PSG; (2) clinical symptoms of OSA; and (3) no other comorbid conditions (eg, trauma in the head and neck region, cancer, or neurological disease).
Studies
This review included clinical investigations published in journals with peer-review policy, which analyzed the effects of OMT (or oropharyngeal exercises) intervention alone for OSA patients. Randomized studies comparing OMT with a placebo intervention or a controlled intervention and prospective case–control studies were prioritized and mentioned in more detail. However, prospective case series were also included to explore the interventional models, due to the scarcity in relevant publications.
Outcomes of interest
The primary outcomes of interest for this review were objective measurements derived from PSG and subjective sleep symptoms including snoring, daytime sleepiness, and sleep quality. The secondary outcome of interest was the evaluation of orofacial myofunctional status.
Data screening and extraction
The initial screening of the detected documents was conducted blindly in triplicate. The titles were analyzed, as well as the abstract of the publication when available. Articles were selected if at least one reviewer identified them as relevant. A detailed full-text analysis was conducted and two reviewers extracted data from each paper such as study purpose, design, participants, interventions, and main and secondary outcomes. Disagreements regarding data screening and extraction were resolved by consensus among the three reviewers. Twelve questions were elaborated to guide the analyses (Supplementary material) based on a previous study.Citation48
Results
A total of 161 published articles were retrieved from the initial search. After exclusion of duplicates (83) and ineligible studies (67), 11 studies were included. shows a flowchart of the search process used in this review, which is based on PRISMA guidelines for systematic reviewCitation49 with the exception that other types of studies were also included here along with randomized controlled trial (RCT) studies.
The types of study included were as follows: RCTs (n=4),Citation29–Citation40 prospective randomized (n=1),Citation41 prospective case–control (n=1),Citation1 and prospective case series (n=5).Citation50–Citation54 provides a summary of the reviewed studies.
Table 1 Summary of studies included
All RCT studies, with clinical trials registered, analyzed the effect of OMT in young subjects and adults with mild and/or moderate OSA, and the authors were Brazilians.Citation29–Citation40 Two of them included the same sample to answer distinct research questions.Citation29,Citation40 The prospective randomized and prospective case–control studies analyzed the benefits of OMT for children with SDB residual apneaCitation1,Citation41 and the authors were Italians.
OMT
OMT is a treatment modality applied to subjects with orofacial myofunctional disorders (OMDs), that is, with changes in the orofacial structure, in the cervical musculature, or both, which may interfere with the development or functioning of orofacial structures and functions.Citation55 OMT is based on exercises and other strategies that favor sensitivity, proprioception, mobility, coordination, and strength of orofacial structures, as well as promote an appropriate performance of respiration, mastication, deglutition, and speech.
In 1990, the American Speech and Hearing Association first recognized the role of the speech-language pathologist in providing services to persons with OMD, and the knowledge and skills required to evaluate and treat OMD were later described.Citation56 However, the starting point in the development of OMT was the recognition that the correction of malocclusion requires equilibrium of orofacial muscles.Citation57–Citation59 The mutual interest of orthodontists and speech pathologists in oropharyngeal mechanisms led them to cooperate in the study of some associated problems.Citation59 Since then, the range of health problems, treatment strategies,Citation60 and scientific evidence has expanded.
An example of this is that the patients with sequelae of chronic mouth breathing have been long treated by speech-language pathologistsCitation61 due to the orofacial myofunctional impairments.Citation62,Citation63 However, the use of PSG for the diagnosis of OSA was not widespread, and therefore, only the symptoms and clinical condition of the patients were considered. Since the last decade, due to the dissemination of knowledge, OSA has attracted the attention of these professionals. The reason for this interest lies in the fact that orofacial and pharyngeal muscles dysfunctionCitation64 and impaired oropharyngeal control are possible contributing factors to airway collapse,Citation65 mainly when associated with an anatomical predisposition.
Therapeutic procedures in this area can be selected based on scientific evidence of benefits of a particular treatment or theoretical solidity when clinical efficacy has not been previously documented.Citation66 Thus, when proposing the first exercise protocol for the reduction of obstructive sleep apnea syndrome (OSAS) severity, Guimarães et alCitation38 used the scientific reports available about the pathogenesis of OSA and the empirical foundations of speech therapy. According to the authors,Citation38 the oropharyngeal exercises target soft palate elevation that recruits several upper airway muscles such as the tensor and levator veli palatini, as well as muscle fibers of the palatopharyngeal and palatoglossus muscles, tongue repositioning, and training of mandibular elevation to avoid mouth opening. The results obtained in an RCT showed that, after three months of therapy, patients with moderate OSA had a significant reduction of AHI (22.4±4.8 vs 13.7±8.5), an increase of lowest oxygen saturation (83±6 vs 85±7), as well as a reduction of associated symptoms. These changes did not occur in the control (placebo) group.
This protocol was applied in other studies in fullCitation52 or with some modifications in the exercises.Citation29,Citation40 An author added 12 other exercises,Citation53 although giving no reason for this addition. In another studyCitation39 whose objective was to treat snoring, the original protocol was simplified to facilitate the incorporation of the training into the daily activities. The number of exercises was reduced to 50% and the duration of each training was set to 8 min. This model was later used for the treatment of OSA.Citation54 Although Baz et alCitation50 have maintained the goals of increasing the tone and endurance of the same muscles for the treatment of OSA, they employed different exercises.
In most of the studies reviewed, the therapeutic program for adults lasted three months, with a weekly visit and training at home for threeCitation29,Citation38–Citation40,Citation52 to five times a day.Citation53,Citation54 The authors adopted systems of control of adherence to treatment considering the frequency of supervised sessions and a diary indicating home practice. Only one pilot studyCitation51 lasted two months, with training at the clinic for 5 min twice a day and four times per week. The strategies and exercises used during therapy are listed in .
Table 2 Strategies and exercises for young and adult patients with OSA recommended in the reviewed studies
The program applied to children lasted two months, and the exercises included differed from those applied to adults. One studyCitation41 involved three meetings with the therapist, the first for evaluation and explanation of the exercises, the second for supervision, and the third for reevaluation, while the otherCitation1 involved two monthly meetings. Villa et alCitation41 described the exercises.
OMT and sleep-breathing variables
Among the randomized studies that analyzed the effect of OMT on sleep breathing based on full-PSG data, the investigators detected evidence of AHI reduction in adults with moderate OSA,Citation29,Citation38–Citation40 an increased percentage of lowest oxygen saturation (SaO2),Citation38 and a reduction of arousal index (AI).Citation38 In children, the authors observed a decrease of AHICitation41 and a higher SaO21 after OMT.
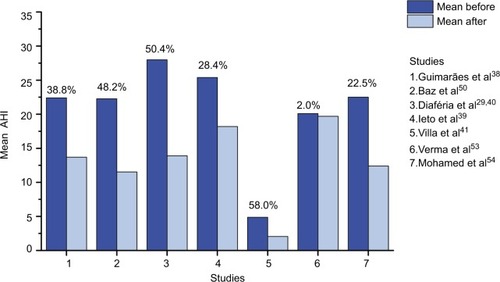
Authors of non-randomized studies also reported that, after OMT, patients with mild or moderate OSA showed a significant reduction of AHICitation50,Citation51,Citation53,Citation54 and AI,Citation50,Citation53,Citation54 and an increase of lowest SaO2 (%)Citation50,Citation51,Citation53,Citation54 compared to baseline. They also observed a decrease in the percentage of the duration of the SaO2<90.Citation50,Citation53,Citation54 Mohamed et alCitation54 analyzed in parallel the effects of OMT in a group of patients with severe OSA, which showed a decrease in mean AHI, but was not statistically significant. The AHI before and after OMT reported by reviewed authors, as well as the ΔAHI%: (AHI before −AHI after)/AHI before × 100) are summarized in .
Figure 2 Apnea–hypopnea index (AHI): mean (or median for Ieto et alCitation39) before and after OMT and percentage of AHI decrease.
OMT and snoring
A studyCitation39 reported a primary efficacy end point of OMT for the objective measurement of snoring in patients with a complaint of snoring and a diagnosis of primary snoring or mild-to-moderate OSA. For this purpose, based on recordings obtained during PSG, the authors established a methodology for the calculation of snore index (total number of snores/total sleep time) and total snore index (sound intensity power generated by all snoring episodes/total sleep time, expressed in arbitrary unit/10Citation7). The results showed that OMT promoted a reduction of snoring index (99.5 vs 48.2, p=0.041) and total snore index (60.4 vs 31.0, p=0.041).Citation39 Other authors also reported a reduction of snoring index and episodes of loud snoring.Citation40,Citation54 Additionally, the intensity and frequency of snoring as reported by the bed partnerCitation39,Citation52 or as perceived by the patient decreased after the therapy.Citation38,Citation39,Citation53
OMT and daytime sleepiness, sleep quality, and life quality
A large number of studies, as noted from the literature as well as observed in the present review, had employed the Epworth Sleepiness Scale (ESS) proposed by JohnsCitation67 that evaluates the propensity to sleep in eight different situations, with a total score ranging from zero (absence) to 24 (intense). Although the ESS has been criticized for its sensitivity and specificity and low predictive value, limiting its value in the screening of patients with sleep apnea,Citation68 in almost all papers analyzed, it was used as a measure of the results of treatment regarding daytime sleepiness.
Patients with a mean baseline ESS score ranging from 12±2.6 to 15.4±2.3 showed a significant improvement after OMT, with a mean reduction of six points.Citation29,Citation38,Citation40,Citation50,Citation52,Citation54 However, no change occurred in a group of patients with a median ESS score of 7.0 (3–11) who were, on average, not sleepyCitation39 or in a group with severe OSA and an ESS score of 20.9±6.2.Citation54
Considering the damage to sleep caused by OSA and its consequences in the daytime and for health in general, other relevant findings were an improved quality of sleep evaluated with the Pittsburg questionnaireCitation38,Citation39 and quality of life.Citation40 The quality of life similarly improved after treatment with OMT alone or combined with CPAP, while no effect was observed in the CPAP-alone group.Citation40 The morning headache symptom was investigated in two studies,Citation50,Citation53 only one of which detected a reduction from 60% to 20% in the number of patients with this complaint.Citation50
All of these reported data concern the effect of therapy measured immediately after the conclusion of OMT. Only Diaféria et alCitation29,Citation40 followed up their patients for three weeks after the end of the intervention and observed that, among the variables that improved after the OMT, the frequency and intensity of snoring were maintained. Another positive contribution of OMT is that it favors adherence to the use of CPAP.Citation29 The educational process and the support received on a weekly basisCitation20 during the sessions probably favored greater adherence to CPAP when combined with OMT (65%) than treatment with CPAP alone (30%).Citation29 Verma et alCitation53 reported differences in response to therapy between men and women. However, the limited number of female participants (n=5) hinders the interpretation.
OMT and orofacial myofunctional status
The efficacy of the OMT for patients with mild–moderate OSA is well characterized by PSG data and questionnaires in the studies analyzed. However, reports about the myofunctional evaluation are limited and not based on a standardized assessment tool that hampers the interpretation and comparison of findings. Only some studies reported the effects promoted by OMT in the oropharyngeal and facial muscles and functions.Citation1,Citation41,Citation51,Citation52
According to Matsumura et al,Citation52 the percentage of patients with an appropriate pattern of structure and functions, such as lowering of the back of the tongue, high soft palate, suprahyoid muscles tonus, nasal breathing, bilateral chewing, and speech, increased in response to the OMT program. An increase in labial closure force resulted from the training specifically applied for this purpose, aiming to promote nasal breathing.Citation51 Diaféria et alCitation29 reported that the Mallampati index improved in the OMT and CPAP+OMT groups and was correlated with the increased strength of the tongue and soft palate, but results of the myofunctional evaluation were not presented.
Villa et alCitation1,Citation41 used a set of exercises divided into three categories: (1) nasal breathing rehabilitation, (2) labial seal and lip tone exercises, and (3) tongue posture exercises. In a group of children with residual apnea, OMT increased the number of patients with a proper labial seal, lip tone, and nasal breathing.Citation41 In another study conducted on subjects with primary snoring or mild–moderate OSA, there was a significant increase in the objective measures of tongue strength, tongue peak, and endurance. Also, the number of children with oral breathing, abnormal tongue rest position, and lip hypotonia decreased after therapy.Citation1
Future studies would determine whether the effects of OMT in patients with SDB are, in fact, related to improved muscle and orofacial functions.Citation65 For this, validated tools that enable identifying, classifying, and grading changes in muscles and functions status would be used, rather than using dichotomous judgment (yes/no), which only inform the frequency of individuals with normal or altered conditions.
Currently, this is possible with the Orofacial Myofunctional Evaluation with Scores (OMES)-expanded protocol validated for OSA patients.Citation65 The OMES protocol, which is the original version, has also shown to be useful for the characterization of the orofacial myofunctional conditions of childrenCitation64 and adults with OSA.Citation34,Citation69 Moreover, objective measures of strength/forceCitation1,Citation51,Citation64 and electromyographyCitation64 can contribute to the myofunctional diagnosis and the assessment of the results of the intervention. Therefore, the parameters for the indication of OMT, as well as the benefits of the therapeutic strategies, could be defined appropriately in the future.
Next, based on the literature, we will consider the possible influences of OMT on the oropharyngeal structures. Because many assumptions still need confirmation, this discussion may suggest new ways for further investigations.
Therapeutic strategies and fundamentals
Most of the authors of the papers reviewed adopted a set of exercises for OMT to cover the various oropharyngeal structures,Citation1,Citation38,Citation39,Citation41,Citation50,Citation52–Citation54 that is, tongue, palate, lateral pharyngeal walls, or epiglottis which, separately or in combination, are involved in the collapse of the pharyngeal airway.Citation16,Citation17
Because the increased soft palate length has been associated with higher rates of obstructive apnea, AHI, and respiratory disturbance index,Citation70 to increase the tone of elongated and floppy soft palate and uvula is one of the goals of training.Citation29,Citation38–Citation40,Citation52–Citation54
Exercises focused on the tongue are widely applied, except for a pilot study,Citation51 and are fully justified by the findings related to the pathogenesis of OSA. The dimensions of the tongue (area and volume) are significantly associated with upper airway collapsibility, which in turn is associated with AHI.Citation17 Tongue fat (in mm3) is the main factor explaining the increased tongue volume in patients with OSA compared to controls.Citation71 The possible implications of this finding are the compromised ability of extrinsic muscles to position the tongue and the reduction of the air space in the retroglossal region, where there is a larger percentage of fat in patients with OSA, increasing the risk of sleep apnea.Citation71 Moreover, the genioglossus muscle activity is significantly reduced during rapid eye movement (REM) sleep, especially phasic REM, coincident with the onset of REM hypopnea/obstructive apnea.Citation15
Thus, we should point out the significant reduction of AHI during REM sleep observed after OMT.Citation38 The exercises, as performed during OMT, with a large number of repetitions at a low level of resistance, may promote adaptation of Type I fatigue-resistant muscles, which represent more than half of the muscle fibers in the posterior region of the tongue.Citation66–Citation72 Thus, the training during wakefulness seems to have contributed to the minimization of hypotonia of the genioglos-sus muscle, the major upper airway dilator muscle, during the most critical phase of sleep when the severity of OSA worsens. According to a recent review, REM sleep apnea may be more important in mediating insulin resistance and the cardiovascular consequences of OSA.Citation18
The suprahyoid muscles, whose adequacy is also mentioned in some studies as a result of OMT,Citation52–Citation54 include the geniohyoid, stylohyoid, mylohyoid, anterior digastric, and posterior digastric muscles.Citation73 These muscles, together with the styloglossus and genioglossus muscles, participate in specific tasks. For example, “placing the tip of the tongue against the front of the palate and sliding the tongue backward” and “forced tongue sucking upward against the palate, pressing the entire tongue against the palate”.Citation38 These tasks are potentially favorable to an increase in resistance and fatigue threshold of the muscles involved and consequently to reach the goal of appropriate tongue positioning during rest and deglutition.
During the oral phase of deglutition, the surface of the tongue rises, gradually expanding the area of tongue–palate contact from the anterior to the posterior region and compressing the bolus in the direction of the pharynx.Citation74 Elevation of the tongue is promoted by the suprahyoid muscles,Citation75 which also displace the hyoid bone in an anterior direction (geniohyoid) and upper direction (mylohyoid).Citation73,Citation76 Therefore, another result of OMT may be a higher positioning of the hyoid bone, which should also be analyzed in future studies because a lower positioned hyoid bone is a common finding in patients with OSA.Citation17
Mouth breathing during sleep lengthens and narrows the upper airway, which in turn may aggravate OSA severity.Citation71 Presumably, for this reason, Suzuki et alCitation51 decided to train labial closure force to the patients. However, this training alone does not seem to be sufficient to modify the signs and symptoms of apnea since the muscles involved in the collapse of the airways are not directly exercised.
Jaw muscles exercisesCitation38,Citation51,Citation53 and training of alternate bilateral mastication and deglutition with teeth in occlusion, focusing on the equilibrium of these functions, have been reported.Citation29,Citation38,Citation39,Citation52–Citation54 However, no statement was made regarding the fact that not all patients have an occlusal condition to chew alternately on the right and left sides. Moreover, patients with OSA may have associated temporomandibular disorders (TMDs), and a prospective cohort study demonstrated that OSA symptoms preceded first-onset TMD.Citation77
Thus, the goals of rehabilitation of mastication and swallowing should take into account the dynamics relationship between occlusions, muscles, and temporomandibular joint.Citation78 In this sense, there should be bilateral occlusal guides without occlusal interference, especially on the balancing side, as well as muscular coordination to grind the food, to transfer it from one side of the oral cavity to the other, and into the oropharynx. Also, during or after mastication, the patients should not have either joint noise or pain.Citation78
Exercise for buccinator muscles has also been included in various studies.Citation29,Citation38,Citation39,Citation52–Citation54 It has been recently suggested that the appearance of the cheeks (volume and flaccidity) may be an additional predictor of OSA risk and a visible signal of fat deposition also affecting the tongue and pharynx.Citation69 However, it would be necessary to demonstrate how the type of cheek exercise can favor the muscles related to OSA since their remote location renders improbable a meaningful contribution to remodeling of the oropharyngeal airway.Citation79
The neck circumference, abdominal circumference measures, and BMI are anthropometric predictors of OSA severity.Citation80 Obese patients with a large neck circumference show worse collapsibility of the upper airway.Citation17 Thus, a significant decrease in neck circumferenceCitation38,Citation39,Citation50,Citation53 correlated with changes in AHI after OMTCitation38,Citation54 indicates that the exercises induced upper airway remodeling.Citation38 Although weight loss is recommended for overweight or obese patients, it is essential to control any variation in BMI when the intention is to analyze the effect of interventions on the severity of OSA, a fact that was not reported in two previous studies.Citation51,Citation54 It should also be pointed out that Verma et alCitation53 emphasized that their experimental design had an advantage over a previous study that did not permit to conclude which set of exercises resulted in maximum benefit for the patients. However, they failed to consider that the effects of training are progressive. Therefore, the highest percentage of improvement of symptoms achieved by patients at the last phase of treatment compared to the previous two does not indicate which set of exercises was most effective.
In summary, the principle of OMT (oropharyngeal exercises) is repetitive muscle training, with specific gains in the coordination, tonicity, and endurance of the muscles, considering the specificity of the exercises adopted (isotonic and isometric). The exercises may improve the condition of muscle fatigue in subjects with OSACitation81 and perhaps act on the equilibrium of contraction between the different muscles that involve the velopharyngeal, oropharyngeal, and hypopharyngeal segments.Citation82 Further, they can decrease the volume of specific structures and fat in the pharynx-dilating muscles, thus also reducing the potential upper airway collapse in apneic subjects. However, these hypotheses have not been verified so far.
As pointed out previously, it needs to be determined whether there is a relationship between tongue exercises and changes in the tongue itself and the palate regarding muscle tone, strength, size, and upper airway volume.Citation44 It would also be relevant to compare different OMT programs in randomized controlled studies. In addition, whether the therapy results are stable over time remains to be determined; however, this is a hard task, given the difficulty of keeping the participants tied to the research team and of convincing them to submit again to the complex exams.
Conclusion
In recent years, OMT for the treatment of patients with OSA has represented a new path in the fight aiming at the minimization or cure of a disease with serious consequences for human beings. The results of randomized studies, although of a limited number, have shown that OMT is effective for the treatment of adult patients with mild and moderate OSA and with primary snoring, and of children with residual apnea. In addition, it provides benefits such as an improved quality of life and increased adherence to CPAP. In general, the goal of the therapy is to induce changes in weak and dysfunctional upper airway muscles, although there is a lack of demonstration based on standardized measures showing whether such changes do occur. New RCTs, including analyses of the effects on the muscles and motor control of the upper airway, could contribute to the dissemination of the indication of OMT for the treatment of OSA.
Author contributions
CMF defined the study design with support from FVSD and LVVT and drafted the article. All authors conducted the literature review, contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was supported by the Provost’s Office for Research of the University of São Paulo (Process No. 11.1.21626.01.7).
Supplementary material
Table S1 Questions for analysis of complete texts
Disclosure
The authors report no conflicts of interest in this work.
References
- VillaMPEvangelistiMMartellaSBarretoMDel PozzoMCan myofunctional therapy increase tongue tone and reduce symptoms in children with sleep-disordered breathing?Sleep Breath2017211025103228315149
- MarcusCLBrooksLJDraperKADiagnosis and management of childhood obstructive sleep apnea syndromePediatrics2012130e714e75522926176
- ThorpyMJClassification of sleep disordersNeurotherapeutics2012968770122976557
- American Academy of Sleep Medicine (AASM)International Classification of Sleep Disorders3rd edDarienAASM2014
- WoodsideDGLinder-AronsonSLundstromAMcWilliamJMandibular and maxillary growth after change mode of breathingAm J Orthod Dentofacial Orthop19911001182069140
- KatzESD’AmbrosioCMPathophysiology of pediatric obstructive sleep apneaProc Am Thorac Soc2008525326218250219
- GuilleminaultCRileyRPowellNObstructive sleep apnea and abnormal cephalometric measurementsChest1984867937946488926
- ArensRMcDonoughJMCostarinoATMagnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndromeAm J Respir Crit Care Med200116469870311520739
- MarcusCLMooreRHRosenCLChildhood Adenotonsillectomy Trial (CHAT)A randomized trial of adenotonsillectomy for childhood sleep apneaN Engl J Med20133682366237623692173
- MarcusCLSleep-disordered breathing in childrenAm J Respir Crit Care Med2001164163011435234
- ChervinRDDillonJEBassettiCGanoczyDAPituchKJSymptoms of sleep disorders, inattention and hyperactivity in childrenSleep199720118511929493930
- GozalDSleep-disordered breathing and school performance in childrenPediatrics19981026166209738185
- SchechterMSTechnical report: diagnosis and management of childhood obstructive sleep apnea syndromePediatrics2002109e6911927742
- EckertDJWhiteDPJordanASMalhotraAWellmanADefining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targetsAm J Respir Crit Care Med2013188996100423721582
- McSharryDGSaboiskyJPDeyoungPPhysiological mechanisms of upper airway hypotonia during REM sleepSleep20143756156924587579
- GentaPRSandsSAButlerJPAirflow shape is associated with the pharyngeal structure causing OSAChest201715253754628651794
- HirataRPSchorrFKayamoriFUpper airway collapsibility assessed by negative expiratory pressure while awake is associated with upper airway anatomyJ Clin Sleep Med2016121339134627448414
- OsmanAMCarterSGCarberryJCEckertDJObstructive sleep apnea: current perspectivesNat Sci Sleep201810213429416383
- TufikSSantos-SilvaRTaddeiJABittencourtLRObstructive sleep apnea syndrome in the São Paulo epidemiologic sleep studySleep Med20101144144620362502
- JordanASMcSharryDGMalhotraAAdult obstructive sleep apnoeaLancet201438373674723910433
- BixlerEOVgontzasANLinHMPrevalence of sleep-disordered breathing in women: effects of genderAm J Respir Crit Care Med200116360861311254512
- EckertDJMalhotraAPathophysiology of adult obstructive sleep apneaProc Am Thorac Soc2008514415318250206
- RomeroEKrakowBHaynesPUlibarriVNocturia and snoring: predictive symptoms for obstructive sleep apneaSleep Breath20101433734319865841
- EpsteinLJKristoDStrolloPJJrClinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adultsJ Clin Sleep Med2009526327619960649
- DuránJEsnaolaSRubioRIztuetaAObstructive sleep apnea–hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yrAm J Respir Crit Care Med200116368568911254524
- ShaharEWhitneyCWRedlineSSleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health studyAm J Respir Crit Care Med2001163192511208620
- DragerLFBortolottoLALorenziMCFigueiredoACKriegerEMLorenzi-FilhoGEarly signs of atherosclerosis in obstructive sleep apneaAm J Respir Crit Care Med200517261361815901608
- VillaMPRizzoliARabascoJRapid maxillary expansion outcomes in treatment of obstructive sleep apnea in childrenSleep Med20151670971625934539
- DiafériaGSantos-SilvaRTruksinasEMyofunctional therapy improves adherence to continuous positive airway pressure treatmentSleep Breath20172138739527913971
- IsacssonGFodorCSturebrandMObstructive sleep apnea treated with custom-made bibloc and monobloc oral appliances: a retrospective comparative studySleep Breath2017219310027380034
- KnappeSWSonnesenLMandibular positioning techniques to improve sleep quality in patients with obstructive sleep apnea: current perspectivesNat Sci Sleep201810657229440942
- Health Quality OntarioOral appliances for obstructive sleep apnea: an evidence-based analysisOnt Health Technol Assess Ser200995151
- Machado-JúniorAJSignorelliLGZancanellaECrespoANRandomized controlled study of a mandibular advancement appliance for the treatment of obstructive sleep apnea in children: a pilot studyMed Oral Patol Oral Cir Bucal201621e403e40726946208
- BragaAGrechiTHEckeliAPredictors of uvulopalatopharyngoplasty success in the treatment of obstructive sleep apnea syndromeSleep Med2013141266127124152797
- VinhaPPEckeliALFariaACXavierSPde Mello-FilhoFVEffects of surgically assisted rapid maxillary expansion on obstructive sleep apnea and daytime sleepinessSleep Breath20162050150826092279
- FariaACda Silva-JuniorSNGarciaLVdos SantosACFernandesMRde Mello-FilhoFVVolumetric analysis of the pharynx in patients with obstructive sleep apnea (OSA) treated with maxillomandibular advancement (MMA)Sleep Breath20131739540122562291
- FariaACGarciaLVSantosACEckeliALGarciaDMMello-FilhoFVDynamic comparison of pharyngeal stability during sleep in patients with obstructive sleep apnea syndrome treated with maxillomandibular advancementSleep Breath201721253027225872
- GuimarãesKCDragerLFGentaPRMarcondesBFLorenzi-FilhoGEffects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndromeAm J Respir Crit Care Med200917996296619234106
- IetoVKayamoriFMontesMIEffects of oropharyngeal exercises on snoring: a randomized trialChest201514868369125950418
- DiafériaGBadkeLSantos-SilvaRBommaritoSTufikSBittencourtLEffect of speech therapy as adjunct treatment to continuous positive airway pressure on the quality of life of patients with obstructive sleep apneaSleep Med20131462863523702236
- VillaMPBrasiliLFerrettiAOropharyngeal exercises to reduce symptoms of OSA after ATSleep Breath20151928128924859614
- GuilleminaultCHuangYSMonteyrolPJSatoRQuoSLinCHCritical role of myofascial reeducation in pediatric sleep-disordered breathingSleep Med20131451852523522724
- ValbuzaJSde OliveiraMMContiCFPradoLBde CarvalhoLBdo PradoGFMethods for increasing upper airway muscle tonus in treating obstructive sleep apnea: systematic reviewSleep Breath20101429930520563659
- CamachoMCertalVAbdullatifJMyofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysisSleep20153866967525348130
- CamachoMGuilleminaultCWeiJMOropharyngeal and tongue exercises (myofunctional therapy) for snoring: a systematic review and meta-analysisEur Arch Otorhinolaryngol201727584985529275425
- SoaresEBPiresJBMenezesMASantanaSKSFragaJSpeech therapy and snore and sleep apneaRev CEFAC201012317325
- MoellerJLPaskayLCGelbMLMyofunctional therapy: a novel treatment of pediatrics sleep-disordered breathingSleep Med Clin20149235243
- SteeleCMAlsaneiWAAyanikalathSThe influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic reviewDysphagia20153022625343878
- MoherDLiberatiATetzlaffJAltmanDGThe PRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med20096e100009719621072
- BazHElshafeyMElmorsySAbu-SamraMThe role of oral myofunctional therapy in managing patients with mild to moderate obstructive sleep apneaPAN Arab J Rhinol201221722
- SuzukiHWatanabeAAkihiroYPilot study to assess the potential of oral myofunctional therapy for improving respiration during sleepJ Prosthodont Res20135719519923522598
- MatsumuraETonisiGABRVecinaALCInocêncioLBGuimarãesKCCNemrNKA percepção do acompanhante e do indivíduo com ronco/saos antes e após fonoterapia [Perception of the bed partner and the individual suffering from SNORING/OSAS before and after speech therapy]Rev CEFAC201416907916 Portuguese [with English abstract]
- VermaRKJohnsonJJrGoyalMBanumathyNGoswamiUPandaNKOropharyngeal exercises in the treatment of obstructive sleep apnoea: our experienceSleep Breath2016201193120126993338
- MohamedASSharsharRSElkolalyRMSerageldinMUpper airway muscle exercises outcome in patients with obstructive sleep apnea syndromeChest201766121125
- Sociedade Brasileira de FonoaudiologiaDocumento do Comitê de Motricidade Orofacial [Document of the Orofacial Motricity Committee]2007 Available from: http://www.sbfa.org.br/portal/pdf/dicionario_mfo.pdf. SpanishAccessed August 6, 2018
- American Speech-Language-Hearing AssociationOrofacial myofunctional disorders: knowledge and skills [guidelines, knowledge and skills]2004 Available from: http://www.asha.org/policyAccessed August 6, 2018
- RogersAPMuscle training and its relation of orthodontiaInt J Orthod19184555577
- RogersAPA restatement of the myofunctional concept in orthodonticsAm J Orthod19503684585514783191
- SubtelnyJDSubtelnyJDMalocclusion, speech, and deglutitionAm J Orthod196248685697
- MasonRMA retrospective and prospective view of orofacial myologyInt J Orofacial Myology20083451419545087
- SchievanoDPuppin-RontaniRMBérzinFInfluence of myofunctional therapy on the perioral muscles – clinical and electromyographic evaluationsJ Oral Rehabil19992656456910445475
- MarchesanIQKrakauerLRThe importance of respiratory activity in myofunctional therapyInt J Orofacial Myology19962223279487822
- ValeraFCPTrawitzkiLVVMattarSEMMatsumotoMAEliasAMAnselmo-LimaWTMuscular, functional and orthodontic changes in pre school children with enlarged adenoids and tonsilsInt J Pediatr Otorhinolaryngol20036776177012791452
- de FelícioCMSilva DiasFVFolhaGAOrofacial motor functions in pediatric obstructive sleep apnea and implications for myofunctional therapyInt J Pediatr Otorhinolaryngol20169051127729152
- FolhaGAValeraFCde FelícioCMValidity and reliability of a protocol of orofacial myofunctional evaluation for patients with obstructive sleep apneaEur J Oral Sci201512316517225780946
- ClarkHMNeuromuscular treatments for speech and swallowing: a tutorialAm J Speech Lang Pathol20031240041514658992
- JohnsMWA new method for measuring daytime sleepiness: the Epworth sleepiness scaleSleep1991145405451798888
- SilABarrGAssessment of predictive ability of Epworth scoring in screening of patients with sleep apnoeaJ Laryngol Otol201212637237922152589
- PrikladnickiAMartinezDBrunettoMGFioriCZLenzMDCSGomesEDiagnostic performance of cheeks appearance in sleep apneaCranio20182118
- LimJSLeeJWHanCKwonJWCorrelation of soft palate length with velum obstruction and severity of obstructive sleep apnea syndromeAuris Nasus Larynx20184549950328807529
- KimEJChoiJHKimKWThe impacts of open-mouth breathing on upper airway space in obstructive sleep apnea: 3-D MDCT analysisEur Arch Otorhinolaryngol201126853353920957487
- ClarkHMSpecificity of training in the lingual musculatureJ Speech Lang Hear Res20125565766722215031
- PearsonWGJrLangmoreSEZumwaltACEvaluating the structural properties of suprahyoid muscles and their potential for moving the hyoidDysphagia20112634535121069388
- MatsuoKPalmerJBAnatomy and physiology of feeding and swallowing: normal and abnormalPhys Med Rehabil Clin N Am20081969170718940636
- ErtekinCAydogduINeurophysiology of swallowingClin Neurophysiol20031142226224414652082
- MillerAJThe neurobiology of swallowing and dysphagiaDev Disabil Res Rev200814778618646019
- SandersAEEssickGKFillingimRSleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohortJ Dent Res20139270S77S23690360
- FelícioCMDesordens temporomandibulares: terapia fonoaudiológica [Temporomandibular disorders: speech-language therapy]FelícioCMTrawitzkiLVVInterfaces da Medicina - Odontologia e Fonoaudiologia no Complexo Cérvico-craniofacial [Interfaces of Medicine, Dentistry and Speech-Language Therapy in the Cervico-Craniofacial Complex]1st edBarueriPró-Fono2009145198 Portuguese
- SteeleCMOn the plausibility of upper airway remodeling as an outcome of orofacial exerciseAm J Respir Crit Care Med200917985885919423718
- PintoJAGodoyLBMarquisVWSonegoTBLeal CdeFArticoMSAnthropometric data as predictors of obstructive sleep apnea severityBraz J Otorhinolaryngol20117751652121860980
- EckertDJLoYLSaboiskyJPJordanASWhiteDPMalhotraASensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apneaJ Appl Physiol20111111644165321885797
- SaboiskyJPButlerJELuuBLGandeviaSCNeurogenic changes in the upper airway of obstructive sleep apnoeaCurr Neurol Neurosci Rep2015151225704006